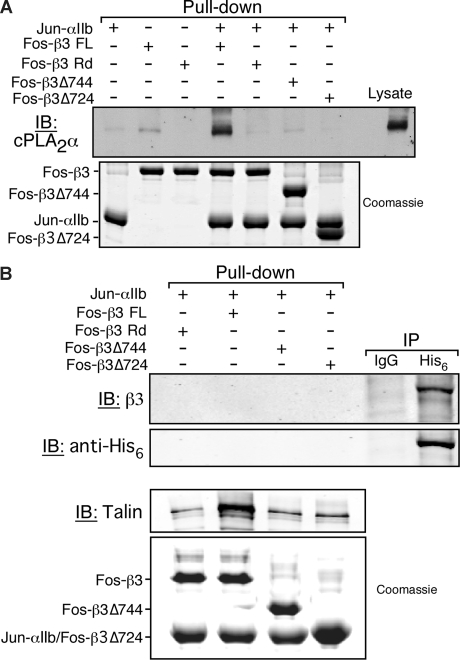
Figure 3.
cPLA2α binding to αIIbβ3 is indirect and depends on αIIb and β3 cytoplasmic tails. Neutravidin beads coated with the indicated integrin cytoplasmic tail model proteins were incubated with platelet lysate or purified His6-cPLA2α as described in “Methods.” (A) cPLA2α was detected by Western blotting. Equal loading of recombinant integrin tails was determined by Coomassie staining. This experiment was performed 3 times. (B) The His6-cPLA2α pull-down assay (first 4 lanes) was performed by incubating 20 μg of purified recombinant His6-cPLA2α with the indicated integrin tails bound to neutravidin beads. The presence of His6-cPLA2α in the pull-down was assayed by immunodetection using an anti-His6 antibody. Simultaneously, His6-cPLA2α was tested for its ability to interact with αIIbβ3 from human platelet lysate in an immunoprecipitation assay (IP) using either an irrelevant isotype match control antibody (IgG) or an anti-His6 antibody (His6; last 2 lanes). Equal loading of recombinant tails was determined by Coomassie staining. This experiment was performed twice.