Abstract
Background
Cognitive impairment in the form of decreased working memory and executive functions has been recognized as a key deficit in schizophrenia. Neurotropic viruses have been associated with focal gray matter deficits in patients with schizophrenia. We evaluated whether such agents alter cognitive function in schizophrenia.
Methods
The sample consisted of 329 patients diagnosed with schizophrenia or schizoaffective disorder. We evaluated associations between exposure to selected agents (Herpes Simplex Viruses 1 and 2 (HSV1, HSV2 respectively) cytomegalovirus (CMV) and Toxoplasma gondii) and scores on the Trail Making Test (TMT), controlling for relevant variables.
Results
Serological evidence of exposure to CMV was associated with impaired performance on TMT part A time to completion (p=0.044), a measure of visual search, working memory, and psychomotor speed. Both CMV and HSV1 were significantly associated with increased errors on TMT part B (p<0.001 for both viruses). HSV2 and Toxoplasma gondii exposure measures were not associated with any of the cognitive functions evaluated using TMT.
Conclusions
Both CMV and HSV1 are associated with impaired cognitive function in schizophrenia as measured by the TMT. Further analyses to evaluate the impact of other illness related variables including genetic variants are warranted.
Keywords: cytomegalovirus, herpes simplex virus, cognitive impairment, trail making test, working memory, schizophrenia, environmental etiology, cognition
1. Introduction
Schizophrenia is a common, debilitating disorder characterized by disturbances in thought, perception, and affect that lead to significant deterioration in function. Cognitive impairment on a broad range of tasks is known to be an important component of schizophrenia (Keefe and Fenton 2007). While psychotic symptoms are variable during the course of the illness, cognitive dysfunction can persist and may be associated with long term outcome (Green et al. 2000; Hyde et al. 1994). Further, cognitive decline is associated with the onset of psychosis in high-risk individuals (Wood et al. 2007). A recent meta-analysis of cognitive remediation therapy suggests that improvement in functional recovery of schizophrenia patients is possible (McGurk et al. 2007). Thus, an understanding of the factors leading to cognitive dysfunction is important (Gray and Roth 2007).
Neurotropic infectious agents have long been studied in schizophrenia (Kirch 1993). Perinatal or neonatal infections with viruses such as Rubella and Coxsackie virus are uncommon but can substantially increase schizophrenia risk (Brown et al. 2001; Koponen et al. 2004; Rantakallio et al. 1997). Although evidence implicating other common infectious agents has been inconsistent, recent studies of cytomegalovirus (CMV), herpes simplex virus 1 (HSV1), mumps virus, Toxoplasma gondi (TOX), and influenza have indicated associations between levels of exposure and increased rates of schizophrenia. (Brown et al. 2005a; Brown et al. 2005b; Dalman et al. 2008; Leweke et al. 2004; Niebuhr et al. 2008; Pearce 2003; Wang et al. 2006).
Dickerson and colleagues have reported that exposure to Herpes Simplex Virus 1, but not other herpes viruses, is an independent predictor of cognitive dysfunction in schizophrenia (Dickerson et al. 2003). Further work showed a similar association in patients with bipolar disorder (Dickerson et al. 2004), and suggested associations of the same cognitive measures with plasma levels of C-reactive protein, a general marker of inflammation (Dickerson et al. 2007). We have previously observed decreased grey matter volumes in the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate gyrus in medication naïve, first-episode schizophrenia patients with serological evidence of HSV1 exposure compared with schizophrenia patients with no serological evidence of HSV1 exposure and healthy controls (Prasad et al. 2007). Taken together, these results suggest that cognitive impairment in schizophrenia may be partly explained by exposure to certain infectious agents that cause central nervous system inflammation. In order to further examine the association of exposure to infectious agents and cognitive impairment among schizophrenia patients, we have evaluated an independent sample of schizophrenia patients and examined a different set of cognitive measures that may be related to prefrontal cortex function than those measured by Dickerson et al (Dickerson et al. 2003). The analyses with HSV1 exposure were contrasted with exposure to three other infectious agents, namely CMV, HSV2 (members of herpes family of viruses), as well as Toxoplasma gondii (TOX). Exposure to each of these agents has previously been found to increase schizophrenia risk (Niebuhr et al. 2008; Torrey and Yolken 2003; Yolken 2004).
2. Methods
2.1 Sample
Our sample consisted of 329 individuals with schizophrenia or schizoaffective disorder recruited at Western Psychiatric Institute and Clinic in Pittsburgh, PA and at other institutions within a 500 mile radius (Chowdari et al. 2002; Talkowski et al. 2008). Clinical information was obtained using the “Diagnostic Interview for Genetic Studies” (DIGS) in combination with medical records and pertinent information from relatives of the participants. DSM-IV criteria for the diagnosis of schizophrenia or schizoaffective disorder were confirmed by board-certified psychiatrists or psychologists (A.P.A. 1994).
The study cohort was 66% male and had a mean age of 38.4 years (standard deviation, (SD) 9.5 years, range 13–75). Our sample was 93% Caucasian, 6% African-American, and 1% of the patients reported other ethnicity or mixed ancestry. The participants had completed a mean of 12.8 years of education (SD 2.5). The mean age of onset was 20.1 years (SD 6.8) making the mean duration of illness 18.3 years (SD 9.0). From the entire list of medications prescribed, we separated participants into non-exclusive groups prescribed clozapine, any other atypical antipsychotic, and older antipsychotics. At the time of testing, 29% of our sample was prescribed clozapine, 38% received another atypical antipsychotic, and 22% of the patients were taking older antipsychotic drugs. Smaller percentages of individuals were prescribed other classes of medications. The average global assessment of function (GAF) scores during the month prior to assessment was 55.4 (SD 13.3), suggesting that the average participant had moderate symptoms or difficulty in social, occupational or school functioning over the past month (Endicott et al. 1976).
All participants provided written informed consent, and all aspects of the study were approved by the Institutional Review Board at the University of Pittsburgh.
2.2 Measures of Cognitive Function
Patients were administered the Trail Making Test (TMT), a widely-used, two-part test of cognitive function (Army Individual Test Battery 1944; Drane et al. 2002). In TMT part A, individuals connect numbered circles in sequence scattered across a page. Both the time taken to complete the test and the number of errors are recorded by an examiner. The test measures visual search, working memory, motor speed, and spatial orientation skills (Crowe 1998). In TMT part B, individuals connect labeled circles switching from numbers to letters and in sequence. In addition to the skills utilized in part A, this procedure tests set-switching, cognitive flexibility, and executive function (Arbuthnott and Frank 2000). Among patients with schizophrenia, speed of performance on TMT part B time is correlated with the Withdrawal-Retardation factor of the Brief Psychiatric Rating scale, a measure of negative symptoms, and TMT part B errors is associated with the Conceptual Disorganization Factor, particularly with working memory (Mahurin et al. 2006).
Individuals were allotted 5 minutes each to finish TMT parts A and B. All individuals in our study completed TMT part A. For participants who took longer than 5 minutes for part B, completion time was recorded as 5 minutes. This was done in order to avoid scores for individuals who took unusually long periods from biasing the results unduly. For individuals who finished over half of part B, which we defined as over 13 of the 25 items, but could not continue further, times and errors were prorated to estimate their performance had they completed the test. In addition to the TMT time and number of errors, we derived a measure of the executive functioning by subtracting time A from time B and dividing by time A ((B-A)/A) (Drane et al. 2002). This procedure has been validated as a more precise measure of executive functioning and prefrontal cortex activity (Shibuya-Tayoshi et al. 2007; Stuss et al. 2001).
2.3 Serological analysis
Specific enzyme based immunoassays for IgG antibodies to HSV1, HSV2, CMV and TOX were performed on serum samples that were available from all cases as previously described (Ashley et al. 1998; Dickerson et al. 2003). An individual was considered to have been exposed to an infectious agent if his or her serum had a level of reactivity which was greater than that of predetermined standards defined for each infectious agent.
Statistical Analysis
2.3.1 Analysis of main cognitive measures
Statistical analyses were conducted using SPSS version 12 and 14 (SPSS 2005). Time scores on TMT parts A and B, and (B-A)/A had substantial positive skew, so we conducted log transformations of these variables, thus yielding a test statistic with normal distribution. We used general linear model analysis of covariance to evaluate the association of antibody titer status with log transformed TMT time and to evaluate and control for the potential impact of age, education, sex, and ethnicity. We included these factors as they have been associated with TMT performance in other studies (Drane et al. 2002; Perianez et al. 2007). Only age and education were found to be significant independent predictors of TMT performance in our sample. Other potential covariates on which we had data, such as age-of-onset, duration of illness, and medications prescribed, were not included as covariates as they were not correlated with TMT performance, and thus would add unnecessary additional degrees of freedom to the analysis. Further, using the same statistical approach we examined the potential interaction effects of the infectious agents.
2.3.2 Analysis of additional measures and missing data
Errors on TMT parts A and B were relatively uncommon events, with the majority of the sample having one or fewer errors, hence outcomes followed a Poisson distribution. We used generalized linear models to evaluate associations of part A and part B errors with antibody titer status while controlling for age, education, sex, and gender.
Missing data on cognitive tasks are a potential problem that is important to address. In this study, some participants did not finish over half of TMT part B, defined as more than 13 of the 25 items. Included in this group were some participants who had stopped after only 1 or 2 items, or who did not finish and for whom it was not possible to verify exactly how many items were completed. Data for TMT part B for these participants were not included in the initial analyses, but were incorporated in an exploratory analysis: we compared the antibody titer status for these individuals with an age, education, and race matched group selected from patients with the fastest 25% of scores on TMT part B using the Fisher’s exact test.
3 Results
3.1 Performance on TMT
The mean time for completion of TMT part A was 45.34 ± 30.63 seconds and the mean number of errors were 0.76 ± 1.19. The mean time on TMT part B was 110.91 ± 68.31 seconds with a mean of 1.63 ± 2.21 errors. These values are consistent with reported values for schizophrenia patients (Perianez et al. 2007). Younger age and as well as longer duration of education were significantly associated with faster performance on TMT parts A and B (p < 0.001). Higher educational status was also associated with fewer errors on TMT part B (p < 0.001), as was younger age (p < 0.05). The mean value for the derived measure (B−A)/A was 1.86 ± 2.35. Education was significantly associated with (B−A)/A (p < 0.01), but age was not significantly associated with this measure (p > 0.25). Ethnicity, sex, age at onset, duration of illness, and medications were not significantly associated with any performance measures after controlling for the effect of age and education. Thirty-seven individuals (11.2%) did not finish TMT part B in the allotted 5 minutes. Within this group, seven individuals did finish the test, but required more than five minutes. For these individuals, the times for completion were recorded as five minutes. For eight individuals who finished over half of part B, times and number of errors were prorated to reflect their performance had they completed the test. Complete data were unavailable for twenty-two other individuals, who either declined to continue after completing less than half of TMT part B, or for whom it was not possible to verify exactly how many items they had completed. These individuals were not included in the primary analyses, and were analyzed separately (see statistical analysis section).
3.2 Serum Antibody Titers
Each individual was evaluated for serological evidence of exposure to HSV1, HSV2, CMV, and TOX. A total of 148(45%) of the individuals had serological evidence of exposure to HSV-1, 50 (15.2%) had serological evidence of exposure to HSV2, 118 (35.9%) had serological evidence of exposure to CMV and 30 (9.1%) had serological evidence of exposure to TOX (Table 1). Serological evidence of exposure was significantly inter-correlated among infections agents we tested after using binary logistic regression to adjust for age, race, gender, and education with the exception of CMV and HSV2 (Wald 5.98, p = 0.014, B = 0.80, see Table 1).
Table 1.
Serological status of patients, including co-infection rates.
| TOX + | TOX − | ||||
|---|---|---|---|---|---|
| HSV+ | HSV− | HSV+ | HSV− | ||
| HSV2+ | CMV+ | 4 | 2 | 14 | 8 |
| CMV− | 0 | 1 | 7 | 14 | |
| HSV2− | CMV+ | 6 | 2 | 39 | 43 |
| CMV− | 10 | 5 | 68 | 106 | |
3.3 Association of serological evidence of infection with main cognitive measures
3.3.1 TMT part A time
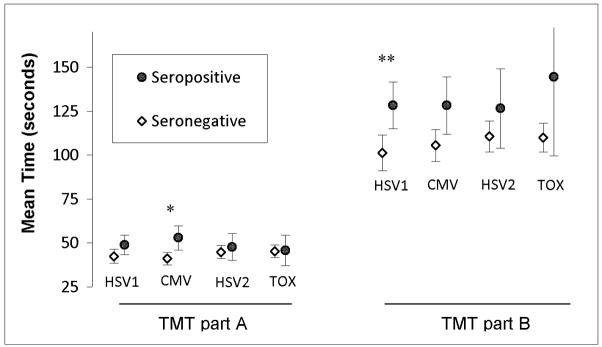
Serological evidence of infection with CMV was associated with slower times on TMT part A after adjusting for age, education, race, and sex (F(1,323) 4.1, p = 0.044, B=−.12, 95% CI [−.23.. −0.003], 1.3 % of variance, see Figure 1). Exposure to HSV1 HSV2, or TOX was not associated with TMT part A time (p = 1.0, 0.92, and 0.97 respectively).
Figure 1. Average times for completion of the Trail Making Test in relation to serological status.
Mean time on trail making test part A and B for individuals with and without serological evidence of HSV1 and CMV exposure are shown. Error bars represent unadjusted 95% confidence interval for means. * p = 0.044 after adjusting for age, education, race, and sex. ** p = 0.040 after adjusting for age and education. HSV1: Herpes Simplex Virus 1. CMV: Cytomegalovirus. HSV2: Herpes Simplex Virus 1. TOX: Toxoplasma gondii.
3.3.2 TMT part B time
Univariate analysis showed association of serological evidence of HSV1 infection with slower times on TMT part B while adjusting for age and education (F(1,303) = 4.3, p = 0.040., B = −0.12, 95% CI [−.24.. −0.006], 1.4% of variance)(Figure 1), but did not attain nominal significance after adding adjustments for sex and race (F(1,303) = 3.7, p = 0.056, B = −0.12, 95% CI [−.23..0.003], 1.2% of variance). Serological evidence of infection with CMV, HSV2 or TOX was not associated with this measure (p = 0.36, 0.46, and 0.29 respectively).
3.3.3 TMT (B-A)/A
Serological evidence of infection with HSV1 was significantly associated with greater (B−A)/A while controlling for age, education, sex, and race (F(1,295) = 5.1, p = 0.025, B = −0.25, 95% CI [−.45.. −0.03], 1.7% of variance, see Figure 2). Serological evidence of infection with CMV, HSV2 or TOX was not associated with this measure (p = 0.87,0.84, and 0.57 respectively).
Figure 2. Average ln(B-A)/A in relation to serological status.
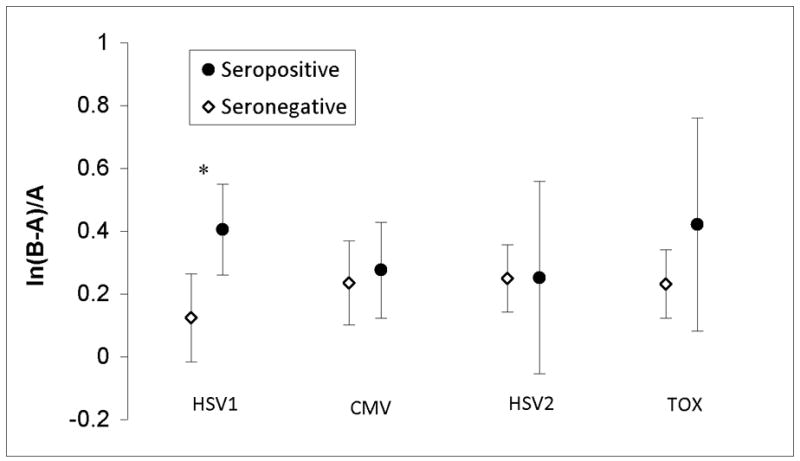
Natural log of trail making test (B-A)/A, a measure of executive function, for individuals with and without serological evidence of HSV1, CMV, HSV1, and TOX exposure are shown. Error bars represent unadjusted 95% confidence interval for mean. * p < 0.05 after adjusting for age, education, race, and sex. HSV1: Herpes Simplex Virus 1. CMV: Cytomegalovirus. HSV2: Herpes Simplex Virus 1. TOX: Toxoplasma gondii.
Further analysis of performance on TMT part A and B and (B−A)/A time indicated that HSV1 and CMV have no significant interaction effect on performance when main effects were taken into account (p-value for interaction effect 0.21 for TMT part A, 0.71 for TMT part B, and 0.44 for (B−A)/A).
3.4 Association of Antibody Titer Status with Additional Measures
3.4.1 TMT errors
Analysis using generalized linear models to adjust for age, education, sex, and race showed that serological evidence of HSV1 infection was associated with significantly more errors on TMT part B (Wald 35.95, p = 2×10−8, B = −0.90, 95% Wald CI [−1.19--0.61]), but not on TMT part A (Figure 3). Serological evidence of CMV infection was also significantly associated with increased errors on TMT parts B (Wald 19.64, p = 1×10−5, B = −0.73, 95% Wald CI [−1.5.. −0.41]), but not on part A (Figure 3). Neither HSV2 nor TOX was significantly associated with errors or either part of the TMT (p = 0.066 and 0.10 respectively).
Figure 3. Average errors on the Trail Making Test in relation to serological status.
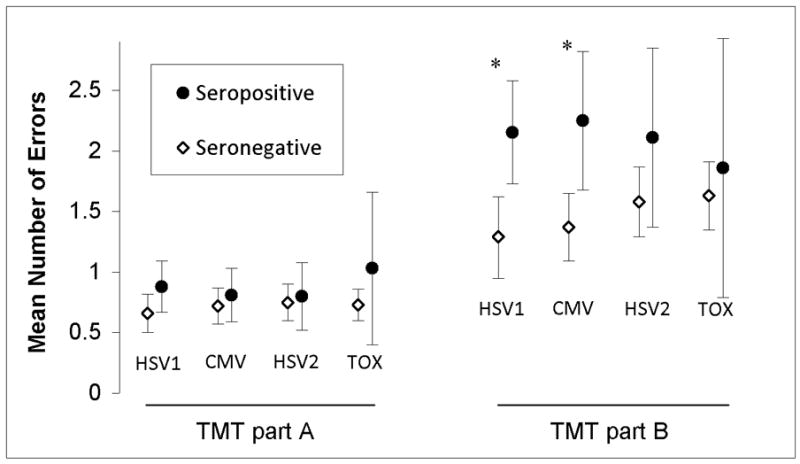
Mean number of errors on trail making test part A and B for individuals with and without serological evidence of HSV1, CMV, HSV1, and TOX exposure are shown. Error bars represent unadjusted 95% confidence interval for mean. * p < 0.001 after adjusting for age, education, race, and sex. HSV1: Herpes Simplex Virus 1. CMV: Cytomegalovirus. HSV2: Herpes Simplex Virus 1. TOX: Toxoplasma gondii.
3.4.2 Inability to complete TMT part B
In exploratory analysis to deal with missing data, those who did not finish at least half of the TMT part B were compared to an age, education, and race matched sub-sample of individuals with the fastest performance on trails B. Non-finishers were significantly more likely to have serological evidence of HSV1 or CMV (p = 0.031and 0.033 respectively, 1 tailed Fisher’s exact test). No significant deviation from expected distribution of TMT non-completers was seen for individuals with serological evidence of infection with HSV2 or TOX (p = 0.50 and 0.83 respectively)
4. Discussion
The present analyses suggest that serological evidence of infection to two neurotropic viral agents, namely HSV1 and CMV, is associated with impaired cognitive functioning among patients with schizophrenia. The associations with HSV1 are consistent with an earlier report (Dickerson et al. 2003). To our knowledge, this is the first study to report that serological evidence of infections with CMV has a significant effect on cognition in patients with schizophrenia. We found a positive association with cognitive impairment that may be qualitatively different from the associations with HSV1 exposure, although others have found similar trends with CMV exposure in schizophrenia patients (Dickerson et al. 2003). The different patterns may be affected by the relatively large variation in the test values noted here. Alternatively, our observation may reflect a larger effect of HSV1 exposure than CMV on performance on these cognitive tasks. The statistically significant associations here explain a relatively small portion of the variability, but are important as they may be remediable. The rates of seropositivity reported here are similar to those found by others (Marshall and Stout 2005; Niebuhr et al. 2008; Yolken 2004).
SZ patients exposed to CMV took longer to complete the TMT A, but did not commit significantly more errors on TMT-A. This suggests that SZ patients exposed to CMV emphasized accuracy over speed when completing the TMT-A. Else, they may experience difficulty in integrating visual scanning, motor coordination and spatial orientation, and perhaps slower processing. With enough time, however, they could perform with comparable number of errors to unexposed patients. On the other hand, SZ patients exposed to CMV committed more errors on the more challenging TMT-B, with no significantly increased time compared to those not exposed. This suggests that SZ patients exposed to CMV may emphasize time over accuracy for complex tasks that involve integration of multiple cognitive processes to generate an appropriate output.
SZ patients exposed to HSV1 had a different pattern of performance. They did not show significant differences in the response time or the errors committed on the TMT-A compared to those not exposed. On the relatively more difficult TMT-B, those exposed to HSV1 took longer and committed more errors. The results may reflect impaired executive subprocesses as suggested by differences in the derived measure ((B-A)/A). This observation is partly supported by the brain regions affected by HSV1 exposure. Whereas CMV is known to affect the limbic regions (Hanshaw 1976), HSV1 primarily affects the prefrontal, parietal and temporal cortical regions (Cleator and Klapper 2004). Although such explanations are speculative at present, the pattern of differences in task performance may be due to real differences in the pattern of impairment, variable effect size associated with each of these viruses, or stochastic variations. Given that these cognitive skills are used in both TMT parts A and B it is surprising that there is no significant difference in TMT part B time for those infected with CMV. These results should be evaluated using other cognitive tests, as the TMT may not discriminate precisely between these skills. Both viruses were also associated with increased errors on TMT part B. Performance on these measures may thus reflect the same cognitive functions influencing time for completion, such as working memory or set switching. It is also possible that our results indicate other areas of cognitive functioning that are influenced by exposure to CMV, as well as HSV1.
The results suggesting worse (B-A)/A and increased errors among individuals exposed to HSV1 are consistent with previous findings, which used the Repeatable Battery for the Assessment of Cognitive Status (RBANS) as a measure of cognitive function in schizophrenia patients. The difference in performance could best be attributed to impaired working memory (Dickerson et al. 2003). They are also consistent with our prior report (Prasad et al. 2007) in which we noted decreased gray matter volumes in the DLPFC among HSV1 seropositive individuals with schizophrenia because some components of working memory are known to be regulated by the DLFPC (Barch et al. 2001; Callicott et al. 2003). Taken together, our observations suggest that HSV1 may mediate impaired cognitive functions through abnormalities in the DLPFC (Brodmann areas 9 and 46). However, the structure-function correlation may be more complex. Demakis (Demakis 2004) in a metaanalysis elegantly points out the complexity in associating neuropsychological test performance purported to tap the frontal lobe function with the function of this region in comparison to non-frontal regions. Surprisingly, this metaanalysis shows statistically significant association of TMT-A but not TMT-B performance with frontal lobe function. The derived value ((B-A)/A) that represents executive function was not included in this metaanalysis. However, as Demakis (Demakis 2004) points out the studies included in the metaanalysis tended to collapse all frontal regions into frontal lobe, which may be an oversimplification of a functionally complex lobe. An integrative study using concurrent functional imaging of the brain while performing these tests may help answer this question.
For individuals with serological evidence of exposure to TOX or HSV2, there was a trend towards slower times to completion, but these results were not statistically significant. The number of individuals with serological evidence of exposure was much smaller for these infectious agents, so our sample had less power to effectively evaluate associations with for effect sizes similar to those observed here for HSV1 and CMV.
Antibody titers do not indicate the precise time when infection occurs. Our serological analysis is based on samples that were taken well after the diagnosis of schizophrenia. Our explanation is that reactivation of latent infection maintains elevated antibody levels and allows us to distinguish with reasonable accuracy between infection exposed and unexposed individuals. However, from our data we are unable to address the important question of whether cognitive effects are due to acute influences on brain functioning or disturbances in brain development related to exposure to these infectious agents.
There are few reports of the impact of HSV1 and CMV on cognitive function among other diagnostic groups or among individuals who do not manifest any overt disorder but have serological evidence of viral exposure. Hence it is uncertain whether our results indicate a specific effect among individuals with schizophrenia. Recently, HSV1 and CMV have been associated with decreased cognitive functioning in elderly patients (Aiello et al. 2006; Strandberg et al. 2003). In addition, Dickerson and colleagues have found impaired memory to be associated with serological evidence of HSV-1 infection in individuals without a psychiatric disease (Dickerson et al. 2008). Impairment in cognitive function due to viral infection may not be specific to schizophrenia pathogenesis, but only noticeable when there is already baseline cognitive deficit, such as that caused by the aging process. Cognitive dysfunction in schizophrenia is relatively stable, and does not progress with age differently than it does in similarly aged healthy subjects (Eyler Zorrilla et al. 2000; Nayak Savla et al. 2006). On the other hand, cognitive dysfunction appears to be an early manifestation of schizophrenia, unrelated to age of onset or duration of illness (Heaton et al. 1994; O’Donnell 2007).
The mechanisms by which viruses cause cognitive dysfunction are likewise unclear. We have found that genetic variants in some genes that are associated with schizophrenia are also associated with HSV1 antibody titer status (Shirts et al. 2007a; Shirts et al. 2007b), suggesting there may be similar mechanisms associated with viral central nervous system infection and schizophrenia. It appears that infections such as HSV1 act directly on the central nervous system or through local immune mediators as there are measurable gray matter volume changes in schizophrenia cases infected with HSV1 (Prasad et al. 2007). However, infections may also act indirectly through activation of systemic cytokines and stress factors, whose levels have been found to be differently expressed in schizophrenia cases and which are known to modulate cognitive functioning (Leonard 2007; Potvin et al. 2008).
Further analysis of cognitive function and infection may be necessary to determine if viral influence on cognition is a general phenomenon or specific to schizophrenia. Regardless of specificity to schizophrenia, treatment of HSV1 and CMV exposed schizophrenia patients with antivirals could potentially improve cognitive function and clinical outcomes. Randomized controlled studies will be necessary to determine the clinical benefit of such treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- A.P.A. 1994. Diagnostic and Statistical Manual of Mental Disorders -- DSM-IV.
- Aiello AE, Haan M, Blythe L, Moore K, Gonzalez JM, Jagust W. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54(7):1046–54. doi: 10.1111/j.1532-5415.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- Arbuthnott K, Frank J. Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol. 2000;22(4):518–28. doi: 10.1076/1380-3395(200008)22:4;1-0;FT518. [DOI] [PubMed] [Google Scholar]
- Army Individual Test Battery. Manual of directions and scoring. Washington D.C: War Department, Adjuntant General’s Office; 1944. [Google Scholar]
- Ashley RL, Wu L, Pickering JW, Tu MC, Schnorenberg L. Premarket evaluation of a commercial glycoprotein G-based enzyme immunoassay for herpes simplex virus type-specific antibodies. J Clin Microbiol. 1998;36(1):294–5. doi: 10.1128/jcm.36.1.294-295.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, 3rd, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58(3):280–8. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic Evidence of Prenatal Influenza in the Etiology of Schizophrenia. Obstet Gynecol Surv. 2005a;60(2):77–78. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Brown AS, Cohen P, Harkavy-Friedman J, Babulas V, Malaspina D, Gorman JM, Susser ES. A.E. Bennett Research Award. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol Psychiatry. 2001;49(6):473–86. doi: 10.1016/s0006-3223(01)01068-x. [DOI] [PubMed] [Google Scholar]
- Brown AS, Schaefer CA, Quesenberry CP, Jr, Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005b;162(4):767–73. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160(12):2209–15. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Chowdari KV, Mirnics K, Semwal P, Wood J, Lawrence E, Bhatia T, Deshpande SN, B KT, Ferrell RE, Middleton FA, Devlin B, Levitt P, Lewis DA, Nimgaonkar VL. Association and linkage analyses of RGS4 polymorphisms in schizophrenia. Hum Mol Genet. 2002;11(12):1373–80. doi: 10.1093/hmg/11.12.1373. [DOI] [PubMed] [Google Scholar]
- Cleator GM, Klapper PE. Herpes Simplex. In: Zuckerman AJ, Banatvala JE, Pattison JR, editors. Principles and Practice of Clinical Virology. New York: John Wiley and Sons, Ltd; 2004. pp. 27–51. [Google Scholar]
- Crowe SF. The differential contribution of mental tracking, cognitive flexibility, visual search, and motor speed to performance on parts A and B of the Trail Making Test. J Clin Psychol. 1998;54(5):585–91. doi: 10.1002/(sici)1097-4679(199808)54:5<585::aid-jclp4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Dalman C, Allebeck P, Gunnell D, Harrison G, Kristensson K, Lewis G, Lofving S, Rasmussen F, Wicks S, Karlsson H. Infections in the CNS during childhood and the risk of subsequent psychotic illness: a cohort study of more than one million Swedish subjects. Am J Psychiatry. 2008;165(1):59–65. doi: 10.1176/appi.ajp.2007.07050740. [DOI] [PubMed] [Google Scholar]
- Demakis GJ. Frontal lobe damage and tests of executive processing: a meta-analysis of the category test, stroop test, and trail-making test. J Clin Exp Neuropsychol. 2004;26(3):441–50. doi: 10.1080/13803390490510149. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93(1–3):261–5. doi: 10.1016/j.schres.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Sullens A, Origoni A, Leister F, Krivogorsky B, Yolken R. Association between cognitive functioning, exposure to Herpes Simplex Virus type 1, and the COMT Val158Met genetic polymorphism in adults without a psychiatric disorder. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.04.156. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Cole S, Krivogorsky B, Yolken RH. Infection with herpes simplex virus type 1 is associated with cognitive deficits in bipolar disorder. Biol Psychiatry. 2004;55(6):588–93. doi: 10.1016/j.biopsych.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Ruslanova I, Yolken RH. Association of serum antibodies to herpes simplex virus 1 with cognitive deficits in individuals with schizophrenia. Arch Gen Psychiatry. 2003;60(5):466–72. doi: 10.1001/archpsyc.60.5.466. [DOI] [PubMed] [Google Scholar]
- Drane DL, Yuspeh RL, Huthwaite JS, Klingler LK. Demographic characteristics and normative observations for derived-trail making test indices. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15(1):39–43. [PubMed] [Google Scholar]
- Endicott J, Spitzer R, Fleiss J, Cohen J. The Global Assessment Scale. Archives of General Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Eyler Zorrilla LT, Heaton RK, McAdams LA, Zisook S, Harris MJ, Jeste DV. Cross-sectional study of older outpatients with schizophrenia and healthy comparison subjects: no differences in age-related cognitive decline. Am J Psychiatry. 2000;157(8):1324–6. doi: 10.1176/appi.ajp.157.8.1324. [DOI] [PubMed] [Google Scholar]
- Gray JA, Roth BL. Molecular targets for treating cognitive dysfunction in schizophrenia. Schizophr Bull. 2007;33(5):1100–19. doi: 10.1093/schbul/sbm074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119–36. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Hanshaw JB. Cytomegalovirus. In: Remington JS, Kelin JO, editors. Infectious diseases of the fetus and newborn infant. Philadelphia (PA): Saunders; 1976. p. 127. [Google Scholar]
- Heaton R, Paulsen JS, McAdams LA, Kuck J, Zisook S, Braff D, Harris J, Jeste DV. Neuropsychological deficits in schizophrenics. Relationship to age, chronicity, and dementia. Arch Gen Psychiatry. 1994;51(6):469–76. doi: 10.1001/archpsyc.1994.03950060033003. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Nawroz S, Goldberg TE, Bigelow LB, Strong D, Ostrem JL, Weinberger DR, Kleinman JE. Is there cognitive decline in schizophrenia? A cross-sectional study. Br J Psychiatry. 1994;164(4):494–500. doi: 10.1192/bjp.164.4.494. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Fenton WS. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr Bull. 2007;33(4):912–20. doi: 10.1093/schbul/sbm046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirch DG. Infection and autoimmunity as etiologic factors in schizophrenia: a review and reappraisal. Schizophrenia Bulletin. 1993;19(2):355–70. doi: 10.1093/schbul/19.2.355. [DOI] [PubMed] [Google Scholar]
- Koponen H, Rantakallio P, Veijola J, Jones P, Jokelainen J, Isohanni M. Childhood central nervous system infections and risk for schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2004;254(1):9–13. doi: 10.1007/s00406-004-0485-2. [DOI] [PubMed] [Google Scholar]
- Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007;32(10):1749–56. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Gerth CW, Koethe D, Klosterkotter J, Ruslanova I, Krivogorsky B, Torrey EF, Yolken RH. Antibodies to infectious agents in individuals with recent onset schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2004;254(1):4–8. doi: 10.1007/s00406-004-0481-6. [DOI] [PubMed] [Google Scholar]
- Mahurin RK, Velligan DI, Hazleton B, Mark Davis J, Eckert S, Miller AL. Trail making test errors and executive function in schizophrenia and depression. Clin Neuropsychol. 2006;20(2):271–88. doi: 10.1080/13854040590947498. [DOI] [PubMed] [Google Scholar]
- Marshall GS, Stout GG. Cytomegalovirus seroprevalence among women of childbearing age during a 10-year period. Am J Perinatol. 2005;22(7):371–6. doi: 10.1055/s-2005-872590. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791–802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak Savla G, Moore DJ, Roesch SC, Heaton RK, Jeste DV, Palmer BW. An evaluation of longitudinal neurocognitive performance among middle-aged and older schizophrenia patients: use of mixed-model analyses. Schizophr Res. 2006;83(2–3):215–23. doi: 10.1016/j.schres.2005.12.851. [DOI] [PubMed] [Google Scholar]
- Niebuhr DW, Millikan AM, Cowan DN, Yolken R, Li Y, Weber NS. Selected infectious agents and risk of schizophrenia among u.s. Military personnel. Am J Psychiatry. 2008;165(1):99–106. doi: 10.1176/appi.ajp.2007.06081254. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF. Cognitive impairment in schizophrenia: a life span perspective. Am J Alzheimers Dis Other Demen. 2007;22(5):398–405. doi: 10.1177/1533317507304745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce BD. Can a virus cause schizophrenia? : facts and hypotheses. Boston: Kluwer Academic; 2003. [Google Scholar]
- Perianez JA, Rios-Lago M, Rodriguez-Sanchez JM, Adrover-Roig D, Sanchez-Cubillo I, Crespo-Facorro B, Quemada JI, Barcelo F. Trail Making Test in traumatic brain injury, schizophrenia, and normal ageing: sample comparisons and normative data. Arch Clin Neuropsychol. 2007;22(4):433–47. doi: 10.1016/j.acn.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801–8. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Shirts BH, Yolken RH, Keshavan MS, Nimgaonkar VL. Brain morphological changes associated with exposure to HSV1 in first-episode schizophrenia. Mol Psychiatry. 2007;12(1):105–13. 1. doi: 10.1038/sj.mp.4001915. [DOI] [PubMed] [Google Scholar]
- Rantakallio P, Jones P, Moring J, Von Wendt L. Association between central nervous system infections during childhood and adult onset schizophrenia and other psychoses: a 28-year follow-up. Int J Epidemiol. 1997;26(4):837–43. doi: 10.1093/ije/26.4.837. [DOI] [PubMed] [Google Scholar]
- Shibuya-Tayoshi S, Sumitani S, Kikuchi K, Tanaka T, Tayoshi S, Ueno S, Ohmori T. Activation of the prefrontal cortex during the Trail-Making Test detected with multichannel near-infrared spectroscopy. Psychiatry Clin Neurosci. 2007;61(6):616–21. doi: 10.1111/j.1440-1819.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- Shirts BH, Kim JJ, Reich S, Dickerson FB, Yolken RH, Devlin B, Nimgaonkar VL. Polymorphisms in MICB are associated with human herpes virus seropositivity and schizophrenia risk. Schizophr Res. 2007a;94(1–3):342–53. doi: 10.1016/j.schres.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Shirts BH, Wood J, Yolken RH, Nimgaonkar VL. Comprehensive evaluation of positional candidates in the IL-18 pathway reveals suggestive associations with schizophrenia and herpes virus seropositivity. Am J Med Genet B Neuropsychiatr Genet. 2007b;18:18. doi: 10.1002/ajmg.b.30603. [DOI] [PubMed] [Google Scholar]
- SPSS. Statistical Package for Social Sciences. Chicago, Illinois: 2005. [Google Scholar]
- Strandberg TE, Pitkala KH, Linnavuori KH, Tilvis RS. Impact of viral and bacterial burden on cognitive impairment in elderly persons with cardiovascular diseases. Stroke. 2003;34(9):2126–31. doi: 10.1161/01.STR.0000086754.32238.DA. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Bisschop SM, Alexander MP, Levine B, Katz D, Izukawa D. The Trail Making Test: a study in focal lesion patients. Psychol Assess. 2001;13(2):230–9. [PubMed] [Google Scholar]
- Talkowski ME, Kirov G, Bamne M, Georgieva L, Torres G, Mansour H, Chowdari KV, Milanova V, Wood J, McClain L, Prasad K, Shirts B, Zhang J, O’Donovan MC, Owen MJ, Devlin B, Nimgaonkar VL. A network of dopaminergic gene variations implicated as risk factors for schizophrenia. Hum Mol Genet. 2008;17(5):747–58. doi: 10.1093/hmg/ddm347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Yolken RH. Toxoplasma gondii and schizophrenia. Emerg Infect Dis. 2003;9(11):1375–80. doi: 10.3201/eid0911.030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Wang GH, Li QY, Shu C, Jiang MS, Guo Y. Prevalence of Toxoplasma infection in first-episode schizophrenia and comparison between Toxoplasma-seropositive and Toxoplasma-seronegative schizophrenia. Acta Psychiatr Scand. 2006;114(1):40–8. doi: 10.1111/j.1600-0447.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Brewer WJ, Koutsouradis P, Phillips LJ, Francey SM, Proffitt TM, Yung AR, Jackson HJ, McGorry PD, Pantelis C. Cognitive decline following psychosis onset: data from the PACE clinic. Br J Psychiatry Suppl. 2007;51(7):s52–7. doi: 10.1192/bjp.191.51.s52. [DOI] [PubMed] [Google Scholar]
- Yolken R. Viruses and schizophrenia: a focus on herpes simplex virus. Herpes. 2004;11(2):83A–88A. [PubMed] [Google Scholar]