Abstract
Poloxamer 407 (P-407) is a copolymer surfactant that induces a dose-controlled dyslipidemia in both mice and rats. Human macrophages cultured with P-407 exhibit a concentration-dependent reduction in cholesterol efflux to apolipoprotein A1 (apoA1) linked to down-regulation of the ATP-binding cassette transporter A1 (ABCA1). Activators of peroxisome proliferator-activated receptor gamma (PPARγ), as well as PPARα, increase expression of liver X receptor alpha (LXRα) in macrophages and promote the expression of ABCA1, which, in turn, mediates cholesterol efflux to apoA1. The present study investigates whether P-407 interferes with this signaling pathway. A transactivation assay was used to evaluate whether P-407 can either activate or inhibit the transcriptional activity of PPARγ. Since triazolidinedione drugs (PPARγ agonists) improve glycemic control in Type 2 diabetes by reducing blood glucose concentrations, P-407 was also evaluated for its potential to alter plasma insulin and blood glucose concentrations following its administration to both wild-type and PPARγ-deficient mice. Additionally, triazolidinediones attenuate free fatty acid (FFA) release from adipocytes and, consequently, circulating plasma levels of FFAs decrease. Therefore, plasma concentrations of circulating FFAs were also determined in P-407-treated mice.
P-407 was unable to modulate PPARγ activity in cell-based transactivation assays. Furthermore, P-407 did not perturb plasma insulin and blood glucose concentrations following administration to mice. However, P-407 caused a significant increase in the serum concentration of FFAs in mice beginning 3 h after administration and lasting >24 h post-dosing by, as yet, an unknown mechanism. It is concluded that P-407 does not interfere with the functional activity of PPARγ following administration to mice.
Keywords: Free fatty acid, Glucose, Insulin, Peroxisome proliferator-activated receptor (PPAR), Transactivation assay
INTRODUCTION
The peroxisome proliferator-activated receptors PPARα and PPARγ are nuclear receptors that, upon heterodimerization with the retinoid X receptor (RXR), function as ligand-activated transcriptional regulators of genes controlling lipid and glucose metabolism (Pineda et al., 1999). PPARα, which is activated by fibrates, fatty acids, and eicosanoids (Chinetti et al., 2000), is most highly expressed in liver, heart, muscle, and kidney, while PPAR-γ1 is expressed in many tissues and cells, including white and brown adipose tissue, skeletal muscle, intestine, and macrophages (Auwerx, 1999; Gonzalez, 1997; Kersten et al., 2000; Saltiel and Olefsky, 1996; Spiegelman and Flier, 1996). A splice variant, PPAR-γ2, is primarily expressed in both white and brown adipose tissue (Chawla et al., 1994; Kliewer et al., 1994; Tontonoz et al., 1994). PPARγ is also expressed in pancreatic β-cells, but its level of expression is much lower than elsewhere (Braissant et al., 1996). Agonist-induced activation of PPARγ/RXR is known to increase insulin sensitivity (Lehmann et al., 1995; Mukherjee et al., 1997), and synthetic ligands of PPARγ, thiazolidinedione drugs (TZD’s), which have the ability to directly bind and activate PPARγ (Lehmann et al., 1995) and stimulate adipocyte differentiation (Okuno et al., 1998; Spiegelman and Flier, 1996), are used clinically to reduce insulin resistance and improve hyperglycemia in Type 2 diabetes mellitus (T2DM).
TZD’s (PPARγ agonists) are often used to improve hyperglycemia associated with the metabolic syndrome of T2DM. The metabolic syndrome is characterized by a) central obesity, b) atherogenic dyslipidemia [i.e., increased plasma triglyceride (TG), with a simultaneous reduction in high-density lipoprotein (HDL) cholesterol], c) hypertension, d) insulin resistance or glucose intolerance, e) a prothrombotic state, and f) a proinflammatory state. We have developed a chemically-induced alternative animal model of hyperlipidemia and atherosclerosis using poloxamer 407 (P-407) [a copolymer surfactant] that replicates one of the features observed in the metabolic syndrome; specifically, atherogenic dyslipidemia (Johnston et al., 1998; Johnston, 2004; Palmer et al., 1997). Recently, we demonstrated that P-407 down-regulates the gene expression of ATP-binding-cassette transporter A1 (ABCA1) and inhibits cholesterol efflux from human macrophages in cell culture (Johnston et al., 2006). Hence, we wondered whether P-407 might also modulate, either directly or indirectly, the functional activity of PPARγ in the PPAR-LXR-ABCA1 signaling pathway. This would have enormous consequence with regard to cellular cholesterol homeostasis in this particular mouse model of atherogenesis (Johnston et al., 1998; Johnston, 2004; Palmer et al., 1997), since TZD’s are used, in part, to promote cholesterol efflux from macrophages in patients with T2DM.
The present work was conducted to further understand the pharmacological effects of P-407 in our animal model of atherogenesis. Because P-407, or some intermediate which may potentially be activated in a biochemical or metabolic cascade following P-407 administration, could conceivably function as either a PPARγ agonist (similar to TZD’s) or antagonist, we first determined whether P-407 could directly modulate PPARγ transcriptional activity using an in vitro transactivation assay. Next, we determined whether blood glucose and plasma insulin levels were perturbed in both wild-type (C57BL/6) and PPARγ-deficient mice following P-407 administration, since PPARγ agonists are used to treat hyperglycemia associated with T2DM. Lastly, we explored the possibility that P-407 acts through PPARγ to effectuate the mobilization of free fatty acids (FFAs) from adiopcytes.
Our desire to investigate the possibility that P-407 acts via PPARγ to cause the release of FFAs from adipocytes and, thereby, increase the concentration of circulating FFAs, is based on the following information. PPARγ is activated by prostaglandins, leukotrienes, and TZD’s and affects the expression of many genes involved in the storage of FFAs. If either P-407, or some intermediate involved in a biochemical or metabolic cascade downstream of P-407 administration, functioned as either a PPARγ agonist or antagonist, then the expression of genes involved in the storage of FFAs may possibly be modulated. This potential pharmacological action of P-407 is based on two previous observations. First, Wasan et al. demonstrated a significant increase in the activity of lecithin cholesterol acyltransferase (LCAT) in the plasma of P-407-treated rats relative to controls (Wasan et al., 2003). LCAT catalyzes the formation of cholesteryl esters from lecithin (phosphatidylcholine) and cholesterol. Secondly, Nash et al. observed a significant decrease in the plasma concentrations of both TG and total cholesterol when nicotinic acid and P-407 were simultaneously administered to rats (Nash et al., 1996). Nash et al. suggested that P-407 may cause hyperlipidemia in rodents, in part, by stimulating the release of FFAs from the adipocyte for at least 24 h following its administration (Nash et al., 1996), although these authors never measured circulating FFA levels in P-407-treated animals. Nicotinic acid is an effective hypolipidemic agent that functions primarily by reducing lipolysis in adipocytes, which results in a reduction in the plasma concentration of FFAs; an essential substrate for both TG and cholesterol biosynthesis. The findings of Wasan et al. (Wasan et al., 2003) and Nash et al. (Nash et al., 1996) suggest that P-407 may influence, either directly or indirectly, the mobilization and storage of FFAs by modulating the functional activity and/or gene expression of PPARγ. Therefore, we determined whether P-407 treatment affected the concentration of circulating FFAs in wild-type mice.
MATERIALS AND METHODS
Materials
Plasmids were obtained from the same sources as previously reported (Maloney and Waxman, 1999; Shipley and Waxman, 2004a; Shipley et al., 2004b). Troglitazone (Rezulin), a potent PPARγ agonist, was obtained from Parke-Davis Pharmaceuticals (Ann Arbor, MI). Male C57BL/6 and PPARγ-deficient (strain name = B6.129-Ppargtm2Rev/J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and weighed approximately 18 g. Test strips, which were inserted into the test strip chamber of the blood glucose monitor, were CHEMSTRIP bG® reagent strips, Cat. No. 502, and were obtained from Boehringer Mannheim Corporation (Indianapolis, IN). For determination of plasma insulin concentrations, commercially-available Coat-A-Count® radioimmunoassay kits were obtained from Diagnostic Products Corporation (Los Angeles, CA). An in vitro enzymatic, colorimetric assay kit (NEFA-C) for the determination of serum non-esterified or FFAs was purchased from Wako Diagnostics, Inc. (Richmond, VA).
Transactivation Assay
The transactivation assay described earlier (Maloney and Waxman, 1999; Shipley et al., 2004b) was used to assess the effect of P-407 on PPARγ activity. Briefly, COS-1 cells (American Type Culture Collection, Rockville, MD) were passaged in 100-mm tissue culture dishes (Greiner Labortechnik, Germany) in DMEM supplemented with 10% FBS (Gibco, Grand Island, NY) and 50 U/ml penicillin/streptomycin (Gibco). Cells were cultured overnight at 37 °C and then reseeded at 2000 to 4000 cells/well in a 96-well tissue culture plate (Greiner Labortechnik) in DMEM containing 10% FBS. The cells were grown for 24 h and then transfected as described previously (Chang and Waxman, 2005; Maloney and Waxman, 1999), using FuGENE 6 transfection reagent (Roche Diagnostics Corp., Indianapolis, IN), which provides higher transfection efficiencies and more consistent results when compared to calcium phosphate transfection methods.
Twenty-four h after P-407 treatment, cells were washed once in cold phosphate-buffered saline (pH 7.4), and then lysed by incubation at 4 °C in passive cell lysis buffer for 15-30 min (Promega). Firefly and Renilla luciferase activities were measured in the cell lysate using the Dual Luciferase Activity Kit (Promega).
In Vivo Experiments
In order to determine whether PPARγ were involved with any potential changes in plasma insulin and blood glucose concentrations following administration of P-407, we used four groups of mice. Groups 1 and 2 consisted of normal C57BL/6 mice, which were treated with either saline or P-407 (0.5 g/kg), respectively. Groups 3 and 4 were comprised of PPARγ-deficient mice and were also administered either saline or P-407 (0.5 g/kg), respectively. It should be noted that disruption of the gene for PPARγ does not cause any significant changes in blood insulin and glucose concentrations relative to these same parameters in controls (He et al., 2003). All procedures for P-407 administration and subsequent blood collection were in accordance with the institution’s guide for the care and use of laboratory animals, and the treatment protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Missouri-Kansas City.
To determine whether P-407 caused any change in the concentrations of plasma insulin and blood glucose in normal mice, as well as whether any potential changes to the concentrations of plasma insulin and blood glucose were mediated through PPARγ, twelve C57BL/6 mice and twelve PPARγ-deficient mice were randomly divided into the four groups described above. All mice were administered 0.5 ml of either normal saline (Groups 1 and 3) or P-407 (0.5 g/kg) (Groups 2 and 4) by intraperitoneal injection. Blood samples were obtained from all mice by tail vein sampling at 0 h (prior to P-407 administration), and then at 2, 4, 8, 16, and 24 h post-dosing. Fifty μl of blood was collected into heparinized tubes at each sampling time point. One drop was immediately used for determination of blood glucose as described below, while the remainder of the blood sample (∼ 40 μl) was centrifuged, the plasma obtained, and the plasma samples stored at -80 °C until the time of insulin analysis.
The concentration of glucose in each blood sample was determined using a commercially-available blood glucose monitor (model 792 Accu-Chek® II; Boehringer Mannheim Corp., Indianapolis, IN). One drop of blood was placed on a glucose reagent strip, the strip allowed to stand at room temperature for one minute, and, finally, the strip inserted into the test strip chamber of the monitor for determination of blood glucose in mg/dl. Blood glucose concentrations were then expressed in units of mM.
The concentration of insulin in all plasma samples was determined using a radioimmunoassay kit according to the manufacturer’s instructions. In the plasma insulin determination procedure, [125I]insulin competes with insulin in the plasma sample for sites on insulin-specific antibody immobilized to the wall of the polypropylene tube. After incubation, isolation of the antibody-bound fraction was achieved by simply decanting the supernatant. The tube was then counted in a model LS 6500 Beckman gamma scintillation counter (Fullerton, CA), the counts being inversely related to the amount of insulin present in the plasma sample. The quantity of insulin in the sample was then determined by comparing the counts to a standard curve (Package Insert M-097, Diagnostic Products Corp., Los Angeles, CA, Sept. 5, 1991). Finally, the plasma insulin concentrations were calculated and expressed in units of pM.
Analysis of Serum Free Fatty Acids
An additional group of six C57BL/6 mice were utilized to determine whether P-407 caused a change in the circulating levels of FFAs in the serum. In these experiments, the mice were all allowed to fast for twelve hours prior to the experiment. On the day of the experiment, 50 μl of blood was collected from the tail vein of each mouse and served as the pre-injection (time t = 0 h) control or baseline FFA concentration for each mouse. Next, 0.5 ml of P-407 (0.5 g/kg) was administered by intraperitoneal injection to each mouse and blood samples (50 μl) collected from the tail vein of each fasting mouse at 3, 6, 12, and 24 h post-dosing. To rule out whether fasting influenced the serum levels of FFAs, another group of six C57BL/6 mice were similarly fasted, injected with normal saline (0.5 ml) at time t = 0 h, and blood samples obtained at the same time points as mice treated with P-407. All blood samples were placed into microcentrifuge tubes on ice and allowed to clot. Blood samples were then centrifuged at 10,000 × g for 20 min at 4 °C and the serum supernatant removed and frozen at -80 °C until the time of FFA analysis.
Analysis of serum samples for FFA utilized a 96-well microtiter plate adapted procedure supplied by the manufacturer. Briefly, an aliquot (5 μl) of each serum sample was placed into a separate well and 100 μl of color reagent A added to each well. Next, samples were mixed and incubated at 37 °C for 5 min, after which time, 200 μl of color reagent B was added to each well. The plate was again incubated at 37 °C for 5 min, removed from the incubator, and 5 min later, the plate read at 550 nm using a model 450 microplate reader (Bio-Rad; Richmond, CA). Both a reagent blank and calibration standard, as well as a specimen blank (to correct for lipemic samples) was included in the assay. Data was processed as described below.
Data Analysis
To determine whether P-407 could modulate mouse and human PPARγ in the transactivation assays, we utilized a classic one-way analysis of variance (ANOVA) to uncover any significant differences in the mean values associated with the individual P-407 concentrations tested relative to the vehicle (Figs. 1A and 1B). ANOVA was also utilized to determine whether increasing concentrations of P-407 would inhibit troglitazone’s capacity to activate human PPARγ (Fig. 2). Lastly, similar to the treatment of the data obtained from the transactivation assays, ANOVA was used to compare the plasma insulin and blood glucose concentration vs. time profiles. Using the blood glucose concentration vs. time profiles (Fig. 3A) as an example, we determined whether each blood glucose concentration at a given time point was different from the rest of the blood glucose concentrations at that same time point between the four groups of mice. This statistical analysis was performed for the four blood glucose concentrations at each of the 5 sampling time points and any significant (p<0.05) differences between the four concentrations at a specific time point were appropriately designated on the resulting graph. Plasma insulin concentration vs. time profiles (Fig. 3B) were analyzed in a similar manner.
Figure 1.
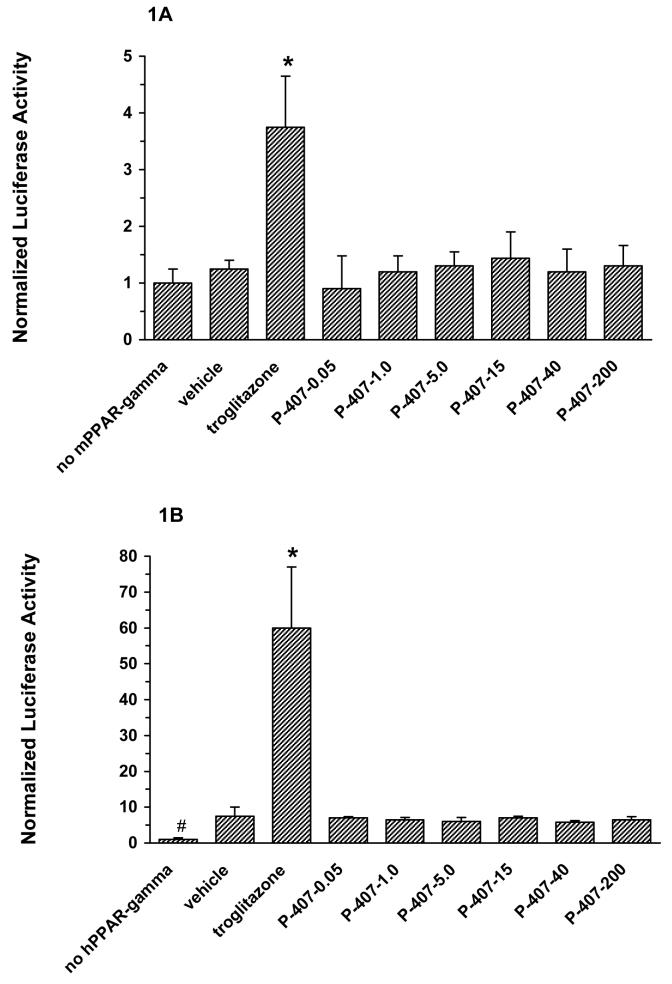
The effect of P-407 on mouse (A) and human (B) PPARγ activity in vitro. All P-407 concentrations are in μM. Data represents the mean ± standard deviation. First bar, no PPARγ control; second bar, cells transfected with either mouse (A) or human (B) PPARγ expression plasmid and then treated with vehicle; other bars, same as for bar 2, except cells were stimulated for 24 hr with troglitazone or P-407 at the micromolar concentrations indicated. * indicates that the mean value of bar 3 (troglitazone) was significantly (p < 0.05) greater than the mean value of all other bars. # indicates that the mean value of bar 1 (no hPPARγ) in (B) was significantly (p < 0.05) less than the mean value of all other bars.
Figure 2.
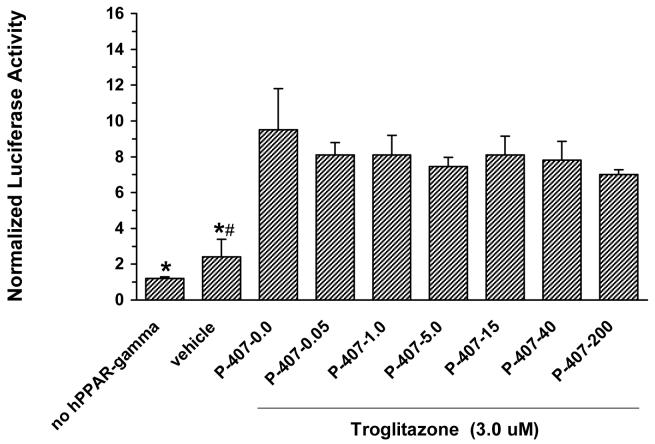
The effect of P-407 on human PPARγ activity stimulated by the PPARγ-activator, troglitazone. All P-407 concentrations are in μM. Data represents the mean ± standard deviation. * indicates that the mean value of bars 1 (no PPARγ) and 2 (vehicle) were significantly (p < 0.05) less than the mean values of all other bars. # indicates that the mean value of bar 2 (vehicle) was significantly (p < 0.05) greater than the mean value of bar 1 (no PPARγ).
Figure 3.
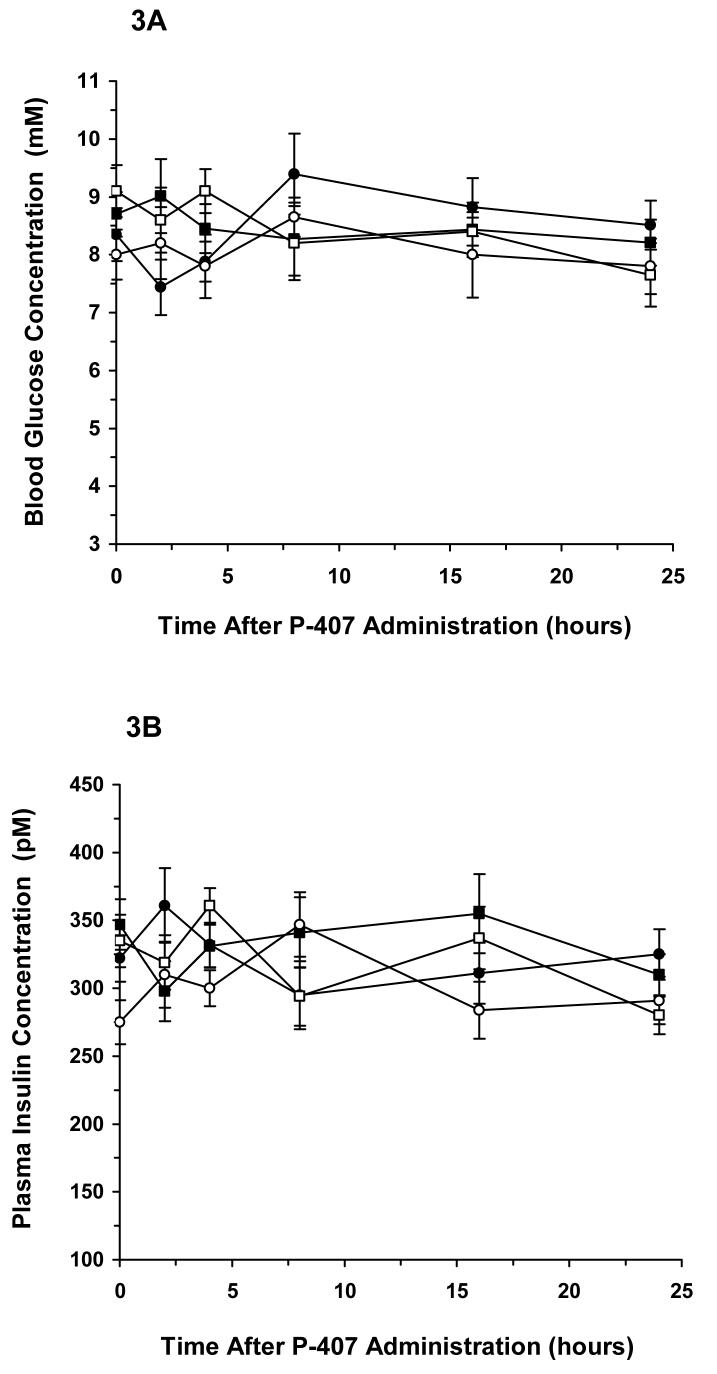
Blood glucose (A) and plasma insulin (B) concentrations following administration of normal saline (squares) to either C57BL/6 (∎) or PPARγ-deficient (□) mice and P-407 [0.5 g/kg] (circles) to either C57BL/6 (●) or PPARγ-deficient (◯) mice. Data represents the mean ± standard deviation.
Data obtained from the analysis of serum FFAs were first corrected using the reagent and specimen blanks as per the manufacturer’s instructions. The resulting mean value of the serum FFA concentration at each time point for P-407-treated mice was then individually compared to the mean serum FFA concentration at time t = 0 h (i.e., the pre-injection level; = 0.83 mEq/l) using the Student’s t-test, with results being deemed statistically significant if p<0.05. Additionally, the mean value of the serum FFA concentration at each time point for P-407-treated mice was compared to the corresponding mean serum FFA concentration for saline-treated mice using the Student’s t-test and deemed significantly different if p<0.05.
RESULTS
Transactivation Assay
As determined in a cell-based transactivation assay, P-407 did not directly modulate the activity of either mouse or human PPARγ relative to vehicle (Figs. 1A and 1B, respectively). Additionally, Figure 2 demonstrates that P-407, over a concentration range of 0.05 to 200 μM, did not inhibit a known PPARγ agonist (troglitazone) from activating human PPARγ.
PPARγ-deficient Mouse Experiments
These experiments were designed to assess whether P-407 could indirectly modulate PPARγ activity and, thereby, alter plasma insulin and blood glucose concentrations. As shown in Figure 3A, blood glucose concentration-time profiles for both C57BL/6 and PPARγ-deficient mice treated with P-407 were no different than corresponding profiles obtained when mice were treated with saline. The concentration-time profiles were overlapping and grouped around an average blood glucose concentration of approximately 8.5 mM. Similar to the blood glucose results, plasma insulin concentration-time profiles were also overlapping and appeared to be grouped around an average plasma insulin concentration of approximately 328 pM (Figure 3B). No significant differences were noted for concentration-time profiles in Figures 3A and 3B when analyzed using an ANOVA.
Serum FFA Concentrations
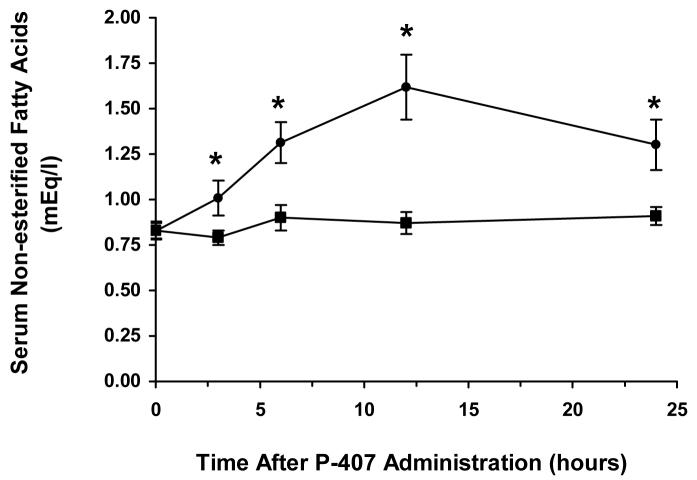
Administration of P-407 to C57BL/6 mice caused a significant (p<0.05) increase in the serum concentration of FFAs as soon as 3 h after injection when compared to corresponding FFA concentrations in saline-treated controls (Fig. 4). The serum FFAs appeared to reach an apparent maximum concentration of 1.61 mEq/l at 12 h following P-407 administration, with FFA concentrations remaining significantly elevated for as long as 24 h post-dosing. The baseline (pre-injection) FFA concentration in fasted C57BL/6 mice administered P-407 was 0.83 mEq/l. This pre-injection, baseline serum FFA concentration in P-407-treated mice (0.83 mEq/l) was not significantly different than the average serum FFA concentration in saline-treated mice (∼ 0.87 mEq/l).
Figure 4.
Serum non-esterified (free) fatty acid concentration following administration of either P-407 (0.5 g/kg) (●) or saline (∎) to fasted (12 h) C57BL/6 mice. Data represents the mean ± standard deviation. * indicates a significant (p<0.05) increase in the serum concentration of FFAs relative to both the pre-injection level (0.83 mEq/l) in P-407-treated mice and the average serum FFA concentration in saline-treated (control) mice.
DISCUSSION
The present study demonstrated that P-407 does not directly activate either mouse or human PPARγ in vitro. Moreover, as assessed using a transactivation assay, P-407 does not inhibit the capacity of a known PPARγ agonist, troglitazone, to activate human PPARγ. These observations suggest that our previous finding of reduced cholesterol efflux by macrophages cultured with P-407 is a result of down-regulation in the gene expression of ABCA1 as proposed (Johnston et al., 2006), and not due to interference with the functional activity of PPARγ. Additionally, it should be noted that we recently demonstrated that P-407 neither modulated PPARα activity in vitro (using a transactivation assay), nor altered the plasma concentrations of total cholesterol, HDL-cholesterol, non-HDL-cholesterol, and triglycerides in PPARα-deficient mice relative to P-407-treated, wild-type (C57BL/6) mice (Johnston and Waxman, 2008).
The second portion of this study focused on whether P-407 might potentially perturb plasma insulin and blood glucose concentrations in mice and, if so, whether this outcome might be mediated through PPARγ. Even though the transactivation assays demonstrated that P-407 was unable to activate both mouse and human PPARγ activity in vitro, we still wished to know whether P-407 could indirectly modulate PPARγ in vivo. This concern was based on the fact that a compound’s ability to modulate PPARs is not always predicted from the results of an in vitro transactivation assay. For example, Peters et al. (Peters et al., 1996) demonstrated that dehydroepiandrosterone-3 beta-sulfate (DHEA-S) does not modulate PPARα in vitro, as assessed using a transactivation assay, yet, in studies using PPARα-knockout mice, PPARα was obligatory for DHEA-S-stimulated hepatic peroxisomal gene induction.
When we initiated the present study, we had no a priori knowledge as to whether the administration of P-407 to C57BL/6 mice would cause any changes in the plasma insulin and blood glucose concentrations. Our data suggest that P-407 has no capacity to modulate either plasma insulin or blood glucose concentrations following administration to normal C57BL/6 mice. To assess whether any potential P-407-induced changes in insulin and glucose concentrations were mediated through PPARγ, we also included a group of P-407-treated, PPARγ-deficient mice and determined the plasma insulin and blood glucose concentrations vs. time post-dosing. Our findings revealed no P-407-mediated perturbations in the plasma insulin and blood glucose concentration-time profiles for P-407-treated, PPARγ-deficient mice when compared to P-407-treated, C57BL/6 mice. This was also true when the profiles were individually compared to the corresponding profiles for saline-treated, PPARγ-deficient and saline-treated, C57BL/6 mice. Therefore, this strongly suggests that P-407 has no capacity to either indirectly activate or inhibit PPARγ, and serves to corroborate our in vitro data obtained from the transactivation assays. As an example, if P-407 had acted as a PPARγ agonist similar to TZD’s, then blood glucose concentrations would have been significantly reduced following the administration of P-407. As mentioned above, this outcome did not occur in the present study.
In accordance with the idea that PPARγ ligands elicit their effects primarily through adipose tissue, it has been demonstrated that PPARγ agonists alter the expression of genes that are involved in lipid uptake, lipid metabolism, and insulin action in adipocytes (Rangwala and Lazar, 2004). As a result, they enhance adipocyte insulin signaling, lipid uptake, and anabolic lipid metabolism, and attenuate lipolysis and FFA release. Consequently, lipid levels in adipose tissue increase, whereas, the concentration of circulating FFAs decrease (Bays et al., 2004). By repartitioning lipids away from liver and muscle, the two primary tissues that are responsible for insulin-mediated glucose disposal and metabolism, PPARγ agonists improve glycemic control by reversing lipotoxicity-induced insulin resistance (Berger et al., 2005). Because of these multiple adipocentric actions, PPARγ agonists (e.g., TZD’s) decrease blood glucose concentrations. As shown in the present study, P-407 did not affect either the plasma insulin or blood glucose concentrations following administration to both wild-type (C57BL/6) and PPARγ-deficient mice. Therefore, P-407 is neither functioning as a PPARγ agonist, nor does it appear to be functioning as a PPARγ antagonist, since P-407 was not able to block the action of the PPARγ agonist, troglitazone, from activating PPARγ in the transactivation assays.
Finally, as stated above, PPARγ agonists cause a reduction in the circulating levels of FFAs. In the present investigation, we demonstrated that P-407 increased the level of FFAs in the serum for up to 24 h post-dosing. Because P-407 causes a decrease in cellular cholesterol efflux (Johnston et al., 2006), which is just opposite to the action of both PPARα and PPARγ agonists (Chawla et al., 2001; Chinetti et al., 2001), perhaps cholesterol homeostasis is maintained, in part, by a P-407-mediated release of FFAs (an essential substrate for both TG and cholesterol synthesis) from adipocytes, as well as by an increase in cholesterol synthesis due to a temporary (up to 48 h following P-407 administration) up-regulation in the activity of 3-hydroxy-3-methylglutaryl Coenzyme A reductase (Johnston and Palmer, 1997; Leon et al., 2006). Future work will examine potential mechanisms responsible for the elevation of serum FFAs following P-407 administration to mice.
In conclusion, P-407 neither activated or inhibited PPARγ in vitro, nor did it interfere with activation of human PPARγ by a known PPARγ agonist (troglitazone). Furthermore, following administration to both control and PPARγ-deficient mice, P-407 did not perturb plasma insulin and blood glucose concentrations, suggesting that P-407 does not indirectly activate or inhibit PPARγ activity in vivo. Lastly, in contrast to a PPARγ agonist, P-407 administration to mice increased the concentration of circulating FFAs by, as yet, an unknown mechanism, but one that is probably unrelated to the inhibition of PPARγ activity. Therefore, since our previous work has shown that a) P-407 does not interfere with an LXRα agonist’s ability to enhance cholesterol efflux from human macrophages (Johnston et al., 2006), and b) ABCA1 gene expression is significantly reduced by P-407 (Johnston et al., 2006), and our present work has demonstrated that P-407 is both unable to activate or inhibit PPARγ in vitro, as well as perturb plasma insulin and blood glucose levels following administration to mice, it is concluded that a) P-407 does not modulate cellular cholesterol efflux at the level of PPARγ in the PPAR-LXR-ABCA1 signaling pathway, and b) P-407 does not interfere with the functional activity of PPARγ following administration to mice.
ACKNOWLEDGEMENTS
Supported, in part, by the Superfund Basic Research Program at Boston University, NIH grant 5 P42 ES07381 (to D.J.W.). The authors thank C. S. Chen for assistance with transactivation assays.
REFERENCES
- Auwerx J. PPARγ, the ultimate thrifty gene. Diabetologia. 1999;42:1033–1049. doi: 10.1007/s001250051268. [DOI] [PubMed] [Google Scholar]
- Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J. Clin. Endocrinol. Metab. 2004;89:463–478. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol. Sci. 2005;26:244–250. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): Tissue distribution of PPAR-α, -β, and -γ in the adult rat. Endocrinol. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- Chang TK, Waxman DJ. Pregnane X receptor-mediated transcription. Methods Enzymol. 2005;400:588–598. doi: 10.1016/S0076-6879(05)00033-9. [DOI] [PubMed] [Google Scholar]
- Chawla A, Schwarz EJ, Dimaculangan DD, Lazar MA. Peroxisome proliferator-activated receptor (PPAR) γ: Adipose-predominant expression and induction early in adipocyte differentiation. Endocrinol. 1994;135:798–800. doi: 10.1210/endo.135.2.8033830. [DOI] [PubMed] [Google Scholar]
- Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): Nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm. Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Pineda TI, et al. PPARα and PPARγ activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat. Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ. Recent update on the PPAR α-null mouse. Biochimie. 1997;79:139–144. doi: 10.1016/s0300-9084(97)81506-4. [DOI] [PubMed] [Google Scholar]
- He W, Barak Y, Hevener A, Olson P, Liao D, Le J, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver, but not in muscle. Proc. Natl. Acad. Sci. USA. 2003;100(26):15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston TP. The P-407-induced murine model of dose-controlled hyperlipidemia and atherosclerosis: A review of findings to date. J. Cardiovasc. Pharmacol. 2004;43:595–606. doi: 10.1097/00005344-200404000-00016. [DOI] [PubMed] [Google Scholar]
- Johnston TP, Palmer WK. Effect of poloxamer 407 on the activity of microsomal 3-hydroxy-3-methylglutaryl CoA reductase in rats. J. Cardiovasc. Pharmacol. 1997;29:580–585. doi: 10.1097/00005344-199705000-00003. [DOI] [PubMed] [Google Scholar]
- Johnston TP, Emeson EE, Palmer WK. Poloxamer 407-induced atherogenesis in the C57BL/6 mouse. Atherosclerosis. 1998;136:115–123. doi: 10.1016/s0021-9150(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Johnston TP, Jaye M, Webb CL, Krawiec JA, Alom-Ruiz SP, Sachs-Barrable K, et al. Poloxamer 407 (P-407)-mediated reduction in the gene expression of ATP-binding-cassette transporter A1 may contribute to increased cholesterol in peripheral tissues of P-407-treated rats. Eur. J. Pharmacol. 2006;536:232–240. doi: 10.1016/j.ejphar.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Johnston TP, Waxman DJ. The induction of atherogenic dyslipidemia in poloxamer 407-treated mice is not mediated through PPARα. J. Pharm. Pharmacol. 2008;60:753–759. doi: 10.1211/jpp.60.6.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc. Natl. Acad. Sci. USA. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Wilson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ) J. Biol. Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Leon C, Wasan KM, Sachs-Barrable K, Johnston TP. Acute P-407 administration to mice causes hypercholesterolemia by inducing cholesterolgenesis and down-regulating low-density lipoprotein receptor expression. Pharm. Res. 2006;23:1597–1607. doi: 10.1007/s11095-006-0276-8. [DOI] [PubMed] [Google Scholar]
- Maloney EK, Waxman DJ. trans-activation of PPARα and PPARγ by structurally diverse environmental chemicals. Toxicol. Appl. Pharmacol. 1999;161:209–218. doi: 10.1006/taap.1999.8809. [DOI] [PubMed] [Google Scholar]
- Mukherjee R, Davies PJ, Crombie DL, Bischoff ED, Cesario RM, Jow L, et al. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature. 1997;386:407–410. doi: 10.1038/386407a0. [DOI] [PubMed] [Google Scholar]
- Nash VJ, Johnston TP, Palmer WK. Effects of nicotinic acid on poloxamer 407-induced hyperlipidemia. Pharmacotherapy. 1996;16(1):10–15. [PubMed] [Google Scholar]
- Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J. Clin. Invest. 1998;101:1354–1361. doi: 10.1172/JCI1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer WK, Emeson EE, Johnston TP. The poloxamer 407-induced hyperlipidemic atherogenic animal model. Med. Sci. Sports Exer. 1997;29:1416–1421. doi: 10.1097/00005768-199711000-00005. [DOI] [PubMed] [Google Scholar]
- Peters JM, Zhou YC, Ram PA, Lee SS, Gonzalez FJ, Waxman DJ. Peroxisome proliferator-activated receptor alpha required for gene induction by dehydroepiandrosterone-3 beta-sulfate. Mol. Pharmacol. 1996;50:67–74. [PubMed] [Google Scholar]
- Pineda TI, Gervois P, Staels B. Peroxisome proliferator-activated receptor alpha in metabolic disease, inflammation, atherosclerosis, and aging. Curr. Opin. Lipidol. 1999;10:151–159. doi: 10.1097/00041433-199904000-00009. [DOI] [PubMed] [Google Scholar]
- Rangwala SM, Lazar MA. Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol. Sci. 2004;25:331–336. doi: 10.1016/j.tips.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Olefsky JM. Thiazolidinediones in the treatment of insulin resistance and Type II diabetes. Diabetes. 1996;45:1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- Shipley JM, Waxman DJ. Simultaneous, bidirectional inhibitory crosstalk between PPAR and STAT5b. Toxicol. Appl. Pharmacol. 2004a;199:275–284. doi: 10.1016/j.taap.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Shipley JM, Hurst CH, Tanaka SS, DeRoos FL, Butenhoff JL, Seacat AM, et al. trans-activation of PPARα and induction of PPARα target genes by perfluorooctane-based chemicals. Toxicol. Sci. 2004b;80:151–160. doi: 10.1093/toxsci/kfh130. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Adipogenesis and obesity: Rounding out the big picture. Cell. 1996;87:377–389. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPARγ 2: Tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- Wasan KM, Subramanian R, Kwong M, Goldberg IJ, Wright T, Johnston TP. Poloxamer 407-mediated alterations in the activities of enzymes regulating lipid metabolism in rats. J. Pharm. Pharmaceut. Sci. 2003;6(2):108–116. [PubMed] [Google Scholar]