Abstract
Group IVA cytosolic phospholipase A2 (cPLA2α) catalyzes release of arachidonic acid from glycerophospholipids, leading to thromboxane A2 (TxA2) production. Some platelet agonists stimulate cPLA2α, but others require fibrinogen binding to αIIbβ3 to elicit TxA2. Therefore, relationships between cPLA2α and αIIbβ3 were examined. cPLA2α and a cPLA2α binding partner, vimentin, coimmunoprecipitated with αIIbβ3 from platelets, independent of fibrinogen binding. Studies with purified proteins and with recombinant proteins expressed in CHO cells determined that the interaction between cPLA2α and αIIbβ3 was indirect and was dependent on the αIIb and β3 cytoplasmic tails. Fibrinogen binding to αIIbβ3 caused an increase in integrin-associated cPLA2α activity in normal platelets, but not in cPLA2α-deficient mouse platelets or in human platelets treated with pyrrophenone, a cPLA2α inhibitor. cPLA2α activation downstream of αIIbβ3 had functional consequences for platelets in that it was required for fibrinogen-dependent recruitment of activated protein kinase Cβ to the αIIbβ3 complex and for platelet spreading. Thus, cPLA2α and αIIbβ3 interact to reinforce each other's functions during αIIbβ3 signaling. This provides a plausible explanation for the role of αIIbβ3 in TxA2 formation and in the defective hemostatic function of mouse or human platelets deficient in cPLA2α.
Introduction
αIIbβ3 is the most abundant integrin in platelets, mediating such essential functions as aggregation and adhesive spreading. Binding of multivalent ligands, such as fibrinogen or von Willebrand factor (VWF), to αIIbβ3 triggers “outside-in” signals that promote cytoskeletal reorganization and granule release to enhance stability of the primary platelet plug.1 αIIbβ3 has been shown to interact directly or indirectly with cytoskeletal components, such as talin,2,3 skelemin,4 actin,5,6 and myosin7 as well as molecules involved in cell signaling, such as Src, Syk,8 PTP-1B,9 protein kinase Cβ (PKC-β),10 PP1c,11 and CIB.12 Whereas work with gene-targeted mice indicates that some of these interactions are dynamic and biologically relevant, the full range of protein interactions involving αIIbβ3 during hemostasis remains incompletely characterized.
During platelet activation, phospholipases are activated to release several key second messengers from membrane phospholipids, including diacylglycerol, inositol-1,4,5-triphosphate (IP3), and arachidonic acid (AA).13 AA is released from the sn-2 position of glycerophospholipids by the action of phospholipase A2 (PLA2) and AA is a precursor of the platelet agonist, thromboxane A2 (TxA2).14,15 Platelets are known to contain one cytosolic phospholipase A2 form (cPLA2α, also known as group IVA PLA2) and one secreted phospholipase A2 (sPLA2, also known as group IIA PLA2).16 AA production in platelets is dependent on cPLA2α but not sPLA2.17 Highlighting the clinical importance of this enzyme, a patient was recently reported to have a functional deficiency of cPLA2α associated with recurrent small intestinal ulcerations and diminished platelet aggregatory and secretory responses in response to adenosine diphosphate (ADP) and collagen.18 Mice deficient in cPLA2α have prolonged bleeding times and are resistant to thromboembolism induced by injection of a mixture of ADP and collagen, further indicating a role for this enzyme in platelet adhesive and hemostatic functions.19 There is indirect evidence of a link between αIIbβ3 outside-in signaling and cPLA2α activation in agonist-treated platelets: cPLA2α activity is reduced or lost in the presence of αIIbβ3-blocking agents, such as antibodies20,21 or small molecules.22,23 Conversely, induction of fibrinogen binding to platelets with an αIIbβ3-activating antibody stimulates TxA2 formation.24 Studies of other cell types suggest a more general role for integrins in cPLA2α activation. In HeLa cells, clustering of β1 integrins with antibodies leads to AA production25; adhesion of NIH3T3 or RBL cells to a fibronectin matrix triggers cPLA2α activation26,27; and production of AA in bovine pulmonary artery endothelial cells is inhibited by an αVβ3 function-blocking antibody.28 In these cells as in platelets, the precise relationships between cPLA2α and integrins are unclear.
The goal of the present study was to clarify the relationships between cPLA2α and αIIbβ3 in platelets. Biochemical and functional studies were carried out with purified proteins, human platelets, platelets from cPLA2α-deficient mice, and CHO cells expressing recombinant cPLA2α and αIIbβ3. When indicated, we also used pyrrophenone, a pyrrolidine-derived inhibitor of cPLA2α.29 The results establish that a pool of cPLA2α is constitutively associated with αIIbβ3 in platelets, with functional consequences for integrin signaling.
Methods
Chemicals, reagents, and kits
Biotin was from Research Organics (Cleveland, OH). ADP, thrombin, prostaglandin E1 (PGE1), apyrase, heparin, p-nitrophenyl phosphate, isopropyl-beta-D-thiogalactopyranoside, and antitalin antibody were from Sigma-Aldrich (St Louis, MO). Protease inhibitor cocktail was from Roche Diagnostics (Indianapolis, IN). Pentobarbitol was from Ovation Pharmaceuticals (Deerfield, IL). AA, U46619, SQ29,548, 2-aminoethoxydiphenyl borate (2-APB), and a cPLA2α assay kit were from Cayman Chemical (Ann Arbor, MI). Integrilin was from COR Therapeutics (South San Francisco, CA). Nickel resin was from EMD Biosciences (San Diego, CA). The bovine serum albumin used to block nitrocellulose membranes was from MP Biomedicals (Solon, OH). N,N-[1,2-ethanediylbis(oxy-2,1-phenylene)]bis[N-[2-[(acetyloxy)methoxy]-2-oxoethyl]]-bis[(acetyloxy)methyl]-ester (BAPTA-AM), rhodamine-phalloidin, Alexa-Fluor 546-conjugated fibrinogen, and β-BODIPY FL C5-HPC were from Invitrogen (Carlsbad, CA). Purified fibrinogen was from Enzyme Research Laboratories (South Bend, IN). Silica gel thin layer chromatography plates were from Whatman (Clifton, NJ). Methanol-free formaldehyde was from Polysciences (Warrington, PA). Mounting medium (Citifluor) was from Ted Pella (Redding, CA). Horseradish peroxidase (HRP) conjugation kit was from KPL (Gaithersburg, MD). Neutravidin beads were from Pierce Chemical (Rockford, IL). Pyrrophenone was a gift from Drs Takashi Ono and Kaoru Seno (Shionogi, Osaka, Japan). Collagen-related peptide (CRP) was a gift from Dr Peter J. Newman (Blood Research Institute, Milwaukee, WI). The murine PAR4 receptor-activating peptide, AYPGKF, was synthesized by the Scripps Laboratories (San Diego, CA).
SSA6, a murine monoclonal anti–human β3 antibody used for immunoprecipitations, was affinity-purified using a kit from Pierce Chemical. PMI-1, a mouse monoclonal used for the immunodetection of αIIb, was described previously.30 Antibodies used for immunoprecipitation of mouse β3 or for immunodetection of β3, PKC-β1, vimentin, and vinculin were from Santa Cruz Biotechnology (Santa Cruz, CA). Western blotting antibodies to c-Src and histidine tag were from Millipore (Billerica, MA), and antibodies to phospho c-Src Tyr418 and hemagglutinin epitope tag were from Invitrogen. An immunoprecipitating antibody to cPLA2α was from Abcam (Cambridge, MA), and the corresponding Western blotting antibody from Cell Signaling Technology (Danvers, MA). Secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA) if HRP-conjugated, LI-COR Biosciences (Lincoln, NE) if IRDye-labeled, and Invitrogen if Alexa-Fluor–labeled. We received University of California, San Diego Institutional Review Board approval for both human platelet and mouse studies.
Plasmids and constructs
Sequences for the His-Avi-Fos-β3 and His-Avi-Jun-αIIb recombinant integrin αIIb and β3 cytoplasmic tails are based on previous work31 and detailed in Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). All constructs were cloned into a modified pET15b (pET15bm). HA2-tagged cPLA2α was subcloned from pVL1393-cPLA2α32 into the pCDM8 mammalian expression plasmid and subsequently subcloned into blunted AgeI/PshAI-open FG12.33
Cell lines
CHO cells stably expressing either αΙΙbβ3, αΙΙbΔ996β3 or αΙΙbβ3Δ724 were described previously.34,35 All cell lines were grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, penicillin, streptomycin, and nonessential amino acids. CHO cells were transduced with cPLA2α using lentivirus FG12 and studied 4 days later.
Platelet isolation
Venous blood was collected from healthy human donors in Walsh buffer (137 mM NaCl, 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 5.6 mM dextrose, 1 g/L bovine serum albumin, 1 mM MgCl2, 2.7 mM KCl, 3.3 mM NaH2PO4, pH 7.4). Mice deficient in cPLA2α were described previously19 and were backcrossed more than 35 times. All experiments with platelets from these mice included age- and sex-matched wild-type controls. For platelet isolation, mice were anesthetized with 1 mg of pentobarbital per 10 g body weight. Blood was drawn from the portal vein, anticoagulated with 15 U/mL of heparin, diluted with one volume of wash buffer,9 and finally resuspended in Walsh buffer. In some experiments, platelets in Walsh buffer were pretreated with specified inhibitors for 15 minutes at room temperature before addition of a platelet agonist. When aspirin-treated platelets were required, platelet-rich plasma was incubated with 1 μM of acetylsalicylic acid for 30 minutes at room temperature before preparation of washed platelets.
Fibrinogen binding assay
Washed platelets were adjusted to a final concentration of 108/mL in Walsh's buffer supplemented with 1 mM of CaCl2. For fibrinogen binding measurements, 50 μL of platelet suspension was incubated with 150 μg/mL of Alexa-Fluor 546-conjugated fibrinogen in the presence or absence of a platelet agonist for 30 minutes at room temperature. Specific fibrinogen binding was assessed by flow cytometry.36
Immunoprecipitation and Western blotting
Washed platelets were pelleted by centrifugation at 9279g for 1 second and lysed in NP-40 lysis buffer (1% NP-40, 150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 1 mM Na3VO4, 0.1 mM NaF, Roche protease inhibitor cocktail). Immunoprecipitations were carried out as described.9 Experiments in which purified His6-cPLA2α was used as a bait for platelet integrin β3 were performed as follows: 20 μg of His6-cPLA2α was added to 1-mg platelet lysate in 400 μL of NP-40 lysis buffer in the presence of either 5 μg mouse IgG or 5 μg of anti-His6 antibody, and incubated overnight at 4°C. Samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and immunoblotting was performed with either HRP-conjugated secondary antibodies for chemiluminescent detection using ECL reagent (Pierce Chemical) or IRDye-labeled secondary antibodies for scanning in an Odyssey infrared imaging system (LI-COR Biosciences).
Measurements of cPLA2α activity
cPLA2α activity in platelet lysates or in αIIbβ3 immunoprecipitates was measured with an assay kit from Cayman Chemical. For studies with cells in suspension, 100 μL of platelets (4 × 108/mL) or 2 mL of CHO cells (2 × 106/mL) in Walsh buffer with 1 mM CaCl2 was sedimented and sonicated in buffer containing 50 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, 1 mM ethylenediaminetetraacetic acid, and protease inhibitor cocktail. Samples were dialyzed in 50 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4 and 1 mM ethylenediaminetetraacetic acid to remove thiol species and thiol scavengers, and cPLA2α activity was measured in triplicate on 10-μL samples following the manufacturer's directions. For each experiment, a baseline readout for the assay was determined in buffer alone, and this value was subtracted to obtain the cPLA2α activity derived specifically from the cells.
To study the effect of platelet adhesion on cPLA2αactivity, but to limit secondary cPLA2α activation in adherent platelets by released TxA2 or ADP, mouse platelets in plasma were incubated for 30 minutes at room temperature with 180 μg/mL of aspirin and resuspended at 6 × 108 platelets/mL in Walsh's buffer supplemented with 1 mM of CaCl2 and 1 U/mL of apyrase. Cells were plated for 30 minutes in plastic wells that had been precoated with 100 μg/mL of human fibrinogen, 10 μg/mL of murine VWF dimeric A1A2 domain, or 5 mg/mL of bovine serum albumin.37 After removing nonadherent cells, adherent platelets were lysed in NP-40 lysis buffer and cPLA2α activity was determined on 3 μg of lysate protein.
cPLA2α activity in platelet immunoprecipitates was determined by incubating cPLA2α or β3 immunoprecipitates at 37°C for 3 hours in the presence of buffer containing 200 ng β-BODIPY FL C5-HPC, 13.7 mM NaCl, 0.537 mM KCl, 25 μM Na2HPO4, 44 μM KH2PO4, 1 mM MgSO4, and 4.2 mM NaHCO3, pH 7.2. Lipids were extracted by adding 3 vol of 2:1 chloroform:methanol and vortexing. After centrifugation for 30 seconds at 16 000g, the solvent phase was recovered and evaporated in a speed vac set on low dry. Lipids were resuspended in 5 μL of chloroform and 0.2-μL samples were spotted onto a 60-Å silica gel thin layer chromatography plate. Thin layer chromatographic separation was performed in 50:40:2:0.2 toluene/diethyl ether/ethanol/acetic acid and the plate analyzed using a fluorescence scanner.
Fluorescence microscopy
Platelet adhesion to fibrinogen-coated glass slides, fixation, permeabilization and staining were carried out as described.8 Cells were stained with anti-β3 antibody (at 1/100 in 10% serum phosphate-buffered saline [PBS]) or antiphosphotyrosine (pTyr) 4G10 (1/1000 in 10% serum-PBS) for 45 minutes at 37°C and then with Alexa-Fluor 488-labeled secondary antibody (1/1000) and rhodamine-phalloidin (1/40 in 10% serum-PBS) for 45 minutes at 37°C. Negative controls included cells stained with an isotype control primary immunoglobulin and cells stained with secondary antibody only. After mounting in Citifluor, β3/actin-stained images were captured on an Olympus T-2000 equipped with a 60× objective, and pTyr/actin-stained images on a Deltavision RT deconvolution microscope equipped with a 100× objective. Spreading was quantified by measuring platelet surface areas using National Institutes of Health ImageJ software.38
Protein expression and purification
C-terminal His6-tagged cPLA2α was expressed using recombinant baculovirus in a suspension culture of Sf9 insect cells. The cell pellet was lysed in 25 mM Tris-HCl, pH 8.0, 150 mM NaCl, 2 mM β-mercaptoethanol, and 2 mM ethyleneglycoltetraacetic acid, and the insoluble portion was removed by centrifugation at 12 000g for 30 minutes. The supernatant was passed through a column composed of 6 mL of nickel-nitrilotriacetic acid agarose (Qiagen, Valencia, CA). The protein in the native state was eluted in 25 mM Tris-HCl, pH 8.0, 100 mM NaCl, 125 mM imidazole, and 2 mM dithiothreitol. Protein concentration was measured using the Bradford assay (Bio-Rad, Hercules, CA), and activity was verified using mixed micelles in a modified Dole assay.
Interaction of cPLA2α with recombinant integrin cytoplasmic tails
Recombinant, biotin-labeled integrin αIIb and β cytoplasmic tails were expressed and purified as described previously (Figure S1).39 After conjugation of integrin tails to neutravidin agarose beads, beads were incubated with either 2 mg of platelet lysate or 20 μg of purified recombinant His6-tagged cPLA2α in NP-40 lysis buffer overnight at 4°C. After 3 washes in NP-40 lysis buffer, integrin tail beads were boiled in Laemmli buffer, and cPLA2α and other proteins pulled down by the beads were identified by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western blotting, as described.39
Results
Fibrinogen binding to αIIbβ3 triggers cPLA2α activation in platelets
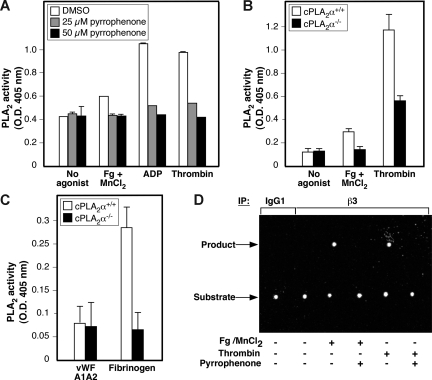
Fibrinogen binding to αIIbβ3 stimulates intracellular enzymes in apparent physical proximity to the integrin, including PKC-β, Src family kinases, Syk, and PP1c.1,8,10,11 Therefore, to investigate the relationship between cPLA2α and αIIbβ3 in human platelets, we first assessed the effect of fibrinogen binding to αIIbβ3 on cPLA2α activity measured in platelet lysates using arachidonoyl thio-phosphatidyl choline as a cPLA2α substrate.40 To determine the direct effects of fibrinogen binding, washed human platelets were incubated with 1 mM MnCl2 and 300 μg/mL fibrinogen for 30 minutes, the former to convert αIIbβ3 to a high-affinity state without the necessity of adding a more global platelet agonist41 and the latter to saturate fibrinogen binding sites in αIIbβ3.42 Fibrinogen binding triggered a modest but consistent and statistically significant increase in platelet cPLA2α activity (P ≤ .003), and this response was prevented by preincubation of the platelets with either pyrrophenone, an inhibitor of cPLA2α29 (Figure 1A), or integrilin, a selective αIIbβ3 antagonist. Incubation of platelets with either fibrinogen or MnCl2 alone had no effect on cPLA2α activity (data not shown). As expected, incubation of platelets with agonists, such as ADP or thrombin, yielded a more robust cPLA2α response that was largely inhibited by pyrrophenone (Figure 1A). To establish unambiguously that the cPLA2α response to fibrinogen reflected the activity of cPLA2α, platelets from cPLA2α−/− knockout mice, or cPLA2α+/+ wild-type littermates were studied. Fibrinogen binding induced by MnCl2 caused a reproducible increase in cPLA2α activity in cPLA2α+/+ platelets, but not in cPLA2α−/− platelets (Figure 1B). To determine whether platelet adhesion to immobilized fibrinogen was sufficient to activate cPLA2α, washed mouse platelets were treated with aspirin and apyrase to limit potential secondary activation of cPLA2α by TxA2 and ADP during the adhesion process. Under these conditions, platelet adhesion to fibrinogen triggered an approximate 3-fold increase in cPLA2α activity in wild-type platelets, but not in cPLA2α−/− platelets (Figure 1C). Furthermore, the cPLA2α response in wild-type platelets was not a general feature of platelet adhesion because it was not observed in cells plated on dimeric VWF A1A2 domain, which engages platelets via GP Ib-V-IX but not αIIbβ3.37 Taken together, these results establish that cPLA2α can become activated in platelets in response to fibrinogen binding to αIIbβ3, independently of released ADP or TxA2.
Figure 1.
Fibrinogen binding to αIIbβ3 promotes activation of cPLA2α in platelets. (A-C) Values on the y-axis represent the average cPLA2α activity of each sample minus the baseline cPLA2α activity determined for reaction buffer alone. (A) Washed human platelets were preincubated with DMSO vehicle or with 25 or 50 μM of pyrrophenone before incubation at 37°C for 30 minutes in the presence or absence of the indicated agonists (300 μg/mL of fibrinogen (Fg) + 1 mM of MnCl2, 20 μM of ADP, or 1 U/mL of thrombin). cPLA2α activity in platelet lysates was measured as described in “Methods.” Data represent the mean plus or minus SEM of 2 independent experiments, each performed in triplicate. (B) cPLA2α activity was measured in platelet lysates from cPLA2α−/− and wild-type cPLA2α+/+ littermates. Platelets were treated while in suspension with saline (no agonist), 100 μg/mL fibrinogen + 1 mM MnCl2, or 1 U/mL of thrombin. Data represent mean plus or minus SEM of triplicate determinations carried out in individual mice (2 mice for each genetic background) on 2 separate occasions. (C) cPLA2α activity was measured in platelets from either wild-type or cPLA2α−/− mice. The assay was performed on lysates from platelets adherent to either recombinant murine VWF A1A2 domain or human fibrinogen. As a further control, platelets incubated on immobilized bovine serum albumin failed to adhere. Data represent mean plus or minus SEM of triplicate determinations carried out in individual mice (2 mice for each genetic background) on 2 separate occasions. (D) Lysates from human platelets treated with either 300 μg/mL of fibrinogen + 1 mM of MnCl2 or 1 U/mL of thrombin in the presence or absence of 50 μM of pyrrophenone were immunoprecipitated with anti-β3 SSA6 antibody or a nonimmune murine IgG1 control. Immunoprecipitates were incubated with 200 ng of the PLA2 substrate β-BODIPY FL C5-HPC for 3 hours at 37°C. The presence of αIIbβ3-associated PLA2 activity was monitored by thin layer chromatography detection of a cleaved product of β-BODIPY FL C5-HPC, an assay strictly qualitative in nature. This experiment was performed twice with identical results.
cPLA2α is constitutively associated with and regulated by αIIbβ3
Based on these results, we wondered if there might be a proximal interaction between αIIbβ3 and cPLA2α, such that fibrinogen binding to the former led to activation of the latter. To explore this possibility, platelets were incubated in the absence or presence of 1 mM of MnCl2 and 300 μg/mL of fibrinogen, or with 1 U/mL of thrombin as a general platelet activator. Then αIIbβ3 was immunoprecipitated with an anti-β3 antibody under conditions where the αIIbβ3 complex is maintained, and the precipitate was examined for PLA2 activity by thin layer chromatography using the PLA2 fluorescent substrate, β-BODIPY FL C5-HPC43 (Figure 1D). This substrate remained uncleaved after incubation with the β3 immunoprecipitate from unstimulated platelets. In contrast, a cleavage product was obtained in αIIbβ3 immunoprecipitates from platelets incubated with either MnCl2/fibrinogen or thrombin (Figure 1D). However, no such substrate cleavage was observed if the platelets were pretreated with the cPLA2α inhibitor, pyrrophenone.
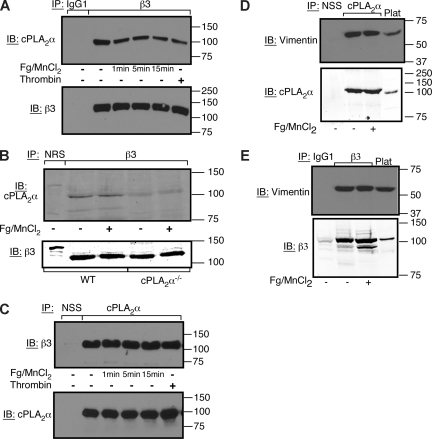
To further evaluate a proximal interaction between cPLA2α and αIIbβ3, αIIbβ3 immunoprecipitates were examined for the presence of cPLA2α. cPLA2α specifically coimmunoprecipitated with the integrin both from resting human platelets and platelets treated with MnCl2/fibrinogen or thrombin (Figure 2A). Similar results were obtained with αIIbβ3 immunoprecipitates from cPLA2α+/+ mouse platelets. No specific immunoreactive cPLA2α band was detected in αIIbβ3 immunoprecipitates from cPLA2α−/− platelets (Figure 2B). cPLA2α association with αIIbβ3 was also observed if cPLA2α immunoprecipitates from human platelets were examined for the presence of β3 (Figure 2C). Collectively, these results indicate that a pool of cPLA2α is constitutively associated with αIIbβ3 in platelets, and it becomes activated in response to fibrinogen binding.
Figure 2.
cPLA2α is constitutively associated with αIIbβ3 in human and mouse platelets. (A,C) Coimmunoprecipitation of αIIbβ3 and cPLA2α was assayed in washed human platelets treated with 300 μg/mL of fibrinogen + 1 mM of MnCl2 for 0 to 15 minutes at 37°C as indicated, or with 1 U/mL of thrombin for 5 minutes. Each experiment was performed 3 times with identical results. NSS indicates normal sheep serum. (B) cPLA2α−/− or wild-type littermate platelets were incubated in the presence or absence of 300 μg/mL of fibrinogen + 1 mM of MnCl2 for 5 minutes at 37°C. Then αIIbβ3 immunoprecipitates were examined by Western blotting for the presence of cPLA2α. NRS indicates normal rabbit serum. This experiment was performed twice. (D,E) cPLA2α and vimentin coimmunoprecipitate with αIIbβ3. Washed human platelets were incubated in the presence or absence of 300 μg/mL of fibrinogen + 1 mM of MnCl2 for 10 minutes at room temperature. Platelets were lysed in NP-40 lysis buffer, and lysates were immunoprecipitated with an antibody to cPLA2α (D) or β3 (E). Immunoblots were probed with an antibody to vimentin, cPLA2α, or β3. This experiment was performed twice.
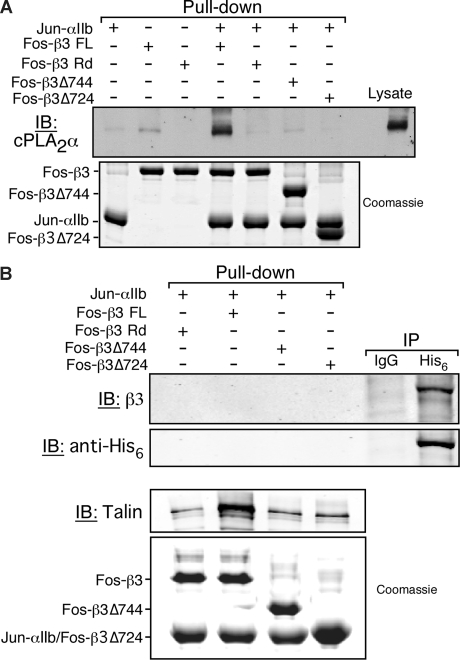
Given the intracellular location of cPLA2α, these results suggest that this protein might interact with the cytoplasmic tails of αIIb and/or β3. To determine whether cPLA2α can interact with isolated αIIb and/or β3 tails, a pull-down assay was used with cPLA2α from platelet lysate and solid-phase, recombinant integrin cytoplasmic tails (Figure S1).39 cPLA2α was specifically pulled down by a recombinant heterodimer of the αIIbβ3 tails, but not by the αIIb tail alone or a randomized β3 tail sequence (β3 Rd) (Figure 3A). Full-length β3 (β3 FL) alone interacted only weakly with cPLA2α, and truncation mutants of the heterodimeric αIIbβ3 tails containing either the 7 or 27 most membrane-proximal β3 tail residues (Δ724 and Δ744, respectively) failed to bind cPLA2α (Figure 3A).
Figure 3.
cPLA2α binding to αIIbβ3 is indirect and depends on αIIb and β3 cytoplasmic tails. Neutravidin beads coated with the indicated integrin cytoplasmic tail model proteins were incubated with platelet lysate or purified His6-cPLA2α as described in “Methods.” (A) cPLA2α was detected by Western blotting. Equal loading of recombinant integrin tails was determined by Coomassie staining. This experiment was performed 3 times. (B) The His6-cPLA2α pull-down assay (first 4 lanes) was performed by incubating 20 μg of purified recombinant His6-cPLA2α with the indicated integrin tails bound to neutravidin beads. The presence of His6-cPLA2α in the pull-down was assayed by immunodetection using an anti-His6 antibody. Simultaneously, His6-cPLA2α was tested for its ability to interact with αIIbβ3 from human platelet lysate in an immunoprecipitation assay (IP) using either an irrelevant isotype match control antibody (IgG) or an anti-His6 antibody (His6; last 2 lanes). Equal loading of recombinant tails was determined by Coomassie staining. This experiment was performed twice.
To evaluate whether the interaction between cPLA2α and the cytoplasmic tails of αIIbβ3 is direct or indirect, recombinant integrin tails were used as bait to pull down purified recombinant His6-tagged cPLA2α. Whereas the full-length αIIbβ3 tail heterodimer was capable of pulling down talin (a protein known to interact directly with αIIbβ3), it failed to interact with the purified cPLA2α (Figure 3B). However, when purified His6-tagged cPLA2α was added to platelet lysate, β3 could be recovered along with His6-tagged cPLA2α in immunoprecipitates prepared using an anti-His antibody (Figure 3B). These results suggest that cPLA2α interacts indirectly with the cytoplasmic tails of αIIbβ3 through one or more intermediary proteins. One such protein may be vimentin because it was shown previously to interact with cPLA2α44 and integrin cytoplasmic tails,45–48 and it coimmunoprecipitated with both αIIbβ3 and cPLA2α from platelets (Figure 2D,E).
To examine the role of the αIIb and β3 cytoplasmic tails in cells, studies were performed in CHO cells expressing cPLA2α-HA and either wild-type αIIbβ3, αIIbΔ996β3, which lacks most of the αIIb tail, or αIIbβ3Δ724, which lacks most of the β3 tail.34,35 Although cPLA2α-HA (and endogenous vimentin) was readily detected in αIIbβ3 immunoprecipitates from αIIbβ3-CHO cells, the amount of cPLA2α-HA associated with the integrin in αIIbΔ996β3-CHO and αIIbβ3Δ724-CHO cells appeared to be reduced (Figure S2). Furthermore, αIIbβ3-associated cPLA2α activity was stimulated by fibrinogen binding to αIIbβ3-CHO cells but not by fibrinogen binding to αIIbΔ996β3-CHO or αIIbβ3Δ724-CHO cells (Figure S2). Thus, full association of cPLA2α with αIIbβ3 and its stimulation by fibrinogen binding require intact integrin cytoplasmic tails.
Functional consequences of cPLA2α activation during outside-in αIIbβ3 signaling
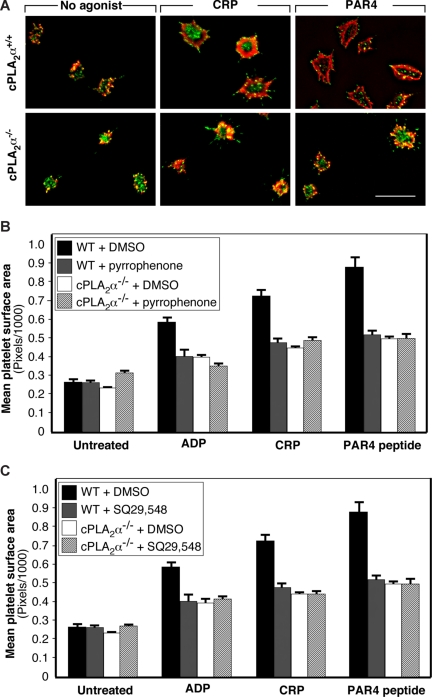
A prominent response of platelets after attachment to immobilized fibrinogen is spreading driven by actin polymerization and rearrangements.6 To determine whether cPLA2α is involved in this process, cPLA2α−/− and cPLA2α+/+ platelets were plated on fibrinogen for 30 minutes, fixed, stained for integrin β3, F-actin, and phosphotyrosine-containing proteins, and examined by fluorescence microscopy. As is typical of normal mouse platelets, unstimulated wild-type and cPLA2α−/− platelets demonstrated various degrees of filopodial extension but minimal lamellipodial extension (Figure 4A). Both wild-type and cPLA2α−/− platelets underwent progressive lamellipodial extension and spreading in response to 20 μM of ADP, 10 ng/mL of CRP, or 1 mM of PAR4 receptor-activating peptide. However, the cPLA2α−/− platelets spread less well as determined by computerized image analysis of platelet surface areas (Figure 4B). Indeed, the spreading of agonist-treated cPLA2α−/− platelets never exceeded 60% of that observed for cPLA2α+/+ platelets. Furthermore, the reduced spreading of pyrrophenone-treated cPLA2α+/+ platelets was similar to that observed for cPLA2α−/− platelets (Figure 4B), supporting a conclusion that it was the lack of cPLA2α activation that was responsible for defective spreading of cPLA2α−/− platelets.
Figure 4.
Role of cPLA2α in platelet spreading on fibrinogen. Washed platelets were allowed to adhere to fibrinogen-coated slides for 30 minutes at 37°C in the presence or absence of 20 μM of ADP, 10 μg/mL of CRP, or 1 mM of PAR4 receptor-activating peptide. (A) Platelets were stained with anti-pTyr antibody (green) and rhodamine-phalloidin (red). Images were captured on a deconvolution microscope equipped with a 100× objective. Each panel is representative of 3 separate experiments. Bar represents 10 μm. (B) Pyrrophenone was used at a concentration of 50 μM. (C) SQ29 548 was used at a concentration of 10 μM. In panels B and C, platelets were stained with anti-β3 antibody and rhodamine-phalloidin. Images were captured on an Olympus T-2000 microscope, and platelet surface areas were quantified by computerized image analysis. Data are mean plus or minus SEM of at least 100 platelets per treatment condition.
Integrin-dependent cPLA2α activation should result in phospholipid hydrolysis, AA release, and TxA2 production. To determine whether cPLA2α-dependent TxA2 production was contributing to the spreading response of platelets on fibrinogen, cPLA2α+/+ platelets were preincubated with SQ29,548, an antagonist of the platelet TP TxA2 receptor. This compound caused a spreading defect in wild-type platelets comparable with that caused by pyrrophenone and comparable with that observed in cPLA2α−/− platelets (Figure 4B,C). Because full platelet spreading on fibrinogen requires signaling downstream of αIIbβ3 and agonist receptors,49 these results indicate that cPLA2α and production of TxA2 are required for this costimulatory response.
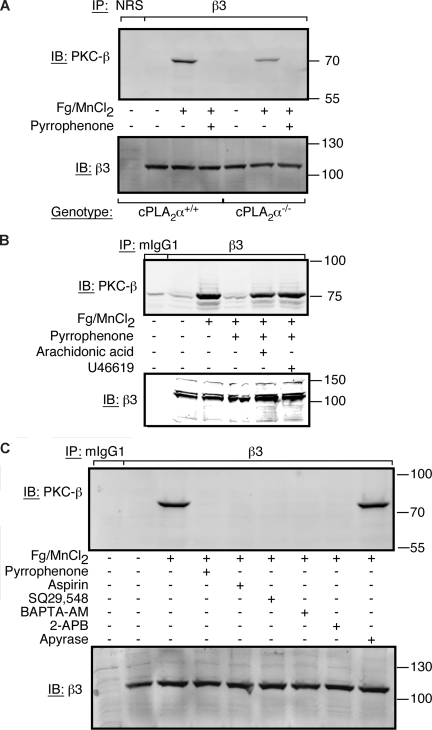
Fibrinogen binding to αIIbβ3 triggers at least 2 signaling pathways: one involving Src kinases, which like cPLA2α is constitutively associated with αIIbβ3,8,9 and the other involving activated PKC-β, which inducibly associates with β3 via the adapter molecule, RACK1.10 Because inhibition of integrin-associated Src activity or PKC-β recruitment each results in a platelet spreading defect,9,10 we investigated the potential contribution of cPLA2α to these events. Inhibition of cPLA2α by pyrrophenone had no effect on c-Src activation in human platelets in response to fibrinogen binding (not shown). On the other hand, pyrrophenone blocked fibrinogen-dependent recruitment of PKC-β to β3 in cPLA2α+/+ platelets, and PKC-β recruitment was reduced in cPLA2α−/− platelets (Figure 5A). Similarly, pyrrophenone blocked PKC-β recruitment to β3 in human platelets, and this effect could be rescued by addition of either 100 μM of AA or 10 μM of U46619, a TP TxA2 receptor agonist (Figure 5B). Similarly, aspirin, which blocks cyclooxygenase and AA conversion to prostaglandin endoperoxides and TxA2, or inhibitors of calcium signaling (intracellular Ca2+ chelator BAPTA-AM; IP3 receptor antagonist 2-ABP) blocked fibrinogen-dependent recruitment of PKC-β to β3, whereas the ADP scavenger, apyrase, had no effect (Figure 5C). These results indicate that PKC-β recruitment to β3 requires an integrin-dependent signaling pathway that involves sequential cPLA2α activation, AA release, TxA2 production, and TP receptor-mediated calcium transients. Accordingly, it is attenuation of this pathway that may contribute to the spreading defect of platelets in which cPLA2α has been genetically deleted or blocked by pyrrophenone.
Figure 5.
PKC-β recruitment to αIIbβ3 is dependent on AA/thromboxane A2 generation by cPLA2α. Where indicated, platelets were treated with 300 μg/mL of human fibrinogen + 1 mM MnCl2 for 30 minutes at 37°C. Immunoprecipitation was carried out with an anti-β3 antibody or normal rabbit serum (NRS) as a control as indicated in “Methods.” (A) Platelets were collected from 9 mice for each background and resuspended in Walsh buffer at a final concentration of 8 × 108/mL. Platelets were then treated with fibrinogen/MnCl2 in the presence or absence of 50 μM of pyrrophenone. The experiment was performed 3 times. (B) Washed human platelets were incubated in the presence or absence 50 μM of pyrrophenone and fibrinogen/MnCl2, fibrinogen/MnCl2 + 100 μM of AA, or fibrinogen/MnCl2 + 10 μM of U46619. This experiment was performed twice. (C) Washed human platelets were preincubated with 50 μM of pyrrophenone, 0.18 g/L of aspirin, 10 μM of SQ29,548, 100 μM of BAPTA-AM, 100 μM of 2-APB, or 1 U/mL of apyrase for 15 minutes at room temperature. The results are representative of 4 separate experiments.
cPLA2α and inside-out αIIbβ3 signaling
Wong et al have reported a defect in collagen-induced aggregation of cPLA2α−/− platelets, implying a role for cPLA2α in inside-out αIIbβ3 signaling.19 Therefore, platelets from wild-type and cPLA2α−/− mice were tested for their ability to bind Alexa-Fluor 546–labeled fibrinogen in response to ADP, CRP, or a PAR4 thrombin receptor-activating peptide. A wide range of concentrations was tested: ADP: 0.1 to 20 μM; CRP: 0.5 to 10 ng/μL; PAR4-activating peptide: 0.05 to 1 mM. Figure S3 displays representative flow cytometry histograms for lower and higher concentrations of CRP (2.5 and 10 μg/mL), PAR4 receptor-activating peptide (0.1 and 0.5 mM), and ADP (0.2 and 20 μM). Platelets from cPLA2α−/− mice showed impaired fibrinogen binding in response to CRP or the lower concentration of PAR4 peptide but normal binding in response to ADP or the higher concentration of PAR4 peptide.
Discussion
By studying both human and gene-targeted mouse platelets and using complementary biochemical and functional techniques, the present study has provided new insights into the relationship between αIIbβ3 and cPLA2α. The results establish that (1) fibrinogen binding to αIIbβ3 is sufficient to promote the activation of cPLA2α in platelets; (2) integrin-dependent cPLA2α activation may involve a pool of enzyme that is associated indirectly with αIIbβ3; (3) cPLA2α and the TxA2 generated as the result of AA release are required for certain outside-in αIIbβ3 signaling responses of platelets, notably the recruitment of activated PKC-β to αIIbβ3 and platelet spreading; and (4) cPLA2α is required for normal inside-out activation of αIIbβ3 in response to collagen and submaximal concentrations of a PAR4 thrombin receptor agonist. Thus, cPLA2α and αIIbβ3 appear to reinforce each other's activation, and such reinforcement may help to explain why platelets from cPLA2α-deficient mice or humans do not function normally during hemostasis and thrombosis.18,19
Activation of cPLA2α downstream of αIIbβ3
The PLA2 response triggered by direct, MnCl2-induced fibrinogen binding to platelets, although modest compared with the responses to ADP or thrombin, was consistent, statistically significant, and involved cPLA2α. Thus, all fibrinogen-dependent increases in PLA2 activity could be blocked by pyrrophenone (Figure 1A,D).29 Furthermore, whereas platelet interaction with fibrinogen stimulated PLA2 activity in wild-type cPLA2+/+ mouse platelets, even after aspirin and apyrase treatment, it failed to do so in platelets from cPLA2α−/− littermates (Figure 1B,C). On the other hand, cPLA2α−/− platelets retained some ability to increase their PLA2 activity in response to thrombin (Figure 1B), suggesting that one or more additional PLA2 species are present in these platelets. This might also explain why cPLA2α−/− platelets can still produce TxA2 in response to agonists19 and why some residual amounts of PKC-β can be recruited to αIIbβ3 after fibrinogen binding to these platelets (Figure 5A). Five cPLA2 isoforms other than cPLA2α have been described,50 and preliminary studies using reverse-transcribed polymerase chain reaction indicate that cPLA2δ is present in platelets (N.P., S.J.S., unpublished data, June 2008). Nonetheless, whatever residual PLA2 species might exist in cPLA2α−/− platelets, they cannot rescue the spreading defect exhibited by these cells (Figure 4).
Physical interaction between cPLA2α with αIIbβ3
When studied by coimmunoprecipitation of platelet lysate, a pool of cPLA2α was found to be associated with αIIbβ3, independent of fibrinogen binding to the integrin. Pull-down experiments with recombinant integrin tail mimics suggested that this interaction is indirect and may be mediated by both αIIb and β3 cytoplasmic tails. These observations raise new issues not addressed by the current studies. First, the protein(s) responsible for the association of cPLA2α with αIIbβ3 remains to be identified. A number of intracellular proteins have been shown to bind directly to the αIIb and/or β3 cytoplasmic tails in vitro,39 but only the intermediate filament protein vimentin has been shown to bind to both the C2 domain of cPLA2α44 and the cytoplasmic domains of integrins, as shown previously for α2β1 and αVβ3.47 Vimentin coimmunoprecipitated from platelet lysates with αIIbβ3 and cPLA2α (Figure 2D,E). Moreover, when cytoplasmic tail deletion mutants of αIIbβ3 were expressed in CHO cells, the interaction between vimentin and the integrin was lost in a manner similar to that of cPLA2α (Figure S2). However, additional studies will be required to determine the precise relationship between vimentin, cPLA2α, and αIIbβ3 in platelets.
A second issue relates to how ligation of αIIbβ3 regulates cPLA2α activity. In nucleated cells, the activity of cPLA2α appears to be regulated by an elevation in levels of free calcium and PIP2 and the subsequent translocation of the protein from the cytosol to the perinuclear region and nuclear envelope.15 In the anucleate platelet, association of cPLA2α with αIIbβ3 may target the enzyme to an ideal cellular location for access to both localized Ca2+ influx and glycerophospholipid substrates. For example, fibrinogen binding to αIIbβ3 triggers Ca2+ entry into platelets.51–53 Possible mediators for the activation of cPLA2α through αIIbβ3-mediated Ca2+ entry include the annexin family of calcium-channel proteins. In this context, annexin I is expressed in platelets54 and can associate with cPLA2α and integrins.55–58
Functions of cPLA2α in αIIbβ3 signaling
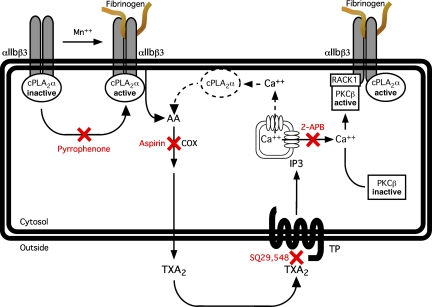
The reduced spreading of fibrinogen-adherent cPLA2α−/− platelets and of wild-type platelets treated with pyrrophenone (Figure 4A,B) suggests that cPLA2α is required for normal outside-in signaling downstream of αIIbβ3 (Figure 6). The observed spreading defect is probably the result of the loss of cPLA2α-mediated TxA2 production because this phenotype was mimicked in wild-type platelets by pretreatment with the TP receptor antagonist, SQ29,548 (Figure 4C). One apparent consequence of the reduction in cPLA2α activity and TxA2 production is a reduction in fibrinogen-dependent recruitment of activated PKC-β to the αIIbβ3 complex, a response that was also blocked by inhibiting cyclooxygenase with aspirin or calcium signaling with BAPTA-AM or 2-APB (Figure 5). Involvement of cPLA2α and TxA2 in this process was further confirmed by the ability of AA or U46619 to rescue PKC-β recruitment to αIIbβ3 in platelets treated with pyrrophenone (Figure 5B). The existence of Ca2+ fluxes downstream of αIIbβ3 has been clearly established,53,59–63 and they are required for the increase in PKC-β activity observed in MnCl2/fibrinogen-treated platelets.10 In the model presented in Figure 6, a distinction is to be made between the calcium mobilization triggered by activation of TP receptors by newly synthetized TxA2 and that triggered downstream of αIIbβ3 by activation of the c-Src/PLCγ2 pathway because the latter does not rely on TxA2.59,62,63 Indeed, neither PKC-β recruitment to β3 nor αIIbβ3-mediated TxA2 production requires Src activity.10,24 A role for PKC-β in the αIIbβ3/cPLA2α pathway depicted in Figure 6 is consistent with the reduced spreading of fibrinogen-adherent platelets observed after pharmacologic blockade or genetic deletion of PKC-β.10
Figure 6.
Model depicting the relationship between cPLA2α and αIIbβ3. MnCl2-induced fibrinogen binding to platelets induces the activation of αIIbβ3-associated cPLA2α, leading to the release of AA from membrane phospholipids and cyclooxygenase-mediated thromboxane A2 production. Activation of the TxA2/prostaglandin H2 receptor by thromboxane A2 leads to the production of inositol-1,4,5-triphosphate and IP3 receptor-mediated release of free calcium from the dense tubular system. The elevation in cytosolic Ca2+ concentration, in turn, promotes the activation of PKC-β and its subsequent recruitment to αIIbβ3 via RACK1. Another consequence of G-protein-mediated Ca2+ release downstream of TxA2, thrombin, or ADP receptors is the activation of additional pools of cPLA2α (dotted lines). COX indicates cyclooxygenase; TXA2, thromboxane A2, TP, thromboxane A2/prostaglandin H2 receptor, IP3, inositol-1,4,5-triphosphate.
Wong et al reported that collagen-induced aggregation of cPLA2α−/− platelets is defective,19 an observation consistent with our findings of reduced fibrinogen binding in response to stimulation of cPLA2α−/− platelets with CRP, a GP VI collagen receptor agonist (Figure S3). Our conclusion that a pool of cPLA2α functions in proximity to and downstream of αIIbβ3 is compatible with previous reports showing that collagen-mediated responses in platelets are dependent on TxA2 production, ADP release and, in some instances, αIIbβ3 engagement by fibrinogen.21,64–66
The physical and functional relationships between cPLA2α and αIIbβ3 detailed here illustrate how intertwined integrin signaling pathways can be. Thus, whereas TxA2 generated through integrin-associated cPLA2α activation promotes specific aspects of outside-in signaling on fibrinogen binding to αIIbβ3, TxA2 is also involved in the activation of αIIbβ3 by certain agonists. Because both inside-out and outside-in αIIbβ3 signaling is required for normal platelet function,1 the placement of cPLA2α at the nexus of αIIbβ3 signaling may explain, at least in part, the prolonged bleeding times and protection from ADP/collagen-induced thrombosis reported in cPLA2α-deficient mice.19 We suggest that it may be fruitful to consider similar relationships between cPLA2α and integrins in other cells.
Supplementary Material
Acknowledgments
The authors thank Dr Jim Clark and Suzana Marusic for the cPLA2α mice, Drs Takashi Ono and Kaoru Seno for pyrrophenone, Dr Peter Newman for CRP, Drs Ana Kasirer-Friede and Zaverio Ruggeri for VWF A1A2 domain, and Nima Yousefi for outstanding technical assistance.
This work was supported by National Institutes of Health grants HL56595, HL78784, HL57900 (S.J.S.) and GM20501 (E.A.D.), and by an American Heart Association fellowship (N.P.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: N.P. designed and performed research, analyzed data, and wrote the paper; J.V.M. performed research and wrote paper; H.K. performed research; J.E.B. and E.A.D. supplied essential reagents and wrote the paper; T.S. supplied essential mice and wrote the paper; and S.J.S. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sanford J. Shattil, Department of Medicine, University of California, San Diego, 9500 Gilman Drive, Mail Code 0726, La Jolla, CA 92093-0726; e-mail: sshattil@ucsd.edu.
References
- 1.Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–1615. doi: 10.1182/blood-2004-04-1257. [DOI] [PubMed] [Google Scholar]
- 2.Calderwood DA, Zent R, Grant R, Rees DJ, Hynes RO, Ginsberg MH. The talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J Biol Chem. 1999;274:28071–28074. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- 3.Tadokoro S, Shattil SJ, Eto K, et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 4.Deshmukh L, Tyukhtenko S, Liu J, Fox JE, Qin J, Vinogradova O. Structural insight into the interaction between platelet integrin αIIbβ3 and cytoskeletal protein skelemin. J Biol Chem. 2007;282:32349–32356. doi: 10.1074/jbc.M704666200. [DOI] [PubMed] [Google Scholar]
- 5.Fox JE. The platelet cytoskeleton. Thromb Haemost. 1993;70:884–893. [PubMed] [Google Scholar]
- 6.Hartwig JH, Barkalow K, Azim A, Italiano J. The elegant platelet: signals controlling actin assembly. Thromb Haemost. 1999;82:392–398. [PubMed] [Google Scholar]
- 7.Jenkins AL, Nannizzi-Alaimo L, Silver D, et al. Tyrosine phosphorylation of the beta3 cytoplasmic domain mediates integrin-cytoskeletal interactions. J Biol Chem. 1998;273:13878–13885. doi: 10.1074/jbc.273.22.13878. [DOI] [PubMed] [Google Scholar]
- 8.Obergfell A, Eto K, Mocsai A, et al. Coordinate interactions of Csk, Src, and Syk kinases with αIIbβ3 initiate integrin signaling to the cytoskeleton. J Cell Biol. 2002;157:265–275. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arias-Salgado EG, Haj F, Dubois C, et al. PTP-1B is an essential positive regulator of platelet integrin signaling. J Cell Biol. 2005;170:837–845. doi: 10.1083/jcb.200503125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buensuceso CS, Obergfell A, Soriani A, et al. Regulation of outside-in signaling in platelets by integrin-associated protein kinase C beta. J Biol Chem. 2005;280:644–653. doi: 10.1074/jbc.M410229200. [DOI] [PubMed] [Google Scholar]
- 11.Vijayan KV, Liu Y, Li TT, Bray PF. Protein phosphatase 1 associates with the integrin alphaIIb subunit and regulates signaling. J Biol Chem. 2004;279:33039–33042. doi: 10.1074/jbc.C400239200. [DOI] [PubMed] [Google Scholar]
- 12.Naik UP, Patel PM, Parise LV. Identification of a novel calcium-binding protein that interacts with the integrin alphaIIb cytoplasmic domain. J Biol Chem. 1997;272:4651–4654. doi: 10.1074/jbc.272.8.4651. [DOI] [PubMed] [Google Scholar]
- 13.Brass L F. Molecular basis for platelet activation. In: Hoffman R, Benz E, Shattil S, et al., editors. Hematology Basic Principles and Practice. 4th ed. New York, NY: Churchill-Livingstone; 2004. pp. 1899–1914. [Google Scholar]
- 14.Kita Y, Ohto T, Uozumi N, Shimizu T. Biochemical properties and pathophysiological roles of cytosolic phospholipase A2s. Biochim Biophys Acta. 2006;1761:1317–1322. doi: 10.1016/j.bbalip.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Ballou LR, DeWitt LM, Cheung WY. Substrate-specific forms of human platelet phospholipase A2. J Biol Chem. 1986;261:3107–3111. [PubMed] [Google Scholar]
- 17.Bartoli F, Lin HK, Ghomashchi F, Gelb MH, Jain MK, Apitz-Castro R. Tight binding inhibitors of 85-kDa phospholipase A2 but not 14-kDa phospholipase A2 inhibit release of free arachidonate in thrombin-stimulated human platelets. J Biol Chem. 1994;269:15625–15630. [PubMed] [Google Scholar]
- 18.Adler DH, Cogan JD, Phillips JA, et al. Inherited human cPLA(2alpha)deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J Clin Invest. 2008;118:2121–2131. doi: 10.1172/JCI30473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong DA, Kita Y, Uozumi N, Shimizu T. Discrete role for cytosolic phospholipase A(2)alpha in platelets: studies using single and double mutant mice of cytosolic and group IIA secretory phospholipase A(2). J Exp Med. 2002;196:349–357. doi: 10.1084/jem.20011443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banga HS, Simons ER, Brass LF, Rittenhouse SE. Activation of phospholipases A and C in human platelets exposed to epinephrine: role of glycoproteins IIb/IIIa and dual role of epinephrine. Proc Natl Acad Sci U S A. 1986;83:9197–9201. doi: 10.1073/pnas.83.23.9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho MJ, Liu J, Pestina TI, et al. The roles of αIIbβ3-mediated outside-in signal transduction, thromboxane A2, and adenosine diphosphate in collagen-induced platelet aggregation. Blood. 2003;101:2646–2651. doi: 10.1182/blood-2002-05-1363. [DOI] [PubMed] [Google Scholar]
- 22.Aoki T, Tomiyama Y, Honda S, et al. Difference of (Ca2+)i movements in platelets stimulated by thrombin and TRAP: the involvement of αIIbβ3-mediated TXA2 synthesis. Thromb Haemost. 1998;79:1184–1190. [PubMed] [Google Scholar]
- 23.Carroll RC, Wang XF, Lanza F, Steiner B, Kouns WC. Blocking platelet aggregation inhibits thromboxane A2 formation by low dose agonists but does not inhibit phosphorylation and activation of cytosolic phospholipase A2. Thromb Res. 1997;88:109–125. doi: 10.1016/s0049-3848(97)00223-5. [DOI] [PubMed] [Google Scholar]
- 24.Jin J, Quinton TM, Zhang J, Rittenhouse SE, Kunapuli SP. Adenosine diphosphate (ADP)-induced thromboxane A(2) generation in human platelets requires coordinated signaling through integrin αIIbβ3 and ADP receptors. Blood. 2002;99:193–198. doi: 10.1182/blood.v99.1.193. [DOI] [PubMed] [Google Scholar]
- 25.Chun JS, Jacobson BS. Spreading of HeLa cells on a collagen substratum requires a second messenger formed by the lipoxygenase metabolism of arachidonic acid released by collagen receptor clustering. Mol Biol Cell. 1992;3:481–492. doi: 10.1091/mbc.3.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark EA, Hynes RO. Ras activation is necessary for integrin-mediated activation of extracellular signal-regulated kinase 2 and cytosolic phospholipase A2 but not for cytoskeletal organization. J Biol Chem. 1996;271:14814–14818. doi: 10.1074/jbc.271.25.14814. [DOI] [PubMed] [Google Scholar]
- 27.Apgar JR. Increased degranulation and phospholipase A2, C, and D activity in RBL cells stimulated through FcepsilonR1 is due to spreading and not simply adhesion. J Cell Sci. 1997;110:771–780. doi: 10.1242/jcs.110.6.771. [DOI] [PubMed] [Google Scholar]
- 28.Bhattacharya S, Patel R, Sen N, Quadri S, Parthasarathi K, Bhattacharya J. Dual signaling by the αVβ3-integrin activates cytosolic PLA(2) in bovine pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1049–L1056. doi: 10.1152/ajplung.2001.280.5.L1049. [DOI] [PubMed] [Google Scholar]
- 29.Ono T, Yamada K, Chikazawa Y, et al. Characterization of a novel inhibitor of cytosolic phospholipase A2alpha, pyrrophenone. Biochem J. 2002;363:727–735. doi: 10.1042/0264-6021:3630727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shadle PJ, Ginsberg MH, Plow EF, Barondes SH. Platelet-collagen adhesion: inhibition by a monoclonal antibody that binds glycoprotein IIb. J Cell Biol. 1984;99:2056–2060. doi: 10.1083/jcb.99.6.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ginsberg MH, Yaspan B, Forsyth J, Ulmer TS, Campbell ID, Slepak M. A membrane-distal segment of the integrin alpha IIb cytoplasmic domain regulates integrin activation. J Biol Chem. 2001;276:22514–22521. doi: 10.1074/jbc.M101915200. [DOI] [PubMed] [Google Scholar]
- 32.Lichtenbergova L, Yoon ET, Cho W. Membrane penetration of cytosolic phospholipase A2 is necessary for its interfacial catalysis and arachidonate specificity. Biochemistry. 1998;37:14128–14136. doi: 10.1021/bi980888s. [DOI] [PubMed] [Google Scholar]
- 33.Qin XF, An DS, Chen IS, Baltimore D. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci U S A. 2003;100:183–188. doi: 10.1073/pnas.232688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leong L, Hughes PE, Schwartz MA, Ginsberg MH, Shattil SJ. Integrin signaling: roles for the cytoplasmic tails of αIIbβ3 in the tyrosine phosphorylation of pp125FAK. J Cell Sci. 1995;108:3817–3825. doi: 10.1242/jcs.108.12.3817. [DOI] [PubMed] [Google Scholar]
- 35.Hughes PE, Renshaw MW, Pfaff M, et al. Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell. 1997;88:521–530. doi: 10.1016/s0092-8674(00)81892-9. [DOI] [PubMed] [Google Scholar]
- 36.Prevost N, Kato H, Bodin L, Shattil SJ. Platelet integrin adhesive functions and signaling. Methods Enzymol. 2007;426:103–115. doi: 10.1016/S0076-6879(07)26006-9. [DOI] [PubMed] [Google Scholar]
- 37.Kasirer-Friede A, Moran B, Nagrampa-Orje J, et al. ADAP is required for normal αIIbβ3 activation by VWF/GP Ib-IX-V and other agonists. Blood. 2007;109:1018–1025. doi: 10.1182/blood-2006-05-022301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hitchcock IS, Fox NE, Prevost N, Sear K, Shattil SJ, Kaushansky K. Roles of focal adhesion kinase (FAK) in megakaryopoiesis and platelet function: studies using a megakaryocyte lineage-specific FAK knockout. Blood. 2007;111:596–604. doi: 10.1182/blood-2007-05-089680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu S, Calderwood DA, Ginsberg MH. Integrin cytoplasmic domain-binding proteins. J Cell Sci. 2000;113:3563–3571. doi: 10.1242/jcs.113.20.3563. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds LJ, Hughes LL, Yu L, Dennis EA. 1-Hexadecyl-2-arachidonoylthio-2-deoxy-sn-glycero-3-phosphorylcholine as a substrate for the microtiterplate assay of human cytosolic phospholipase A2. Anal Biochem. 1994;217:25–32. doi: 10.1006/abio.1994.1079. [DOI] [PubMed] [Google Scholar]
- 41.Takagi J, Petre BM, Walz T, Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599–611. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- 42.Bennett JS, Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979;64:1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hendrickson HS, Kotz KJ, Hendrickson EK. Evaluation of fluorescent and colored phosphatidylcholine analogs as substrates for the assay of phospholipase A2. Anal Biochem. 1990;185:80–83. doi: 10.1016/0003-2697(90)90258-b. [DOI] [PubMed] [Google Scholar]
- 44.Nakatani Y, Tanioka T, Sunaga S, Murakami M, Kudo I. Identification of a cellular protein that functionally interacts with the C2 domain of cytosolic phospholipase A(2)alpha. J Biol Chem. 2000;275:1161–1168. doi: 10.1074/jbc.275.2.1161. [DOI] [PubMed] [Google Scholar]
- 45.Gonzales M, Weksler B, Tsuruta D, et al. Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol Biol Cell. 2001;12:85–100. doi: 10.1091/mbc.12.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Homan SM, Martinez R, Benware A, LaFlamme SE. Regulation of the association of alpha 6 beta 4 with vimentin intermediate filaments in endothelial cells. Exp Cell Res. 2002;281:107–114. doi: 10.1006/excr.2002.5643. [DOI] [PubMed] [Google Scholar]
- 47.Kreis S, Schonfeld HJ, Melchior C, Steiner B, Kieffer N. The intermediate filament protein vimentin binds specifically to a recombinant integrin α2β1 cytoplasmic tail complex and co-localizes with native α2β1 in endothelial cell focal adhesions. Exp Cell Res. 2005;305:110–121. doi: 10.1016/j.yexcr.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 48.Tsuruta D, Jones JC. The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J Cell Sci. 2003;116:4977–4984. doi: 10.1242/jcs.00823. [DOI] [PubMed] [Google Scholar]
- 49.Haimovich B, Lipfert L, Brugge JS, Shattil SJ. Tyrosine phosphorylation and cytoskeletal reorganization in platelets are triggered by interaction of integrin receptors with their immobilized ligands. J Biol Chem. 1993;268:15868–15877. [PubMed] [Google Scholar]
- 50.Ghosh M, Tucker DE, Burchett SA, Leslie CC. Properties of the Group IV phospholipase A2 family. Prog Lipid Res. 2006;45:487–510. doi: 10.1016/j.plipres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Brass LF. Ca2+ transport across the platelet plasma membrane: a role for membrane glycoproteins IIB and IIIA. J Biol Chem. 1985;260:2231–2236. [PubMed] [Google Scholar]
- 52.Sinigaglia F, Bisio A, Torti M, Balduini CL, Bertolino G, Balduini C. Effect of GPIIb-IIIa complex ligands on calcium ion movement and cytoskeleton organization in activated platelets. Biochem Biophys Res Commun. 1988;154:258–264. doi: 10.1016/0006-291x(88)90678-x. [DOI] [PubMed] [Google Scholar]
- 53.Rosado JA, Meijer EM, Hamulyak K, Novakova I, Heemskerk JW, Sage SO. Fibrinogen binding to the integrin αIIbβ3 modulates store-mediated calcium entry in human platelets. Blood. 2001;97:2648–2656. doi: 10.1182/blood.v97.9.2648. [DOI] [PubMed] [Google Scholar]
- 54.Kourie JI, Wood HB. Biophysical and molecular properties of annexin-formed channels. Prog Biophys Mol Biol. 2000;73:91–134. doi: 10.1016/s0079-6107(00)00003-1. [DOI] [PubMed] [Google Scholar]
- 55.Solito E, Romero IA, Marullo S, Russo-Marie F, Weksler BB. Annexin 1 binds to U937 monocytic cells and inhibits their adhesion to microvascular endothelium: involvement of the α4β1 integrin. J Immunol. 2000;165:1573–1581. doi: 10.4049/jimmunol.165.3.1573. [DOI] [PubMed] [Google Scholar]
- 56.Andersen MH, Berglund L, Petersen TE, Rasmussen JT. Annexin-V binds to the intracellular part of the beta(5) integrin receptor subunit. Biochem Biophys Res Commun. 2002;292:550–557. doi: 10.1006/bbrc.2002.6673. [DOI] [PubMed] [Google Scholar]
- 57.Cardo-Vila M, Arap W, Pasqualini R. αVβ5 integrin-dependent programmed cell death triggered by a peptide mimic of annexin V. Mol Cell. 2003;11:1151–1162. doi: 10.1016/s1097-2765(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 58.Wang W, Kirsch T. Annexin V/beta5 integrin interactions regulate apoptosis of growth plate chondrocytes. J Biol Chem. 2006;281:30848–30856. doi: 10.1074/jbc.M605937200. [DOI] [PubMed] [Google Scholar]
- 59.Goncalves I, Hughan SC, Schoenwaelder SM, Yap CL, Yuan Y, Jackson SP. Integrin αIIbβ3-dependent calcium signals regulate platelet-fibrinogen interactions under flow: involvement of phospholipase C gamma 2. J Biol Chem. 2003;278:34812–34822. doi: 10.1074/jbc.M306504200. [DOI] [PubMed] [Google Scholar]
- 60.Ariyoshi H, Salzman EW. Association of localized Ca2+ gradients with redistribution of glycoprotein IIb-IIIa and F-actin in activated human blood platelets. Arterioscler Thromb Vasc Biol. 1996;16:230–235. doi: 10.1161/01.atv.16.2.230. [DOI] [PubMed] [Google Scholar]
- 61.Honda S, Tomiyama Y, Aoki T, et al. Association between ligand-induced conformational changes of integrin αIIbβ3 and αIIbβ3-mediated intracellular Ca2+ signaling. Blood. 1998;92:3675–3683. [PubMed] [Google Scholar]
- 62.Wonerow P, Pearce AC, Vaux DJ, Watson SP. A critical role for phospholipase Cgamma2 in αIIbβ3-mediated platelet spreading. J Biol Chem. 2003;278:37520–37529. doi: 10.1074/jbc.M305077200. [DOI] [PubMed] [Google Scholar]
- 63.Pearce AC, McCarty OJ, Calaminus SD, Vigorito E, Turner M, Watson SP. Vav family proteins are required for optimal regulation of PLCgamma2 by integrin αIIbβ3. Biochem J. 2007;401:753–761. doi: 10.1042/BJ20061508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roger S, Pawlowski M, Habib A, Jandrot-Perrus M, Rosa JP, Bryckaert M. Costimulation of the Gi-coupled ADP receptor and the Gq-coupled TXA2 receptor is required for ERK2 activation in collagen-induced platelet aggregation. FEBS Lett. 2004;556:227–235. doi: 10.1016/s0014-5793(03)01430-3. [DOI] [PubMed] [Google Scholar]
- 65.Moers A, Wettschureck N, Gruner S, Nieswandt B, Offermanns S. Unresponsiveness of platelets lacking both Galpha(q) and Galpha(13): implications for collagen-induced platelet activation. J Biol Chem. 2004;279:45354–45359. doi: 10.1074/jbc.M408962200. [DOI] [PubMed] [Google Scholar]
- 66.Van de Walle GR, Schoolmeester A, Iserbyt BF, et al. Activation of αIIbβ3 is a sufficient but also an imperative prerequisite for activation of α2β1 on platelets. Blood. 2007;109:595–602. doi: 10.1182/blood-2005-11-011775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.