Abstract
Coupling drug carriers to antibodies for targeting endothelial cells (ECs) may improve treatment of vascular and pulmonary diseases. Selecting antibodies that deliver carriers to the cell surface or intracellularly may further optimize specifcity of interventions. We studied antibody-directed targeting of nanocarriers to platelet–endothelial cell adhesion molecule (PECAM)-1, an endothelial glycoprotein containing 6 Ig-like extracellular domains. PECAM-1 antibodies bind to ECs without internalization, but ECs internalize by endocytosis nanocarriers carrying multiple copies of anti-PECAM (anti-PECAM/NCs). To determine whether binding and intracellular transport of anti-PECAM/NCs depend on the epitope engaged, we targeted five PECAM-1 epitopes: mAb35, mAb37 and mAb62 (membrane-distal Ig domain 1), mAbGi34 (Ig domains 2/3), and mAb4G6 (membrane-proximal Ig domain 6). The antibodies bound to ECs regardless of the epitope proximity to the plasmalemma, whereas 130 nm diameter nanocarriers only targeted effectively distal domains (mAb4G6/NCs did not bind to ECs). ECs internalized mAb35, mAb62, and mAbGi34 carriers regardless of their size (0.13 to 5 µm diameter), yet they did not internalize mAb37/NCs. After internalization, mAb62/NCs trafficked to lysosomes within 2–3 h, whereas mAb35/NCs had prolonged residence in pre-lysosomal vesicles. Therefore, endothelial binding, endocytosis, and intracellular transport of anti-PECAM/NCs are epitope-specific. This paradigm will guide the design of endothelial drug delivery systems providing specific cellular localizations.
Keywords: Vascular targeting, Endothelial PECAM-1, Polymer carriers, Endocytosis, Intracellular drug delivery
1. Introduction
Targeting of drugs to endothelial cells (ECs) holds promise to optimize diagnostic and therapeutic means for treatment of vascular, pulmonary and other human diseases, including acute lung injury, pulmonary hypertension, ischemia–reperfusion, inflammation, oxidative stress, and thrombosis [1–4]. Conjugation of drugs or their carriers with antibodies that specifically bind to determinants exposed at the endothelial surface provides an avenue to achieve this important biomedical goal [5–13]. Selecting targeting antibodies that optimally deliver drug carriers to certain cellular destinations (e.g., the cell surface vs intracellular compartments) is a key component of successful drug delivery [1,9,12–19]. The goal of this work was to study these aspects of drug delivery using as an example prototype polymer carriers targeted to platelet–endothelial cell adhesion molecule (PECAM)-1.
Among the surface determinants potentially useful for drug delivery to the endothelium, PECAM-1 (a 130 kDa transmembrane glycoprotein of the Ig superfamily involved in leukocyte transmigration, angiogenesis and signaling [20–24]) is an attractive candidate target [1–3,5]. PECAM-1 is expressed at high levels (millions of molecules per cell) on ECs, where it concentrates at the cell–cell border [20,23,25] and is accessible from the circulation [26–32]. PECAM-1 is constitutively expressed on continuous endothelium of all vessel types [20] and, in contrast to other endothelial determinants, its surface density is relatively stable under pathologic conditions [2,22,33]. Numerous animal studies have shown that PECAM-1 is a robust target for drug delivery to either normal or pathologically altered endothelium, e.g., for prophylactic or therapeutic interventions [2,26–31,34].
ECs do not internalize PECAM-1 antibodies (e.g., mAb62 or mAb4G6 anti-PECAM) [2,30,34] or anti-PECAM scFv fusion proteins [2,27], which is useful for drug targeting to the vascular lumen [2,27,30,34]. On the other hand, ECs internalize anti-PECAM conjugates [2,30] and anti-PECAM-coated nanocarriers (e.g., anti-PECAM/NCs) [34,35] that multivalently engage PECAM-1 in the endothelial plasmalemma. This multivalent binding initiates a unique vesicular internalization pathway, cell adhesion molecule (CAM)-mediated endocytosis, which is distinct from classical clathrin or caveolar endocytosis, phagocytosis and macropinocytosis [35], and permits intracellular drug delivery of reporter and therapeutic agents targeted to PECAM-1 [28–30,34,35]. For instance, endothelial targeting of antioxidant enzymes (e.g., catalase) conjugated with anti-PECAM or loaded to anti-PECAM/NCs provides antioxidant protection in cell cultures and animal models of pulmonary oxidative stress [26,28,29,31]. Drug delivery into ECs via PECAM-1 may improve these and other therapeutic interventions.
Therefore, anti-PECAM/NCs represent a promising platform for intracellular drug delivery to ECs which constitutes a key requirement for the therapeutic action of many drugs and biotherapeutics [36]. The understanding of the mechanisms governing the internalization of anti-PECAM/NCs is of both basic and applied interest. However, a question that still remains to be answered is whether internalization by ECs depends on the specific PECAM-1 epitopes engaged by anti-PECAM/NCs.
Given that active endothelial signaling is necessary for anti-PECAM/NC internalization [35,37], it seemed plausible that selection of specific PECAM-1 epitopes for anti-PECAM/NC binding might play a role in the subsequent internalization process that drives intracellular drug delivery. We utilized multi-label fluorescence microscopy to analyze binding and uptake by ECs of model polystyrene nanocarriers targeted by monoclonal antibodies to five distinct epitopes located in different Ig domains in the extracellular region of PECAM-1 [24,25]. We found that carrier binding to PECAM-1 on the endothelial surface, internalization within ECs, and subsequent intracellular transport to endosomes and lysosomes depend on the selection of the particular extracellular PECAM-1 epitope that is engaged by the multivalent carriers. Hence, precise targeting to one of the different epitopes of a same endothelial determinant, PECAM-1, provides a means to achieve delivery of polymer carriers to the cell surface vs distinct intracellular vesicular compartments (endosomes vs lysosomes) for endothelial drug delivery.
2. Materials and methods
2.1. Antibodies and reagents
The mouse monoclonal antibodies to human PECAM-1 (mAbs) used in this study were mAb62 and mAb35 (kindly provided by Dr. M. Nakada, Centocor, Malvern, PA), mAbGi34 (AXXORA Platform, San Diego, CA), mAb4G6 and mAb37 [25]. Secondary fluorescent anti-bodies were from Jackson ImmunoResearch (West Grove, PA). FITC-labeled polystyrene latex microspheres (0.13, 1, and 5 µm diameter) were from Polysciences (Warrington, PA). Unless otherwise specified, all other reagents were from Sigma-Aldrich (St. Louis, MO).
2.2. Cell culture
Pooled human umbilical vein endothelial cells (HUVECs; Cambrex Corporation, East Rutherford, NJ), endothelial-epithelial hybrid EAhy926 cells [38], and human mesothelioma REN cells stably transfected with human PECAM-1 (RhP)were grown in supplemented medium as described [30,35]. For experiments, cells were seeded and grown to confluence on either plastic (for radioisotope tracing studies) [30] or gelatin-coated glass coverslips [35].
2.3. Preparation and characterization of anti-PECAM carriers
For anti-PECAM conjugates, antibodies were biotinylated with NHS-LC-biotin (Pierce Biotech., Rockford, IL), radiolabeled with Iodogen (Pierce Biotech., Rockford, IL), and conjugated by streptavidin as in our prior work [30]. Alternatively, anti-PECAM and control IgG carriers (anti-PECAM/NCs, IgG/NCs) were prepared by absorption (non-covalent link) of one of the different anti-PECAM antibodies (mAb62, mAb35, mAb37, mAb4G6, or mAbGi34) or non-specific IgG onto FITC-labeled polystyrene particles of several sizes (0.13, 1, or 5 µm diameter), as previously described [34,35,37]. By this protocol each targeting antibody is likely to display an array of different orientations on the particle surface. The diameter of the anti-PECAM/NC preparations was assessed by dynamic light scattering [34,37].
To determine the number of antibodies coating the surface of the polystyrene particles, anti-PECAM was radiolabeled and the amount of 125I-anti-PECAM on the carriers was determined in a gamma counter after centrifugation (12,000 ×g for 5 min) and elimination of non-bound 125I-anti-PECAM from the supernatant [34,37]. Alternatively, the uniformity of the anti-PECAM coat on FITC-labeled particles was assessed by incubation with goat anti-mouse IgG conjugated to Texas red. The samples were washed and imaged by fluorescence microscopy using an Eclipse TE2000-U microscope provided of a 40× objective and filters optimized for FITC and Texas red fluorescence (Nikon, Melville, NY). Images were taken with an Orca-1 CCD camera (Hamamatsu, Bridgewater, NJ) and analyzed using ImagePro 3.0 software (Media Cybernetics, Silver Spring, MD) to quantify the surface fraction of green-fluorescent FITC-carriers which were stained with the Texas red secondary antibody, as described [34,35,37]. For each channel, only a fluorescent signal below saturation and above the threshold value of intensity of the surrounding background was quantified to avoid non-specific noise.
2.4. Binding of anti-PECAM to endothelial cells
Binding of the five distinct PECAM-1 antibodies used in this study to endothelial PECAM-1 was quantified by fluorescence activated cell sorting (FACS) in cells in suspension. HUVECs were grown in gelatin-coated T25 flasks, harvested by trypsin treatment, washed, and incubated for 1 h at 4 °C in cell media containing 25 µg/ml mAb62, mAb35, mAb37, mAb4G6 or mAbGi34 antibodies. The cells were then washed in PBS, incubated for 30 min at 4 °C with FITC-labeled goat anti-mouse secondary antibody, washed again, and finally resuspended in PBS and analyzed by FACS (Flow Cytometry Facility, The Wistar Institute, Philadelphia, PA).
Alternatively, binding of anti-PECAM was confirmed in confluent adherent monolayers of HUVECs by immunofluorescent staining using goat anti-mouse IgG conjugated to Texas red. The total fluorescent signal per cell was determined by fluorescence microscopy using the imaging acquisition and analysis setting described above.
2.5. Binding, endocytosis, and intracellular transport of anti-PECAM carriers
To trace binding of PECAM-1 antibodies and anti-PECAM conjugates, HUVECs, EAhy926 and/or RhP cells were incubated with 125I-labeled counterparts (mAb62, mAb37 and mAb4G6 antibodies or conjugates) for 90 min at 37 °C. After washing unbound material, the isotope was determined in the cell lysates [30].
In the case of anti-PECAM/NCs, confluent HUVECs were first incubated with FITC-labeled anti-PECAM/NCs or control IgG/NCs of different sizes (0.13, 1, or 5 µm diameter) at 37 °C for varying periods of time, from 5 min to 3 h. As a control to determine whether the internalization rate of anti-PECAM carriers may be affected by the sedimentation rate of carriers with different sizes, HUVECs were first incubated with 0.13, 1, or 5 µm diameter mAb62 carriers for 30 min at 4 °C to permit only binding, then washed and warmed to 37 °C to permit internalization of pre-bound carriers. The cells were washed, fixed in cold 2% paraformaldehyde, and stained with Texas red goat anti-mouse IgG to label non-internalized, surface located anti-PECAM/NCs as in our prior work [34,35]. The samples were imaged by fluorescence microscopy as described above and analyzed to quantify double-labeled (Texas red and green FITC) yellow particles located at the cell surface vs single-labeled green FITC particles internalized within the cells [34,35]. To estimate particle numbers, the area of specific fluorescence was normalized to the number of pixels that theoretically correspond to the size of a single particle, viewed under the magnification used to take the image [34]. Phase-contrast was used to delimitate the cell borders.
To examine nanocarrier intracellular transport, HUVECs were treated with anti-PECAM/NCs as described above. Non-internalized anti-PECAM/NCs were stained using goat anti-mouse IgG conjugated to blue AlexaFluor 350, followed by permeabilizing cells with 0.2% Triton X-100 and staining intracellular endosomes with an antibody to early endosome antigen 1 (EEA-1) labeled with Texas red [39,40]. In the case of lysosomes, these were first labeled by incubating HUVECs with Texas red dextran (10,000 MW), followed by washing and treatment with anti-PECAM/NCs. The samples were imaged after labeling non-internalized carriers with blue Alexa Fluor 350 goat anti-mouse IgG [39,40].
2.6. Statistics
Unless otherwise stated, the data were calculated as the mean ± standard error of the mean, where statistical significance was determined by Student's t test.
3. Results
3.1. Differential binding of antibodies vs nanocarriers targeted to membrane-distal vs membrane-proximal PECAM-1 epitopes
Human PECAM-1 consists of a 574 amino acids extracellular region containing six Ig-like domains, numbered from 1 to 6, from the most membrane-distal to the most membrane-proximal domain (Table 1), followed by a short hydrophobic transmembrane domain and a cytoplasmic tail [21]. PECAM-1 molecules in neighboring ECs interact in a homophilic manner, and specific PECAM-1 epitopes located in this extracellular region and differentially involved in homophilic PECAM-1 interactions have been identified [25,41,42].
Table 1.
Monoclonal antibodies (mAb) recognizing distinct extracellular PECAM-1 epitopes
| Antibody | Isotype | PECAM-1 domain | PECAM-1 adhesion |
|---|---|---|---|
| mAb62 | IgG2a | 1 | Inhibition |
| mAb35 | IgG1 | 1 | Augmentation |
| mAb37 | IgG1 | 1 | No effect |
| mAbGi34 | IgG1 | 2/3 | Not known |
| mAb4G6 | IgG2b | 6 | Augmentation |
Previous studies showed that mAb62 directed to PECAM-1 Ig domain 1 (Table 1) efficiently binds to ECs as a monomolecular antibody and also when coupled to sub-micron and micron size protein conjugates and carriers [24,25,28–31,34,35]. In this study we compared mAb62 with four other mAbs directed to different PECAM-1 extracellular epitopes (Table 1): mAb4G6 binds to membrane-proximal domain 6, mAbGi34 binds between domains 2 and 3, and mAb35 and mAb37 bind to membrane-distal domain 1. Previous works revealed different functional effects of mAb62, mAb35 and mAb37 on PECAM-1 homophilic interaction (inhibiting, activating and innocuous, respectively (Table 1)), which indicates that these antibodies bind to distinct PECAM-1 epitopes in the same domain [25].
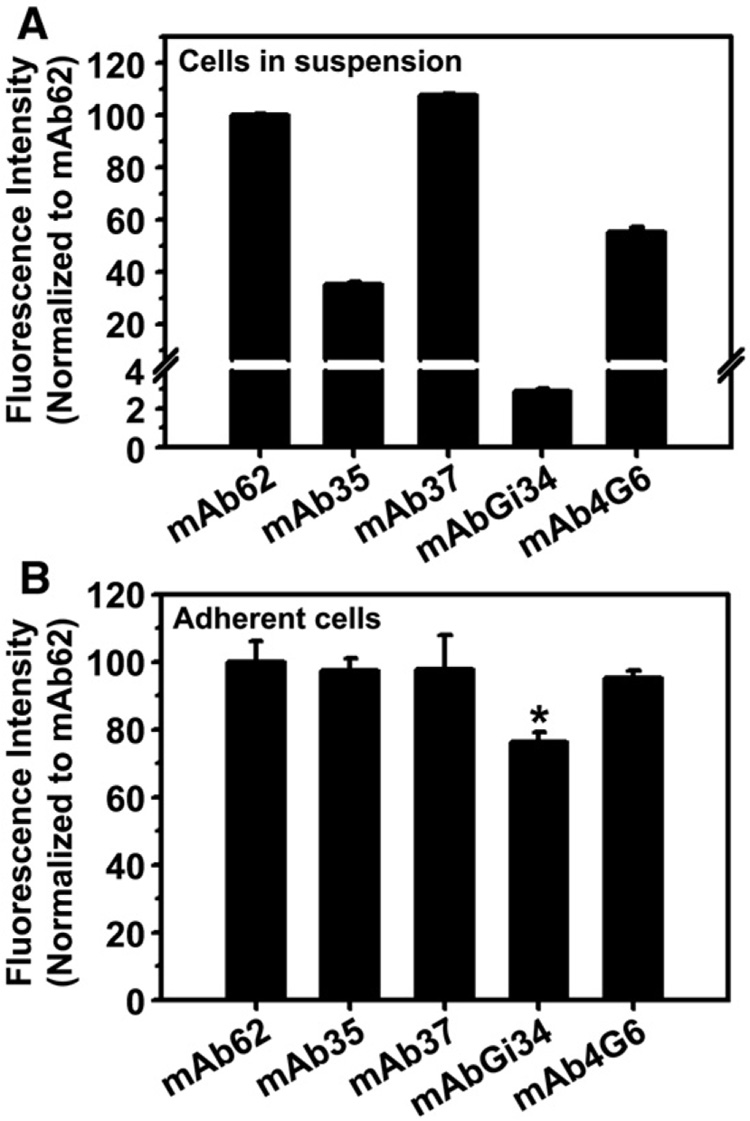
FACS analysis using ECs in suspension showed that all five tested mAbs bind to PECAM-1-positive cells to different degrees (Fig. 1A). Anti-PECAM mAb62 and mAb37 had the highest binding level (85.5±0.4 and 91.6±0.6 fold over IgG control), while mAb35 and mAb4G6 bound to ECs similarly less effectively (30.0±1.0 and 47.0±1.7 fold over IgG control), despite the fact that they recognize the most distal and proximal PECAM-1 domains, respectively. Of note, mAbGi34 recognizing a middle area of PECAM-1 extracellular region showed the lowest level of binding to ECs (2.4±0.1 fold over IgG control (Fig. 1A)).
Fig. 1. Binding of monoclonal antibodies targeted to distinct PECAM-1 extracellular epitopes in endothelial cell cultures.
A: Relative binding of anti-PECAM antibodies to HUVECs in suspension, analyzed by FACS and represented as mean fluorescence intensity values normalized to mAb62. Data are mean ± SD (n = 2). B: Relative binding of anti-PECAM antibodies to confluent, adherent HUVEC monolayers, tested by indirect immunostaining and analyzed by fluorescence microscopy. The mean fluorescence intensity for the different anti-PECAM antibodies are compared to that of mAb62. Data are mean±SEM (n≥10 cells).
However, it is plausible that binding of anti-PECAM to ECs in suspension, used for FACS analysis, may not reflect binding in a more physiological situation, e.g., confluent ECs, particularly because PECAM-1 is involved in homophilic interactions in the cell-cell border. To discern this, we incubated adherent, confluent ECs with anti-PECAM and analyzed binding by fluorescence microscopy after fluorescent immunostaining with a secondary antibody. All five anti-PECAM antibodies bound specifically to confluent monolayers of adherent ECs, yet their relative binding was more similar to that of mAb62 (Fig. 1B). Only binding of mAbGi34 (which bound very poorly to ECs in suspension) to adherent ECs was significantly lower than that of mAb62 (70.3±2.7%).
3.2. Nanocarriers and conjugates targeted to membrane-distal vs membrane-proximal PECAM-1 epitopes display a binding pattern distinct from that of naked anti-PECAM
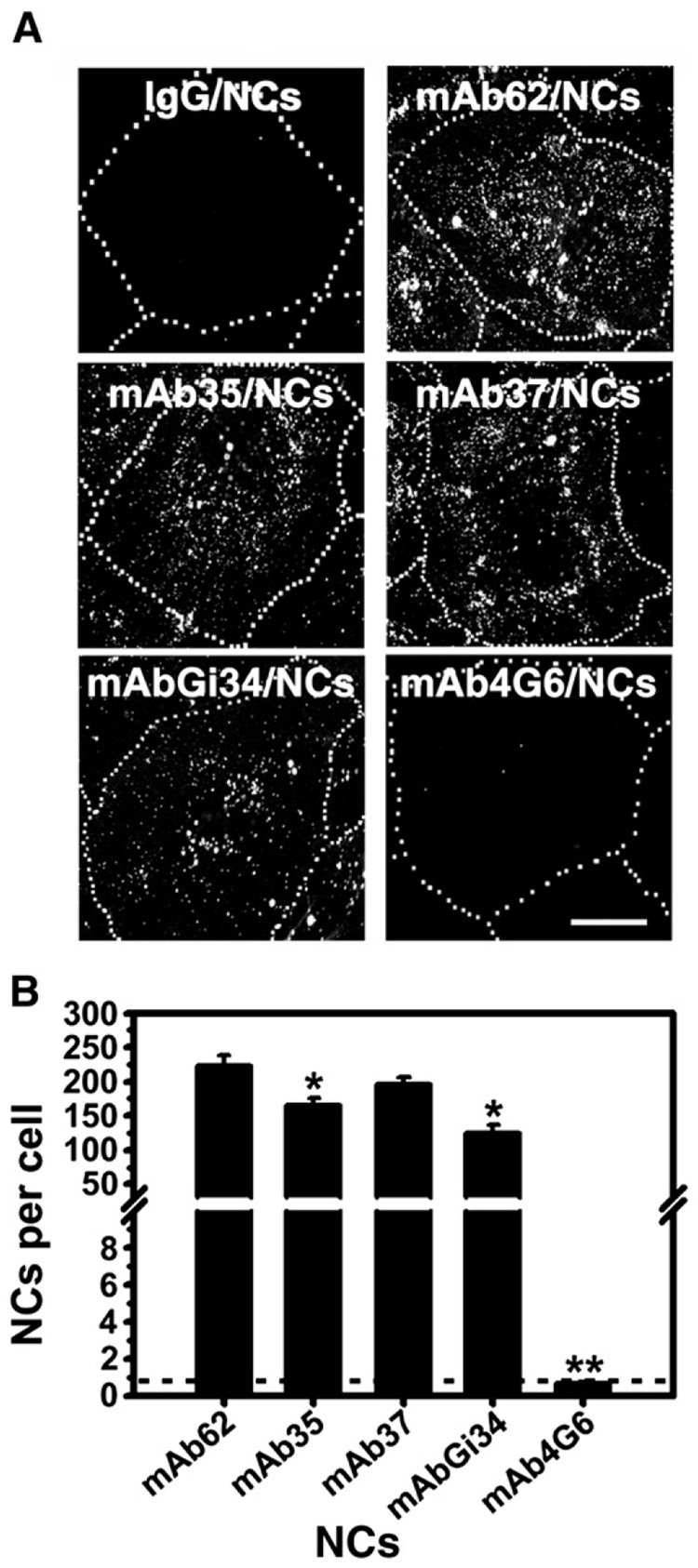
Somewhat unexpectedly, prototype (130 nm diameter) polystyrene nanocarriers targeted to PECAM-1 by these five antibodies had a binding pattern different from that of their corresponding free counterparts (Fig. 2). Binding of anti-PECAM/NCs to ECs was specific vs that of IgG/NCs used as control (0.7±0.3 particles/cell). Among the nanocarrier formulations, mAb62/NCs had the highest binding to ECs (222.5±15.2 particles/cell; 305.4±20.8 fold over IgG/NCs), followed by mAb37/NCs, mAb35/NCs and mAbGi34/NCs (196.2±10.1, 165.8±9.7, and 125.4±11.5 particles/cell; 269.3±13.8, 227.6±13.3, and 172.1±15.7 fold over IgG/NCs, respectively), despite the fact that the two latter antibodies had markedly lower and very low binding to ECs, respectively (compare Fig. 1 and Fig. 2). In contrast, mAb4G6/NCs did not bind to ECs (0.7±0.1 particles/cell; 1.0±0.1 fold of IgG/NCs), despite the fact that the corresponding antibody had relatively effective binding to ECs (compare Fig. 1 and Fig 2).
Fig. 2. Binding of anti-PECAM/NCs targeted to five distinct PECAM-1 extracellular epitopes in endothelial cells.
A: HUVECs were incubated for 1 h at 37 °C with either 130nm diameter FITC-labeled control IgG/NCs or each one of five indicated anti-PECAM/NCs targeted to different PECAM-1 epitotes, then washed, fixed, and analyzed by fluorescence microscopy. The dotted line has been drawn from comparison of the fluorescent images to their phase-contrast counterparts to mark the cell borders. Scale bar=10 µm. B: Quantification of the number of green-fluorescent nanocarriers bound per cell. Data are mean±SEM (n ≥ 25 cells). The dashed line indicates the binding level of control IgG/NCs. * p<0.05, ** p<0.001, by Student's t test (compared to mAb62/NCs).
We determined whether differences in parameters such as the size of anti-PECAM/NCs, the number of anti-PECAM antibodies per carrier particle, and the uniformity of the antibody coating on the particle surface may account for the differences observed regarding binding of anti-PECAM/NC to ECs. Dynamic light scattering, isotope tracing of 125I-anti-PECAM/NCs, and fluorescence microscopy of anti-PECAM on the surface of FITC- nanocarriers using goat anti-mouse IgG conjugated to Texas red, respectively, revealed that anti-PECAM/NCs ranged from 156±3.7 to 285±11.8 nm in diameter and presented from 181±15.8 to 249±3.5 antibody molecules per particle, which were distributed in a uniform manner onto the particle (from 95.2±2.3% to 91.5±3.7% of the surface area) (Table 2). We did not find a correlation between binding of anti-PECAM/NCs to ECs and these parameters (compare Table 2 and Fig. 2B).
Table 2.
Characterization of anti-PECAM/NCs
| Anti-PECAM-1 | Size of anti-PECAM/NCs |
Anti-PECAM molecules per particle | Anti-PECAM coating (% particle surface) | |
|---|---|---|---|---|
| Diameter (nm) | Polydispersity | |||
| mAb62 | 229±9.0 | 0.19±0.01 | 249±3.5 | 95.2±2.3% |
| mAb35 | 285±11.8 | 0.19±0.01 | 181±15.8 | 91.5±3.7% |
| mAb37 | 245±7.0 | 0.16±0.01 | 218±1.4 | 92.1±2.3% |
| mAbGi34 | 156±3.7 | 0.14±0.02 | N/D | 92.5±1.9% |
| mAb4G6 | 211±21.1 | 0.14±0.01 | 206±2.1 | 92.9±1.4% |
The size of the particles prior to coating anti-PECAMwas 130±0.7 nm (polydispersity=0.09±0.003).
Data are mean±SEM (n≥3).
N/D: not-determined.
It is tempting to speculate that ineffective binding of mAb4G6/NCs to ECs may then be due to spatial inaccessibility of the corresponding PECAM-1 membrane-proximal epitope to carrier particles. This notion is supported by the fact that streptavidin conjugation of radiolabeled, biotinylated mAb4G6 also suppressed binding to PECAM-1 expressing cells (by ~50%) compared to the non-conjugated biotinylated antibody (Supplement 1A). In contrast, streptavidin conjugation stimulated binding of biotinylated mAb62 directed to the most membrane-distal PECAM-1 domain (2.6±0.1 fold of the non-conjugated biotinylated mAb, Supplement 1A). Such inhibitory effect of streptavidin conjugation on binding of biotinylated mAb4G6 was also observed in diverse PECAM-1 positive cell types tested, including HUVECs, as well as the endothelial-epithelial hybrid cell line, EAhy926, and PECAM-1 transfected mesothelioma REN cells (RhP) (Supplement 1B).
3.3. Role of targeting specific PECAM-1 extracellular domains in the endothelial internalization of anti-PECAM nanocarriers
We have previously shown using radioactive tracing that naked PECAM-1 antibodies to either membrane-distal (mAb62) or membrane-proximal (mAb4G6) domains are not internalized by PECAM-1 positive cells (e.g., HUVECs or RhP cells) despite effective binding to the cell surface [30,34].
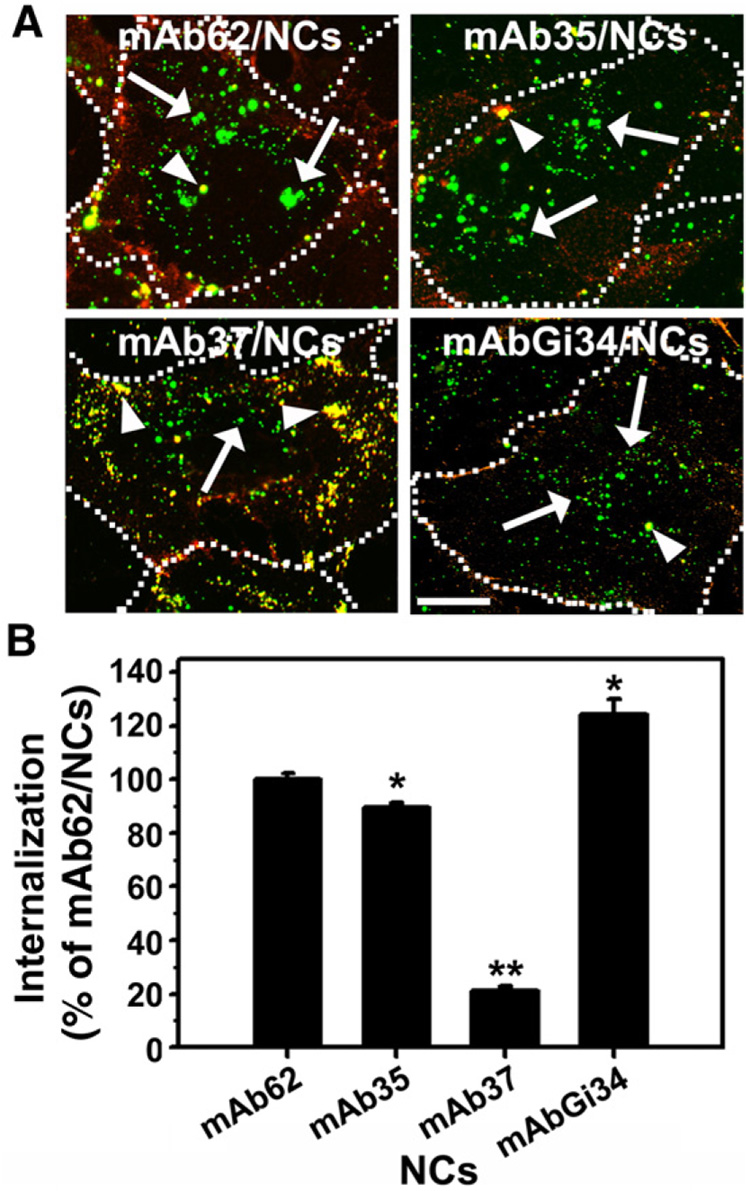
We then used double-label fluorescence microscopy to test internalization of anti-PECAM/NCs incubated for 1 h at 37 °C with ECs (Fig. 3A). Results of the image analysis (performed as described [34,35,37]) were expressed as internalization percent relative to the rate of internalization of mAb62/NCs (Fig. 3B), a formulation that has been extensively utilized in our previous studies and consistently showed high level of internalization (73.1±2.2% of cell-associated mAb62/NCs or 162.6±11.1 mAb62/NCs per cell in this study). Both mAb4G6/NCs and IgG/NCs, which showed no appreciable binding (Fig. 2), were excluded from this and subsequent studies.
Fig. 3. Differential internalization within endothelial cells of anti-PECAM/NCs which target four distinct PECAM-1 extracellular epitopes .
A: HUVECs were incubated for 1 h at 37 °C with the indicated 130 nm diameter FITC-labeled anti-PECAM/NCs, washed, fixed, and incubated with Texas red goat anti-mouse IgG to counterstain non-internalized nanocarriers on the cell surface. Merged fluorescence microscopy images of the samples show internalized anti-PECAM/NCs as single-labeled green particles (arrows) vs surface-bound, double-labeled (yellow) anti-PECAM/NCs particles (arrow-heads). The dotted line has been drawn from comparison of the fluorescent images to their phase-contrast counterparts to mark the cell borders. Scale bar=10 µm. B: Internalization was quantified as percent of internalized anti-PECAM/NCs relative to total number of particles associated to cells, and normalized to previously described mAb62/NCs. Data are mean±SEM (n≥25 cells). * p<0.05, ** p<0.001, by Student's t test (compared to mAb62/NCs).
Internalization of mAb35/NCs was marginally lower than that of mAb62/NCs (89.7±1.4% of mAb62/NCs level), whereas mAbGi34/NCs directed to domains 2/3 were internalized by ECs even more effectively than mAb62/NCs (124.5±6.5% of mAb62/NCs level). Unexpectedly, mAb37/NCs (which had a level of endothelial binding similar to that of other anti-PECAM/NCs targeted to domain 1 (Fig. 2)), was internalized by ECs at a very low level, 21.0±1.3% of mAb62/NCs (Fig. 3B).
As in the case of binding, we did not find a correlation between the internalization of anti-PECAM/NCs by ECs and measured parameters such as the size of anti-PECAM/NCs, or the number of anti-PECAM antibodies per carrier particle (compare Table 2 and Fig. 3B).
3.4. Endothelial internalization of anti-PECAM carriers is effective in a wide size range of carriers
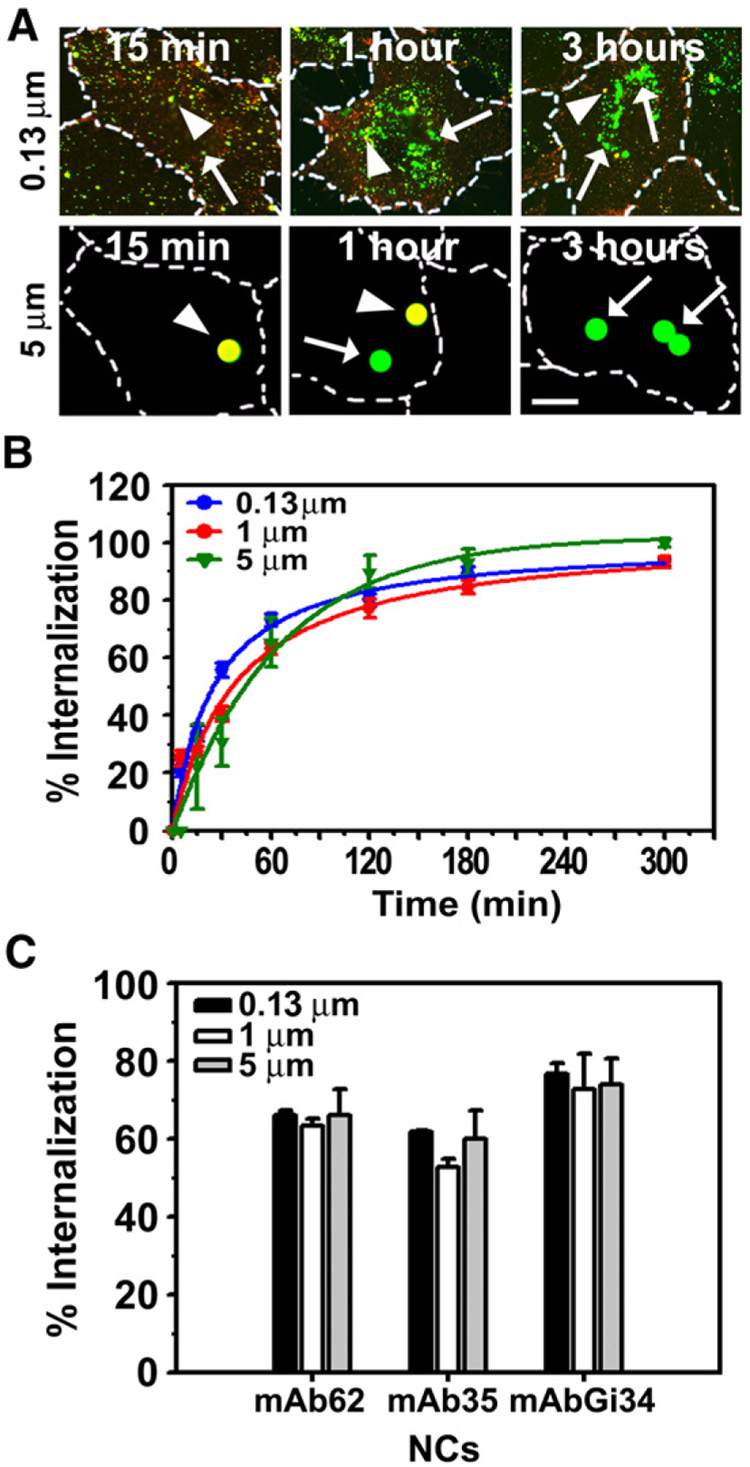
We compared uptake by ECs of “internalizable” mAb62-coated anti-PECAM carriers prepared using model polymer spheres of the same chemistry, yet varying in diameter from 130 nm to 5 µm. To avoid potential differences in the internalization rate of mAb62 carriers due to distinct sedimentation rates of carriers with different sizes, cells were first incubated with mAb62 carriers at 4 °C to permit only binding of the carriers to the cell surface, followed by washing non-bound carriers and warming cells to 37 °C to permit internalization of pre-bound carriers. Implementation of this method revealed a similar level of internalization of mAb62 carriers regardless of their size (Fig. 4A and B). Although 130 nm diameter mAb62 showed detectably faster rate of internalization than micron size counterparts (t1/2~25min, vs 34min and 43min, for 1 and 5 µm respectively), similar maximal level of internalization (~80%) was attained by anti-PECAM carriers of all tested sizes shortly after 1 h of incubation (Fig. 4B).
Fig. 4. Efficient internalization of sub-micron and micron size anti-PECAM carriers which target three “permissive” PECAM-1 extracellular epitopes.
A: HUVECs were incubated at 4 °C in the presence of 0.13 µm or 5 µm diameter FITC-labeled carriers targeted by anti-PECAM mAb62 to permit carrier binding but not internalization. The cells were then washed and warmed to 37 °C either for 15 min, 1 h, or 3 h to allow internalization of pre-bound carriers. The cells were washed, fixed, and incubated with Texas red goat anti-mouse IgG which labels surface-bound carriers (yellow particles, arrowheads) vs internalized counterparts (green particles, arrows). Magnification bar= 10 µm. B: Quantification of the internalization kinetics of pre-bound mAb62 carriers of several sizes (0.13, 1 and 5 µm diameter) by HUVECs. C: Internalization of FITC-labeled carriers of various sizes (0.13, 1 and 5 µm diameter) targeted to PECAM-1 by mAb62, mAb35 or mAbGi34 after incubation with HUVEC for 1 h at 37 °C. Data in B and C are mean±SEM (n≥25 cells).
Efficient internalization of micron size carriers was consistent with all types of internalizable anti-PECAM/NCs, e.g., ECs internalized equally well mAb62/NCs, mAb35/NCs and mAbGi34/NCs with diameter of 0.13, 1 or 5 µm (Fig. 4C). Taken together with data shown above, this result indicates that internalization of anti-PECAM carriers is predominantly dictated by binding to specific accessible PECAM-1 epitopes rather than the carrier size.
3.5. Role of targeting specific PECAM-1 extracellular domains in the intracellular transport of anti-PECAM nanocarriers
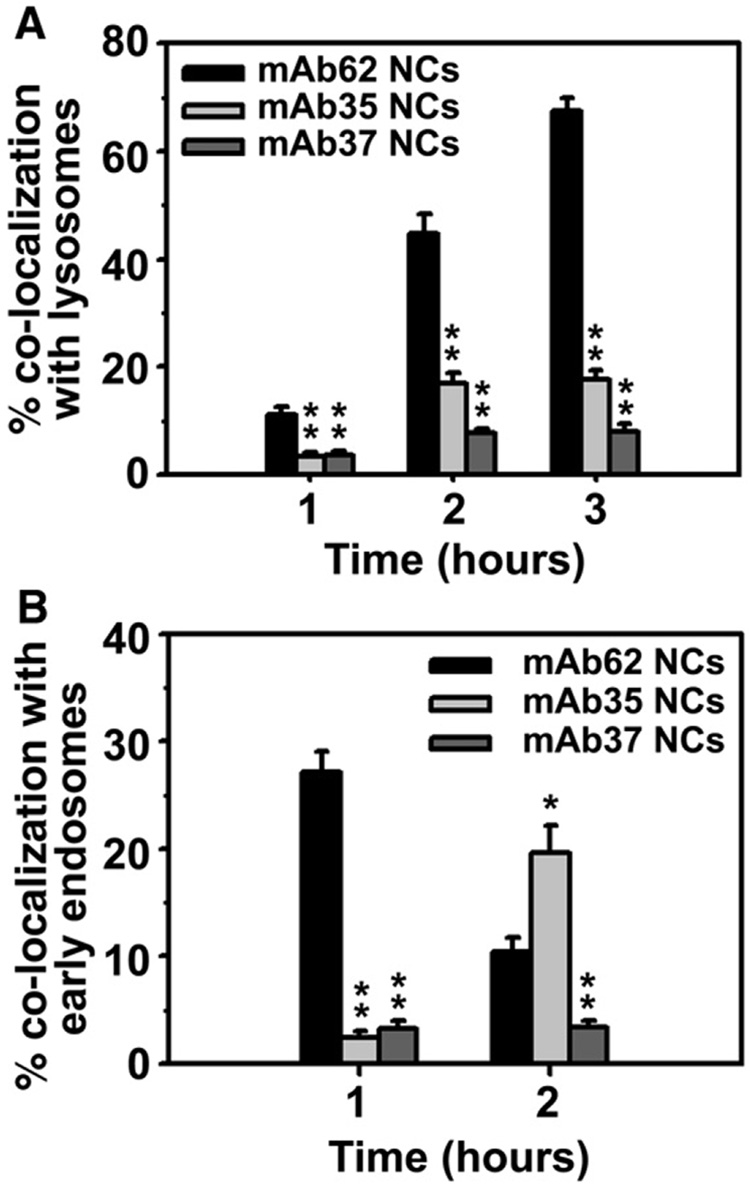
Following internalization, the majority of mAb62/NCs were transported to dextran-labeled lysosomal compartments in ECs within 3 h (67.5±2.3%; Fig. 5A), confirming our previous work [29,39]. However, unexpectedly, transport to lysosomes of internalized mAb35/NCs was significantly decreased (17.5±1.8% at 3 h; Fig. 5A).
Fig. 5. Intracellular transport of anti-PECAM/NCs internalized by endothelial cells.
A: Fluorescence microscopy was used to examine the trafficking in HUVECs of 130 nm diameter FITC-labeled (green) anti-PECAM/NCs to Texas red dextran positive lysosomes, or B: EEA-1-positive early endosomes detected by fluorescent labeling with anti-EEA1. Non-internalized carriers were stained by blue Alexa Fluor 350 goat anti-mouse IgG, whereas endosomal or lysosomal co-localization was visualized as yellow. Data are mean±SEM (n≥25 cells). * p<0.05, ** p<0.001, by Student's t test (compared to mAb62/NCs).
Delayed transport to lysosomes may be due to prolonged residence of internalized carriers in pre-lysosomal compartments, as we have recently observed for nanocarriers of different geometries targeted to a related adhesion molecule, ICAM-1 [54]. Therefore, we examined the co-localization of internalized anti-PECAM/NCs with a marker of early endosomes (early endosome antigen 1 or EEA1; Fig. 5B). We found that, consistent with the rate of their transport to lysosomes, mAb62/NCs appeared in the endosomal fraction at earlier time points after internalization (27.1±1.9% at 1 h) and rapidly disappeared from this location (10.4±1.4% at 2 h),whereas mAb35/NCs entered slowly early endosomes (2.4±0.6% at 1 h) and resided in pre-lysosomal compartments for prolonged periods of time (19.6±2.6% co-localization in early endosomes at 2 h). These results indicate that, although the internalization rate of mAb35/NCs is similar to that of mAb62/NCs, the intracellular transport of mAb35/NCs is considerably slower. Confirming this, mAb35/NCs only appeared in the lysosomal fraction at 5 h after internalization (42.9±3.0%). As a negative control, mAb37/NCs were not visualized within endosomes or lysosomes at the times tested (Fig. 5A and C), confirming the lack of internalization of these nanocarriers.
Once again, we did not find a correlation between intracellular transport of anti-PECAM/NCs and the size of anti-PECAM/NCs or the number of anti-PECAM antibodies per carrier particle (compare Table 2 and Fig. 5).
4. Discussion
Intracellular drug delivery is an important goal of modern bio-medicine [2,3,9,14,18,19,36,43–45]. One of the current paradigms for intracellular drug delivery is based on identification of surface determinants that support internalization by active endocytic processes that involve plasmalemma invagination and vesiculization [18,19,36,46,47]. For this purpose, selection of the target molecule is thought to be a key. For example, endothelial determinants localized in caveoli (e.g., gp60 [48,49]) or in clathrin-coated pits (e.g., transferrin receptor [10,50]) have been shown to support constitutive and ligand-stimulated endocytosis.
Our previous studies suggest that this paradigm may be overly simplistic, at least for some ligands. We have shown that antibody-mediated nanocarrier binding to two constitutive endothelial cell adhesion molecules (PECAM-1 and inter-cellular adhesion molecule-1 (ICAM-1)) triggers an unconventional mechanism for internalization. Despite the fact that ECs do not internalize anti-PECAM or anti-ICAM, multivalent anti-CAM conjugates and nanocarriers effectively enter ECs via CAM-mediated endocytosis, a unique mechanism distinct from previously known internalization pathways [35]. Since certain PECAM-1 functions (e.g., PECAM-1 homophilic binding) are mediated by specific domains of its extracellular region [25,41,42], it seemed reasonable to determine if PECAM-mediated internalization, driving intracellular delivery of anti-PECAM nanocarriers, is also affected by the site of antibody binding.
To address this issue we used a panel of five monoclonal antibodies directed to distinct PECAM-1 epitopes (Table 1). We found that binding of the non-conjugated antibodies to ECs in suspension varied considerably (e.g., lowest binding was for mAbGi34 (Fig. 1A)). However, all antibodies (except for a slight difference observed for mAbGi34) bound with relatively similar efficiency to adherent confluent ECs (Fig. 1B), which suggests a more accessible conformation of PECAM-1 exposed in this more physiological model.
In the case of 130 nm polymer particles and conjugates carrying multiple copies of these antibodies (anti-PECAM/NCs and anti-PECAM conjugates), the binding profile was quite different (Fig. 1 and Fig. 2, and Supplement 1). Anti-PECAM/NCs and conjugates of mAb4G6, directed to PECAM-1 domain 6, showed no appreciable binding to ECs and other cell types expressing PECAM-1. Given the lack of correlation between the size and antibody surface density of anti-PECAM/NCs used in this study and the binding of these particles to ECs (Table 2), the differences observed are likely due to the inaccessibility of membrane-proximal epitopes to the carriers and conjugates.
However, the binding differences between the most and least effective nanocarriers did not exceed 30% among the other anti-PECAM/NC formulations. This result implies that formation of multivalent anti-PECAM/NCs increases the effective affinity of binding to accessible epitopes, thus minimizing differences between antibodies. This outcome is similar to that observed in our previous work testing targeting to another endothelial CAM, ICAM-1. For instance, the affinity of anti-ICAM/NCs exceeded that of the corresponding anti-ICAM by ~100 fold [51]. Binding of anti-ICAM/NCs to cytokine-activated ECs exceeded that to quiescent ECs less than 30%, whereas anti-ICAM bound to cytokine-activated ECs approximately 100-fold better than to quiescent ECs [40,52].
The most striking finding in this study is the observation that, despite binding similarly to the other anti-PECAM/NCs, nanocarriers targeted by mAb37 were markedly inhibited in their ability to stimulate internalization. This observation could not be explained by differences among the size and antibody surface density of mAb37/NCs and that of the internalizable anti-PECAM/NC counterparts (Table 2 and Fig. 3). This result indicates that the epitope recognized by antibody-coated nanocarriers may be critical in determining its ultimate surface vs intracellular localization. There appears to be a high degree of specificity in this regulation, since the epitope for mAb37 is located in the same Ig domain as that of mAb62 and mAb35, both of which were able to trigger nanocarrier internalization (Fig. 3). In addition, there is specificity of regulation of intracellular transport of anti-PECAM/NCs, reflected by the fact that mAb62/NCs were transported to lysosomes within 3 h, whereas mAb35/NCs resided in pre-lysosomal compartments for prolonged periods of time (Fig. 5).
The exact mechanisms of these effects are not known. It has been shown that binding of mAb35 and mAb62 to PECAM-1 Ig domain 1 affects PECAM-1 homophilic interaction, whereas mAb37 (also binding PECAM-1 Ig domain 1) does not [25]. In an independent study, mAb62 was shown to alter development of lung alveoli in rodents, in contrast to mAb37 which did not affect this process [53]. It is possible that binding of mAb35 and mAb62 to endothelial PECAM-1 may induce key signaling events required for internalization, while binding of mAb37 does not (the functional characteristics of mAbGi34 are not known). The fact that mAb62 inhibits PECAM-1 homophilic interaction, whereas mAb35 activates this, suggests a different regulatory role of the PECAM-1 epitopes targeted by these two antibodies. This could explain the differential lysosomal transport of carriers targeted by mAb62 vs mAb35, yet the mechanism governing such a regulation remains to be determined.
Interestingly, the epitope engaged and “stimulated” by anti-PECAM/NCs seems to play a more important role in determining the efficacy of internalization and intracellular transport of these carriers into ECs than other design parameters such as carrier size: polymer particles carrying anti-PECAM mAb62, mAb35 and mAbGi34 whose size ranged from0.13 to 5 µm were similarly internalized by ECs (Fig. 4). This is in agreement with our recent observation that endocytosis of carriers targeted to ICAM-1, which also mediates endocytosis via the CAM-mediated pathway, is mainly limited by carrier shape (spherical vs elliptical disks or irregular protein conjugates) and not by carrier size. For instance, ECs were able to internalize anti-ICAM spherical carriers of several micrometers in size [54].
This is, to the best of our knowledge, the first report showing differential endothelial binding and surface vs intracellular (endosomal vs lysosomal) destination of nanocarriers targeted to specific epitopes of the same target, which is precisely a non-internalizable cell adhesion molecule. Only a few previous studies compared targeting and effects of antibodies directed to distinct epitopes of the same endothelial determinant. For example, Balyasnikova et al. [13] examined targeting of five monoclonal antibodies recognizing different epitopes on rat angiotensin-converting enzyme (ACE), an endothelial determinant involved in the regulation of the blood pressure extensively utilized as a target for specific delivery of drugs to the pulmonary vasculature [13]. Interestingly, efficacy of endothelial binding and potency of ACE inhibition were distinctly epitope-dependent [11,13]. However, contrarily to our results, these authors found that the ability of targeting antibodies to induce internalization by ECs was similar between all five different antibodies binding to the same target [13]. Of note, ECs are known to internalize monomolecular anti-ACE [55] in contrast to anti-PECAM that is not internalizable unless coupled to a multivalent carrier [30].
In conclusion, this study shows the importance of epitope specificity on the endothelial delivery of targeted nanocarriers. The efficacy of endothelial binding, internalization, and intracellular transport of model multivalent polymer carriers targeted to PECAM-1 is very dependent on the specific extracellular epitope of PECAM-1 that is targeted. This new finding can be used as a design parameter to modulate binding, as well as surface vs intracellular destination and rate of intracellular transport of endothelial drug delivery vehicles.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jconrel.2008.06.007.
Acknowledgments
The authors thank Jeffrey Faust (Flow Cytometry Facility, The Wistar Institute, Philadelphia, PA) for his help with FACS analysis. This work was funded by American Heart Association 0435181N, NIH R21 HL85533, and NIH-Upenn Pilot Grant P30 DK47757 (S. Muro), and HL/GM71175 (V. Muzykantov).
References
- 1.Muro S, Muzykantov VR. Targeting of antioxidant and anti-thrombotic drugs to endothelial cell adhesion molecules. Curr. Pharm. Des. 2005;11(18):2383–2401. doi: 10.2174/1381612054367274. [DOI] [PubMed] [Google Scholar]
- 2.Ding BS, Dziubla T, Shuvaev VV, Muro S, Muzykantov VR. Advanced drug delivery systems that target the vascular endothelium. Mol. Interv. 2006;6(2):98–112. doi: 10.1124/mi.6.2.7. [DOI] [PubMed] [Google Scholar]
- 3.Muzykantov VR. Targeting of superoxide dismutase and catalase to vascular endothelium. J. Control. Release. 2001;71(1):1–21. doi: 10.1016/s0168-3659(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 4.Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, Testa JE, Schnitzer JE. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429(6992):629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- 5.Muzykantov VR. Biomedical aspects of targeted delivery of drugs to pulmonary endothelium. Expert Opin. Drug Deliv. 2005;2(5):909–926. doi: 10.1517/17425247.2.5.909. [DOI] [PubMed] [Google Scholar]
- 6.Ghitescu LD, Crine P, Jacobson BS. Antibodies specific to the plasma membrane of rat lung microvascular endothelium. Exp. Cell Res. 1997;232(1):47–55. doi: 10.1006/excr.1997.3490. [DOI] [PubMed] [Google Scholar]
- 7.Bogdanov AA, Jr, Lin CP, Kang HW. Optical imaging of the adoptive transfer of human endothelial cells in mice using anti-human CD31 monoclonal antibody. Pharm. Res. 2007;24(6):1186–1192. doi: 10.1007/s11095-006-9219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khandare JJ, Minko T. Antibodies and peptides in cancer therapy. Crit. Rev. Ther. Drug Carr. Syst. 2006;23(5):401–435. doi: 10.1615/critrevtherdrugcarriersyst.v23.i5.20. [DOI] [PubMed] [Google Scholar]
- 9.Dunehoo AL, Anderson M, Majumdar S, Kobayashi N, Berkland C, Siahaan TJ. Cell adhesion molecules for targeted drug delivery. J. Pharm. Sci. 2006;95(9):1856–1872. doi: 10.1002/jps.20676. [DOI] [PubMed] [Google Scholar]
- 10.Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36(4):555–558. doi: 10.1016/s0896-6273(02)01054-1. [DOI] [PubMed] [Google Scholar]
- 11.Danilov S, Atochina E, Hiemisch H, Churakova T, Moldobayeva A, Sakharov I, Deichman G, Ryan U, Muzykantov VR. Interaction of mAb to angiotensin-converting enzyme (ACE) with antigen in vitro and in vivo: antibody targeting to the lung induces ACE antigenic modulation. Int. Immunol. 1994;6(8):1153–1160. doi: 10.1093/intimm/6.8.1153. [DOI] [PubMed] [Google Scholar]
- 12.Nowak K, Weih S, Metzger R, Albrecht RF, II, Post S, Hohenberger P, Gebhard MM, Danilov SM. Immunotargeting of catalase to lung endothelium via anti-angiotensin-converting enzyme antibodies attenuates ischemia-reperfusion injury of the lung in vivo. Am. J. Physiol., Lung Cell. Mol. Physiol. 2007;293(1):L162–L169. doi: 10.1152/ajplung.00001.2007. [DOI] [PubMed] [Google Scholar]
- 13.Balyasnikova IV, Metzger R, Visintine DJ, Dimasius V, Sun ZL, Berestetskaya YV, McDonald TD, Curiel DT, Minshall RD, Danilov SM. Selective rat lung endothelial targeting with a new set of monoclonal antibodies to angiotensin I-converting enzyme, Pulm. Pharmacol. Ther. 2005;18(4):251–267. doi: 10.1016/j.pupt.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Torchilin VP. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu. Rev. Biomed. Eng. 2006;8:343–375. doi: 10.1146/annurev.bioeng.8.061505.095735. [DOI] [PubMed] [Google Scholar]
- 15.Wilson A, He F, Li J, Ma Z, Pitt B, Li S. Targeted delivery of therapeutic oligo-nucleotides to pulmonary circulation. Adv. Genet. 2005;54:21–41. doi: 10.1016/S0065-2660(05)54002-1. [DOI] [PubMed] [Google Scholar]
- 16.Kabanov AV, Okano T. Challenges in polymer therapeutics: state of the art and prospects of polymer drugs. Adv. Exp. Med. Biol. 2003;519:1–27. doi: 10.1007/0-306-47932-X_1. [DOI] [PubMed] [Google Scholar]
- 17.Shuvaev VV, Christofidou-Solomidou M, Scherpereel A, Simone E, Arguiri E, Tliba S, Pick J, Kennel S, Albelda SM, Muzykantov VR. Factors modulating the delivery and effect of enzymatic cargo conjugated with antibodies targeted to the pulmonary endothelium. J. Control. Release. 2007;118(2):235–244. doi: 10.1016/j.jconrel.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson SC, Wallom KL, Ferguson EL, Deacon SP, Davies MW, Powell AJ, Piper RC, Duncan R. The use of fluorescence microscopy to define polymer localisation to the late endocytic compartments in cells that are targets for drug delivery. J. Control. Release. 2008;127(1):1–11. doi: 10.1016/j.jconrel.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai SK, Hida K, Chen C, Hanes J. Characterization of the intracellular dynamics of a non-degradative pathway accessed by polymer nanoparticles. J. Control. Release. 2008;125(2):107–111. doi: 10.1016/j.jconrel.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller WA, Ratti CM, McDonnell SL, Cohn ZA. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular. J. Exp. Med. 1989;170(2):399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman PJ, Berndt MC, Gorski J, White GC, II, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247(4947):1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- 22.Scalia R, Lefer AM. In vivo regulation of PECAM-1 activity during acute endothelial dysfunction in the rat mesenteric microvasculature. J. Leukoc. Biol. 1998;64(2):163–169. doi: 10.1002/jlb.64.2.163. [DOI] [PubMed] [Google Scholar]
- 23.Vaporciyan AA, DeLisser HM, Yan HC, Mendiguren, Thom SR, Jones ML, Ward PA, Albelda SM. Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science. 1993;262(5139):1580–1582. doi: 10.1126/science.8248808. [DOI] [PubMed] [Google Scholar]
- 24.Yan HC, Pilewski JM, Zhang Q, DeLisser HM, Romer L, Albelda SM. Localization of multiple functional domains on human PECAM-1 (CD31) by monoclonal antibody epitope mapping. Cell Adhes. Commun. 1995;3(1):45–66. doi: 10.3109/15419069509081277. [DOI] [PubMed] [Google Scholar]
- 25.Nakada MT, Amin K, Christofidou-Solomidou M, O'Brien CD, Sun J, Gurubhagavatula I, Heavner GA, Taylor AH, Paddock C, Sun QH, Zehnder JL, Newman PJ, Albelda SM, DeLisser HM. Antibodies against the first Ig-like domain of human platelet endothelial cell adhesion molecule-1 (PECAM-1) that inhibit PECAM-1-dependent homophilic adhesion block in vivo neutrophil recruitment. J. Immunol. 2000;164(1):452–462. doi: 10.4049/jimmunol.164.1.452. [DOI] [PubMed] [Google Scholar]
- 26.Christofidou-Solomidou M, Scherpereel A, Wiewrodt R, Ng K, Sweitzer T, Arguiri E, Shuvaev V, Solomides CC, Albelda SM, Muzykantov VR. PECAM-directed delivery of catalase to endothelium protects against pulmonary vascular oxidative stress. Am. J. Physiol., Lung Cell. Mol. Physiol. 2003;285(2):L283–L292. doi: 10.1152/ajplung.00021.2003. [DOI] [PubMed] [Google Scholar]
- 27.Ding BS, Gottstein C, Grunow A, Kuo A, Ganguly K, Albelda SM, Cines DB, Muzykantov VR. Endothelial targeting of a recombinant construct fusing a PECAM-1 single-chain variable antibody fragment (scFv) with prourokinase facilitates prophylactic thrombolysis in the pulmonary vasculature. Blood. 2005;106(13):4191–4198. doi: 10.1182/blood-2005-05-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dziubla TD, Shuvaev VV, Hong NK, Hawkins BJ, Madesh M, Takano H, Simone E, Nakada MT, Fisher A, Albelda SM, Muzykantov VR. Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomaterials. 2008;29(2):215–227. doi: 10.1016/j.biomaterials.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozower BD, Christofidou-Solomidou M, Sweitzer TD, Muro S, Buerk DG, Solomides CC, Albelda SM, Patterson GA, Muzykantov VR. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat. Biotechnol. 2003;21(4):392–398. doi: 10.1038/nbt806. [DOI] [PubMed] [Google Scholar]
- 30.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, Harshaw DW, Schultz L, Fisher AB, Albelda SM. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc. Natl. Acad. Sci. U. S. A. 1999;96(5):2379–2384. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shuvaev VV, Tliba S, Nakada M, Albelda SM, Muzykantov VR. Platelet-endothelial cell adhesion molecule-1-directed endothelial targeting of superoxide dismutase alleviates oxidative stress caused by either extracellular or intracellular superoxide. J. Pharmacol. Exp. Ther. 2007;323(2):450–457. doi: 10.1124/jpet.107.127126. [DOI] [PubMed] [Google Scholar]
- 32.Scherpereel A, Wiewrodt R, Christofidou-Solomidou M, Gervais R, Murciano JC, Albelda SM, Muzykantov VR. Cell-selective intracellular delivery of a foreign enzyme to endothelium in vivo using vascular immunotargeting. FASEB J. 2001;15(2):416–426. doi: 10.1096/fj.00-0022com. [DOI] [PubMed] [Google Scholar]
- 33.Romer LH, McLean NV, Yan HC, Daise M, Sun J, DeLisser HM. IFN-gamma and TNF-alpha induce redistribution of PECAM-1 (CD31) on human endothelial cells. J. Immunol. 1995;154(12):6582–6592. [PubMed] [Google Scholar]
- 34.Wiewrodt R, Thomas AP, Cipelletti L, Christofidou-Solomidou M, Weitz DA, Feinstein SI, Schaffer D, Albelda SM, Koval M, Muzykantov VR. Size-dependent intracellular immunotargeting of therapeutic cargoes into endothelial cells. Blood. 2002;99(3):912–922. doi: 10.1182/blood.v99.3.912. [DOI] [PubMed] [Google Scholar]
- 35.Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J. Cell Sci. 2003;116(Pt 8):1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 36.Muro S, Koval M, Muzykantov V. Endothelial endocytic pathways: gates for vascular drug delivery. Curr. Vasc. Pharmacol. 2004;2(3):281–299. doi: 10.2174/1570161043385736. [DOI] [PubMed] [Google Scholar]
- 37.Garnacho C, Shuvaev V, Thomas A, McKenna L, Sun J, Koval M, Albelda S, Muzykantov V, Muro S. RhoA activation and actin reorganization involved in endothelial CAM-mediated endocytosis of anti-PECAM carriers: critical role for tyrosine 686 in the cytoplasmic tail of PECAM-1. Blood. 2008;111(6):3024–3033. doi: 10.1182/blood-2007-06-098657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. U. S. A. 1983;80(12):3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muro S, Cui X, Gajewski C, Murciano JC, Muzykantov VR, Koval M. Slow intracellular trafficking of catalase nanoparticles targeted to ICAM-1 protects endothelial cells from oxidative stress. Am. J. Physiol., Cell Physiol. 2003;285(5):C1339–C1347. doi: 10.1152/ajpcell.00099.2003. [DOI] [PubMed] [Google Scholar]
- 40.Muro S, Gajewski C, Koval M, Muzykantov VR. ICAM-1 recycling in endothelial cells: a novel pathway for sustained intracellular delivery and prolonged effects of drugs. Blood. 2005;105(2):650–658. doi: 10.1182/blood-2004-05-1714. [DOI] [PubMed] [Google Scholar]
- 41.Newton JP, Buckley CD, Jones EY, Simmons DL. Residues on both faces of the first immunoglobulin fold contribute to homophilic binding sites of PECAM-1/CD31. J. Biol. Chem. 1997;272(33):20555–20563. doi: 10.1074/jbc.272.33.20555. [DOI] [PubMed] [Google Scholar]
- 42.Wong CW, Wiedle G, Ballestrem C, Wehrle-Haller B, Etteldorf S, Bruckner M, Engelhardt B, Gisler RH, Imhof BA. PECAM-1/CD31 trans-homophilic binding at the intercellular junctions is independent of its cytoplasmic domain; evidence for heterophilic interaction with integrin alphavbeta3 in Cis. Mol. Biol. Cell. 2000;11(9):3109–3121. doi: 10.1091/mbc.11.9.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitchens KM, Foraker AB, Kolhatkar RB, Swaan PW, Ghandehari H. Endocytosis and interaction of poly (amidoamine) dendrimers with Caco-2 cells. Pharm. Res. 2007;24(11):2138–2145. doi: 10.1007/s11095-007-9415-0. [DOI] [PubMed] [Google Scholar]
- 44.Rawat A, Vaidya B, Khatri K, Goyal AK, Gupta PN, Mahor S, Paliwal R, Rai S, Vyas SP. Targeted intracellular delivery of therapeutics: an overview. Pharmazie. 2007;62(9):643–658. [PubMed] [Google Scholar]
- 45.Haider M, Hatefi A, Ghandehari H. Recombinant polymers for cancer gene therapy: a minireview. J. Control. Release. 2005;109(1–3):108–119. doi: 10.1016/j.jconrel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 46.Stan RV. Endocytosis pathways in endothelium: how many? Am. J. Physiol., Lung Cell. Mol. Physiol. 2006;290(5):L806–L808. doi: 10.1152/ajplung.00533.2005. [DOI] [PubMed] [Google Scholar]
- 47.Medina-Kauwe LK. “Alternative” endocytic mechanisms exploited by pathogens: new avenues for therapeutic delivery? Adv. Drug Deliv. Rev. 2007;59(8):798–809. doi: 10.1016/j.addr.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schnitzer JE. gp60 is an albumin-binding glycoprotein expressed by continuous endothelium involved in albumin transcytosis. Am. J. Physiol. 1992;262(1 Pt 2):H246–H254. doi: 10.1152/ajpheart.1992.262.1.H246. [DOI] [PubMed] [Google Scholar]
- 49.John TA, Vogel SM, Minshall RD, Ridge K, Tiruppathi C, Malik AB. Evidence for the role of alveolar epithelial gp60 in active transalveolar albumin transport in the rat lung. J. Physiol. 2001;533(Pt 2):547–559. doi: 10.1111/j.1469-7793.2001.0547a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simionescu M, Simionescu N. Endothelial transport of macromolecules: transcytosis and endocytosis. A look from cell biology. Cell Biol. Rev. 1991;25(1):1–78. [PubMed] [Google Scholar]
- 51.Muro S, Dziubla T, Qiu W, Leferovich J, Cui X, Berk E, Muzykantov VR. Endothelial targeting of high-affinity multivalent polymer nanocarriers directed to intercellular adhesion molecule 1. J. Pharmacol. Exp. Ther. 2006;317(3):1161–1169. doi: 10.1124/jpet.105.098970. [DOI] [PubMed] [Google Scholar]
- 52.Murciano JC, Muro S, Koniaris L, Christofidou-Solomidou M, Harshaw DW, Albelda SM, Granger DN, Cines DB, Muzykantov VR. ICAM-directed vascular immunotargeting of antithrombotic agents to the endothelial luminal surface. Blood. 2003;101(10):3977–3984. doi: 10.1182/blood-2002-09-2853. [DOI] [PubMed] [Google Scholar]
- 53.DeLisser HM, Helmke BP, Cao G, Egan PM, Taichman D, Fehrenbach M, Zaman A, Cui Z, Mohan GS, Baldwin HS, Davies PF, Savani RC. Loss of PECAM-1 function impairs alveolarization. J. Biol. Chem. 2006;281(13):8724–8731. doi: 10.1074/jbc.M511798200. [DOI] [PubMed] [Google Scholar]
- 54.Muro S, Garnacho C, Champion J, Leferovich J, Gajewski C, Schuchman EH, Mitragotri S, Muzykantov V. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol. Ther.Ahead of print. 2008 June 17; doi: 10.1038/mt.2008.127. PMID: 18560419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muzykantov VR, Atochina EN, Kuo A, Barnathan ES, Notarfrancesco K, Shuman H, Dodia C, Fisher AB. Endothelial cells internalize monoclonal antibody to angiotensin-converting enzyme. Am. J. Physiol. 1996;270(5 Pt 1):L704–L713. doi: 10.1152/ajplung.1996.270.5.L704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jconrel.2008.06.007.