Abstract
Background
Autoimmune autonomic ganglionopathy (AAG) is an acquired immune-mediated form of diffuse autonomic failure. Many patients have serum antibodies that bind to the ganglionic acetylcholine receptors (AChRs) that mediate fast synaptic transmission in autonomic ganglia. Previous clinical studies and observations in animal models suggest that AAG is an antibody-mediated neurologic disorder.
Methods
Using whole-cell patch clamp techniques, we recorded ganglionic AChR currents in cultured human IMR-32 cells and examined the effects of bath application of IgG derived from patients with AAG.
Results
IgG from seven patients with AAG all produced a progressive decline in whole-cell ganglionic AChR current, whereas IgG from control subjects had no effect. The effect was abolished at low temperature. Fab antibody fragments had no effect unless a secondary antibody was added concurrently. IgG from one patient also produced a more immediate reduction of ganglionic AChR current.
Conclusions
The characteristics of antibody-mediated inhibition of ganglionic acetylcholine receptor (AChR) current are consistent with modulation and blocking of the membrane AChR, analogous to the effects of muscle AChR antibodies in myasthenia gravis. Our observations demonstrate that antibodies in patients with autoimmune autonomic ganglionopathy (AAG) cause physiologic changes in ganglionic AChR function and confirm that AAG is an antibody-mediated disorder.
Autoimmune autonomic ganglionopathy (AAG; also known as autoimmune autonomic neuropathy or acute pandysautonomia) is an immune-mediated form of widespread and severe autonomic failure. Patients typically present with symptoms related to sympathetic failure (e.g., orthostatic hypotension and anhidrosis), parasympathetic failure (e.g., impaired heart rate variability, dry mouth, and impaired pupil constriction), and gastrointestinal dysmotility.1 Many cases have a rapid onset, but others have a chronic progressive course that may resemble degenerative forms of autonomic failure.
About 50% of patients with AAG have antibodies against the neuronal nicotinic acetylcholine receptor found in autonomic ganglia (ganglionic AChR).2 The ganglionic AChR mediates fast synaptic transmission in all autonomic ganglia and is homologous but genetically and immunologically distinct from the AChR at the neuromuscular junction. Absence of the ganglionic AChR in transgenic mice is associated with severe autonomic failure.3 Several observations suggest that AAG is an antibody-mediated disorder. Clinically, higher levels of serum ganglionic AChR antibodies correlate with more severe autonomic deficits, and some patients improve with therapeutic plasma exchange or IV immunoglobulin.4,5 An animal model of this disorder can be induced in rabbits by immunization with ganglionic AChR subunit proteins.6 Rabbits with experimental autoimmune autonomic ganglionopathy (EAAG) manifest symptoms of autonomic failure similar to those seen in AAG patients and show a deficit in synaptic transmission in autonomic ganglia. Autonomic deficits can also be transferred to mice by passive transfer of ganglionic AChR IgG.7 To help establish the nature of this antibody-mediated disorder, we examined the effects of IgG from seven patients with AAG on the function of the ganglionic AChR.
METHODS
Patient and animal material
Human specimens were collected with informed consent. All protocols were approved by the Institutional Review Board (at each of the participating institutions) and the Animal Care and Use Committee at University of Texas Southwestern Medical Center. Plasma (taken at the time of therapeutic plasma exchange) or serum was collected from seven patients with AAG who were seropositive for ganglionic AChR binding antibodies (table). By immunoprecipitation assay, none of the patients had ganglionic AChR blocking antibodies.2 The clinical features of Patients 2 and 4 have been reported previously.4,8 Control serum or plasma was collected from three healthy volunteers and three patients with other neurologic disorders (table). The patient with Lambert–Eaton syndrome was positive for P/Q-type calcium channel antibodies and had small-cell lung carcinoma. The patient with MG was seropositive for muscle AChR antibodies and did not have thymoma. The patient with autoimmune limbic encephalitis did not have cancer and improved with plasma exchange.
Table.
Human study subjects
| Age, y/sex | Diagnosis | Ganglionic AChR Ab* | Source | |
|---|---|---|---|---|
| Patient 1 | 64/F | AAG | 0.09 | Serum |
| Patient 2 | 41/M | AAG | 0.54 | PLEX |
| Patient 3 | 45/M | AAG | 2.40 | PLEX |
| Patient 4 | 46/F | AAG | 3.42 | PLEX |
| Patient 5 | 68/M | AAG | 5.36 | Serum |
| Patient 6 | 35/F | AAG | 18.8 | PLEX |
| Patient 7 | 44/M | AAG | 28.4 | Serum |
| HC 1 | 39/M | HC | 0.0 | Serum |
| HC 2 | 37/M | HC | 0.0 | Serum |
| HC 3 | 24/F | HC | 0.0 | Serum |
| LES | 71/M | LES | 0.06 | PLEX |
| MG | 71/F | MG | 0.0 | Serum |
| LE | 35/F | LE | 0.0 | PLEX |
Antibody binding activity determined by immunoprecipitation (normal value < 0.05 nmol/L).
AChR = acetylcholine receptor; Ab = antibody; AAG = autoimmune autonomic ganglionopathy; PLEX = therapeutic plasma exchange; HC = healthy control; LES = Lambert—Eaton syndrome; LE = limbic encephalitis.
Female rabbits were immunized with a recombinant fragment of the α3 AChR subunit to produce ganglionic AChR antibodies as previously described.6 Serum from five immune rabbits was pooled and used as a source of ganglionic AChR antibodies. Pooled serum from adjuvant-immunized rabbits was a source of control rabbit IgG.
Preparation of IgG and Fab fragments
Total IgG was isolated from serum or plasma by adsorption to protein A–Sepharose.7 All IgG samples were subsequently eluted in acidic buffer, dialyzed into phosphate-buffered saline, and sterilized by filtration. Material from Patient 3 was used in many of the experiments because of the availability of a large quantity of plasma. To generate Fab fragments, purified IgG was incubated with immobilized papain (Pierce, Rockford, IL) at 37 °C for 4 hours. F(ab′)2 fragments were produced by similar digestion with immobilized pepsin (Pierce). The integrity of Fab and F(ab′)2 binding to ganglionic AChR was confirmed by immunoprecipitation assay.2
Cell culture and electrophysiology
IMR-32 human neuroblastoma cells were obtained from American Type Culture Collection (ATCC, Rockville, MD) and maintained in minimal essential medium supplemented with 10% fetal bovine serum. For electrophysiology, cells were plated on glass coverslips at low density and studied at least 3 days later. IMR-32 cells express neuronal ganglionic AChR and are similar to autonomic neurons in terms of the repertoire of expressed neuronal nicotinic AChR subunits and the characteristics of the nicotinic AChR current in these cells.9 AChR currents were measured using standard patch-clamp techniques. The recording chamber was continuously perfused with extracellular solution consisting of (in mM): 150 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 5 HEPES, adjusted to pH 7.3. Patch pipettes with a resistance of 5 to 8 MΩ were formed from borosilicate glass and filled with a solution containing (in mM): 140 cesium-methanesulfonate, 10 CsCl, 10 EGTA, 4 Na2ATP, and 10 HEPES, pH 7.2. Solutions were applied to the bath by gravity-fed glass tubes, which were moved by a computer-controlled micromanipulator (Fast-Step system; Warner Instrument Co., Hamden, CT). Nicotinic AChR currents were recorded at a holding potential of −70 mV and evoked by rapid application of the nicotinic agonist 1,1-dimethyl-4-phenylpiperazinium (DMPP; 50 μM). DMPP was selected because it preferentially activates the postganglionic bungarotoxin-insensitive ganglionic AChR in autonomic neurons.10 Experiments were usually performed at room temperature (22 °C). For experiments at other temperatures, the bath perfusion solutions were warmed or cooled using a Peltier thermal block, and the bath temperature was continuously monitored.
Currents in IMR-32 cells induced by DMPP were not affected by 100 nM α-bungarotoxin but were inhibited by the ganglionic blockers chlorisondamine and hexamethonium within 30 seconds of application (data not shown). The currents recorded under these conditions are therefore consistent with ganglionic AChR. After recording stable baseline AChR currents, IgG (at a final concentration of 0.2 or 1.0 mg/mL) was added to the solution perfusing the bath. The whole-cell AChR current was recorded after 2 minutes, 5 minutes, and subsequently every 5 minutes throughout the experiment. In other experiments, IgG was added to the cell culture media 16 to 20 hours prior to patch-clamp studies.
Data analysis
Summary data are presented as means ± SE. The number of cells recorded under each experimental condition was typically 4 to 6. The effect of antibody on current was assessed in each cell by recording peak current amplitude and by comparing the evoked current after application of IgG with the initial stable baseline current to calculate either percentage reduction in current or normalized current amplitude. A 20-minute time point was chosen in most of the analyses because patch-clamp recordings in these cells were generally stable and the effect of IgG was easily recognized over this time period. Using percentage current reduction as the primary measure eliminated the effect of variability in peak current amplitude between cells. Although this measure could not exceed 100%, our data met the Kolmogorov–Smirnov test for normality. A one-tailed t test was used to determine if current reduction was significantly greater than zero. In most cases, experimental data were compared with pooled data from healthy control subject IgG using two-tailed t test (p values of <0.05 were considered significant).
RESULTS
Ganglionic AChR IgG inhibits AChR current
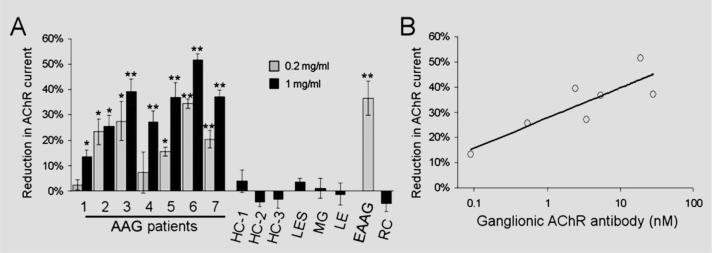
Ganglionic-type AChR currents were readily recorded from cultured IMR-32 cells, and we determined the effect of bath application of human or rabbit IgG (figure 1). IgG from control subjects or from adjuvant-immunized control rabbits (at 1 mg/mL) had no effect on ganglionic AChR currents in these cells. Application of IgG (at 0.2 mg/mL for 20 minutes) from rabbits immunized against the ganglionic AChR resulted in a 36 ± 7% decrease in peak current amplitude. At 0.2 mg/mL, IgG from five of the AAG patients produced a significant reduction in ganglionic AChR current (mean reduction of 18 ± 3%). At higher concentration (1 mg/mL), IgG from all seven AAG patients significantly inhibited the ganglionic AChR current in IMR-32 cells. At this concentration, the mean reduction for all AAG patients was 33 ± 3% after 20 minutes' exposure to IgG. Figure 2A summarizes the effect of IgG on ganglionic AChR current for all subjects. The potency of IgG-mediated inhibition correlated with the level of ganglionic AChR binding antibodies determined by immunoprecipitation assay (figure 2B).2
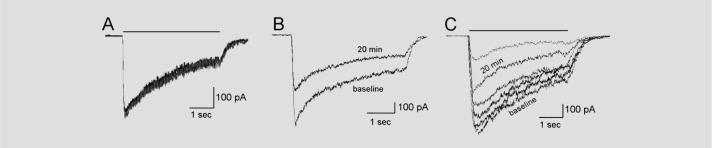
Figure 1. Effect of IgG on ganglionic acetylcholine receptor (AChR) current.
Recordings of whole-cell membrane current in IMR-32 cells at −70-mV holding potential. Application of agonist (1,1-dimethyl-4-phenylpiperazinium 50 μM, 4-second horizontal bar) produces an inward current that decreases during the agonist application owing to receptor desensitization. (A) Currents from IMR-32 cell recorded at baseline and at 20 and 50 minutes after addition of human control IgG (1 mg/mL) to the bath (superimposed traces) remain stable over time, indicating no effect of the control IgG on the AChR current (0% current reduction). (B) Exposure to rabbit ganglionic AChR IgG (0.2 mg/mL) for 20 minutes results in a reduction of peak AChR current by 39%. The steady-state current was similarly reduced. (C) Exposure to autoimmune autonomic ganglionopathy (AAG) IgG produces a gradual decrease in AChR current. Recordings from baseline and 2, 5, 10, 20, and 50 minutes after addition of IgG are shown in this example (IgG from Patient 3, 1 mg/mL).
Figure 2. Summary of IgG effect on ganglionic acetylcholine receptor (AChR) current.
(A) Reduction in peak current after 20-minute exposure to IgG is shown for all subjects. IgG from all seven autoimmune autonomic ganglionopathy (AAG) patients and from rabbits immunized against the ganglionic AChR (experimental AAG [EAAG]) produced a current reduction (*p < 0.05, **p < 0.0001 compared with control IgG). IgG from healthy control (HC), Lambert–Eaton syndrome (LES), MG, autoimmune limbic encephalitis (LE), or adjuvant-control rabbits (RC) had no effect on the ganglionic AChR current. Error bars represent SEMs (number of cells for each data point is 3 to 8). (B) The magnitude of current reduction for each AAG patient IgG (at 1 mg/mL) correlated with the serum ganglionic AChR antibody level (log-linear regression, r2 = 0.73, p = 0.01).
IgG effect on ganglionic AChR current is time dependent
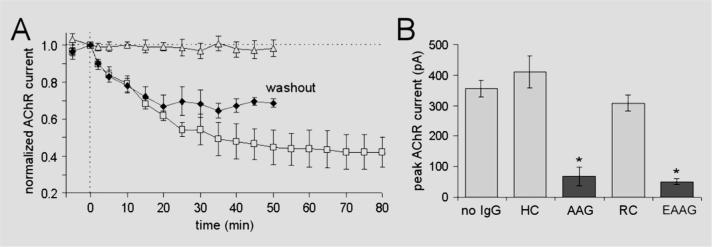
Application of IgG from AAG patients or experimental rabbits produced a gradual reduction in the amplitude of the AChR current (figures 1C and 3A). The AChR current amplitude continued to decline as long as IgG was present in the bath solution (up to 100 minutes). When IgG was washed out of the bath solution after 20 minutes, the AChR current stabilized and showed no further decline or recovery (figure 3A, washout).
Figure 3. Time course of IgG effect on acetylcholine receptor (AChR) current.
(A) AChR current is shown as the peak current amplitude normalized to the baseline current. IgG was added to the bath perfusing solution at time 0. Control IgG (open triangles, pooled data from three healthy control subjects) produced no change in current over time. Autoimmune autonomic ganglionopathy (AAG) IgG (pooled data from Patients 2, 3, and 4) produced a gradual reduction in the AChR current as long as the IgG is present (open squares). When IgG was removed from the bath after 20 minutes (washout, filled diamonds), the current stabilized. (B) Overnight exposure to human control (HC) or normal rabbit control (RC) IgG produced no change in mean AChR current compared with untreated cells (no IgG). Overnight exposure to IgG from Patient 3 (AAG) or experimental AAG rabbit IgG reduced the current amplitude by about 84% (*p < 0.001 compared with control). Error bars are SEMs.
With short application of IgG (2 minutes), most of the ganglionic AChR IgG samples produced only a small reduction in current amplitude (up to 12%). However, IgG from one of the seven AAG patients (Patient 6) consistently produced a larger immediate effect on AChR current (26.5 ± 5.5% reduction at 2 minutes) followed by a further gradual decline in current amplitude. This was unique because IgG from the patient with a higher antibody level (Patient 7) produced only 12% reduction in current amplitude within 2 minutes.
We also recorded AChR current in IMR-32 cells after overnight application of IgG (0.2 mg/mL) added to the culture medium (figure 3B). AChR current was subsequently recorded from these treated cells in standard bath solution without IgG. The current amplitude in cells exposed to IgG from AAG patients was markedly reduced compared with those exposed to control IgG, and AChR currents could not be obtained in about 20% of cells after overnight treatment with AAG IgG. Similar results were found with ganglionic AChR IgG from immunized rabbits (figure 3B). The gradual time course of the IgG effect would be consistent with antigenic modulation (reduction in number) of surface AChR rather than allosteric inhibition or physical blockade of the agonist binding site or ion channel pore.
Inhibition of AChR current is temperature dependent
Previous studies have shown that antibody-mediated internalization of membrane receptors is temperature dependent.11 We found that the inhibition of ganglionic AChR current was abolished at low temperature. At 4 °C, ganglionic AChR currents were readily recorded, but 20-minute exposure to IgG (1 mg/mL) from Patient 3 produced no reduction of current (1.7 ± 1.6% compared with 39.3 ± 4.9% at 22 °C; p < 0.001). The magnitude of current reduction increased at 37 °C (52.3 ± 2.3%, not significant compared with 22 °C).
Antibody-mediated modulation of ganglionic AChR requires cross-linking
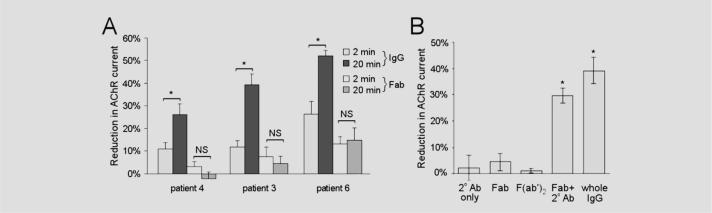
To determine if the antibody-mediated inhibition of AChR current requires intact IgG, we examined the electrophysiologic effects of Fab fragments. The Fab fragments prepared from AAG patient IgG still bind to the ganglionic AChR as determined by immunoprecipitation. Fab produced from IgG of Patient 6 produced an immediate small, but significant, reduction in AChR current (within 2 minutes) after which the current amplitude remained stable (figure 4A). Fab preparations from other AAG patients had no significant effect on ganglionic AChR current.
Figure 4. Effect of antibody fragments on ganglionic acetylcholine receptor (AChR) current.
(A) Total IgG (1 mg/mL) from patients with autoimmune autonomic ganglionopathy (AAG) produces an early (2-minute) small reduction in AChR current. This rapid reduction in current was most apparent with IgG from Patient 6 (26.5 ± 5.5% compared with average of 11.7 ± 1.3% for the other six patients; p = 0.04). The IgG-mediated current inhibition increases over time (*p < 0.05). Fab fragments of the IgG (1 mg/mL) from Patients 3 and 4 caused no significant change in the AChR current. Exposure to Fab from Patient 6 produced a small immediate reduction but no further change in current over time. (B) Incubation with Fab or F(ab′)2 from Patient 3 (at 1 mg/mL) did not affect the AChR current. However, simultaneous application of Fab along with a secondary antibody (goat anti-human IgG) to cross-link the Fab fragments resulted in a reduction in AChR current similar to the effect of whole IgG from the same patient.
Neither monovalent Fab nor divalent F(ab′)2 fragments from the IgG of Patient 3 had any effect on ganglionic AChR currents (figure 4B). However, bath application of Fab along with a secondary antibody (goat anti-human IgG) resulted in a progressive decline in current similar to the effect of intact IgG from the same patient. This finding indicates that the antibody-mediated modulation of AChR function requires specific binding and cross-linking as well as subsequent effects mediated by the IgG Fc domain.
DISCUSSION
We have shown that IgG from patients with AAG have inhibitory effects on the function of ganglionic AChRs in vitro. This IgG-mediated inhibition of AChR current did not require complement or any other immune mediators. Our studies provide some clues into the mechanism of action of the antibody effect. Ganglionic AChR IgG reduced the magnitude of the current but did not affect the whole-cell current desensitization time constant. The main effect was gradual in onset, was abolished at low temperature, and could not be readily reversed by removing antibody from the bath solution. These features suggest that the predominant action of IgG on the AChR is not due to interference with agonist binding or ion permeation but consistent with antigenic modulation (antibody-mediated internalization and depletion of cell surface AChR) as has been described for muscle AChR antibodies in myasthenia gravis. In previous MG studies, IgG-mediated blocking occurs rapidly (within 60 seconds) and is readily reversible.12 Antigenic modulation, on the other hand, develops more slowly. Although it is often studied after prolonged (overnight) exposure to IgG, our study and others indicate that considerable AChR modulation in vitro takes place within minutes.11
Other mechanisms of IgG-mediated inhibition may also contribute. In one case (Patient 6), IgG produced an immediate reduction in ganglionic AChR current as well as more gradual modulation. Fab fragments from this patient's IgG produced the immediate but not the gradual inhibition of current. In this case, binding of IgG (or Fab) to the receptor likely either interferes with agonist binding or allosterically modifies channel kinetics. This patient's serum did not block epibatidine binding to solubilized ganglionic AChR. Similarly, previous studies have shown that IgG from MG patients can rapidly block muscle AChR currents even when the IgG shows no binding or blocking of solubilized AChR.12 Either there are mechanisms of rapid antibody-mediated receptor inhibition that do not involve blocking of agonist or the immunoprecipitation blocking assay lacks the sensitivity to detect lower levels of blocking antibodies.
Antibodies from patients with MG inhibit the function of the muscle AChR by blocking and antigenic modulation.13 We have now shown that IgG from patients with AAG has similar effects on the ganglionic AChR. This effect on ganglionic AChR would be expected to produce a potent impairment in ganglionic cholinergic fast synaptic transmission. Indeed, we have observed that autonomic deficits in animal models of AAG are associated with impaired ganglionic neurotransmission.6,7 It remains to be seen whether long-term exposure to these antibodies has other effects on neurons or if chronic inhibition of synaptic transmission leads to irreversible changes in autonomic function. Our findings further confirm that AAG is an antibody-mediated neurologic disorder. As pathophysiologic effects are mediated by IgG, therapies that reduce levels of pathogenic antibodies, including plasma exchange, are rational treatment for AAG patients with serum ganglionic AChR antibodies.4
ACKNOWLEDGMENT
The authors thank Steve Hopkins, Janice Nhan, and Kim Nickander for their technical assistance.
Supported by NIH grants NS32352 (P.A.L., S.V.), NS48077 (S.V.), and DK68055 (S.V.).
Footnotes
Disclosure: The authors report no conflicts of interest.
REFERENCES
- 1.Klein CM, Vernino S, Lennon VA, et al. The spectrum of autoimmune autonomic neuropathies. Ann Neurol. 2003;53:752–758. doi: 10.1002/ana.10556. [DOI] [PubMed] [Google Scholar]
- 2.Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343:847–855. doi: 10.1056/NEJM200009213431204. [DOI] [PubMed] [Google Scholar]
- 3.Xu W, Gelber S, Orr-Urtreger A, et al. Megacystis, mydriasis, and ion channel defect in mice lacking the alpha3 neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci USA. 1999;96:5746–5751. doi: 10.1073/pnas.96.10.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroeder C, Vernino S, Birkenfeld AL, et al. Plasma exchange for primary autoimmune autonomic failure. N Engl J Med. 2005;353:1585–1590. doi: 10.1056/NEJMoa051719. [DOI] [PubMed] [Google Scholar]
- 5.Smit A, Vermeulen M, Koelman J, Wieling W. Unusual recovery from acute panautonomic neuropathy after immunoglobulin therapy. Mayo Clin Proc. 1997;72:333–335. doi: 10.4065/72.4.333. [DOI] [PubMed] [Google Scholar]
- 6.Lennon VA, Ermilov LG, Szurszewski JH, Vernino S. Immunization with neuronal nicotinic acetylcholine receptor induces neurological autoimmune disease. J Clin Invest. 2003;111:907–913. doi: 10.1172/JCI17429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vernino S, Ermilov LG, Sha L, Szurszewski JH, Low PA, Lennon VA. Passive transfer of autoimmune autonomic neuropathy to mice. J Neurosci. 2004;24:7037–7042. doi: 10.1523/JNEUROSCI.1485-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbons CH, Vernino SA, Kaufmann H, Freeman R. l-DOPS therapy for refractory orthostatic hypotension in autoimmune autonomic neuropathy. Neurology. 2005;65:1104–1106. doi: 10.1212/01.wnl.0000178980.83477.14. [DOI] [PubMed] [Google Scholar]
- 9.Nelson ME, Wang F, Kuryatov A, Choi CH, Gerzanich V, Lindstrom J. Functional properties of human nicotinic AChRs expressed by IMR-32 neuroblastoma cells resemble those of alpha3beta4 AChRs expressed in permanently transfected HEK cells. J Gen Physiol. 2001;118:563–582. doi: 10.1085/jgp.118.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kristufek D, Stocker E, Boehm S, Huck S. Somatic and prejunctional nicotinic receptors in cultured rat sympathetic neurones show different agonist profiles. J Physiol (Lond) 1999;516:739–756. doi: 10.1111/j.1469-7793.1999.0739u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clementi F, Sher E. Antibody induced internalization of acetylcholine nicotinic receptor: kinetics, mechanism and selectivity. Eur J Cell Biol. 1985;37:220–228. [PubMed] [Google Scholar]
- 12.Bufler J, Pitz R, Czep M, Wick M, Franke C. Purified IgG from seropositive and seronegative patients with myasthenia gravis reversibly blocks currents through nicotinic acetylcholine receptor channels. Ann Neurol. 1998;43:458–464. doi: 10.1002/ana.410430408. [DOI] [PubMed] [Google Scholar]
- 13.Drachman DB, Angus CW, Adams RN, Michelson JD, Hoffman GJ. Myasthenic antibodies cross-link acetylcholine receptors to accelerate degradation. N Engl J Med. 1978;298:1116–1122. doi: 10.1056/NEJM197805182982004. [DOI] [PubMed] [Google Scholar]