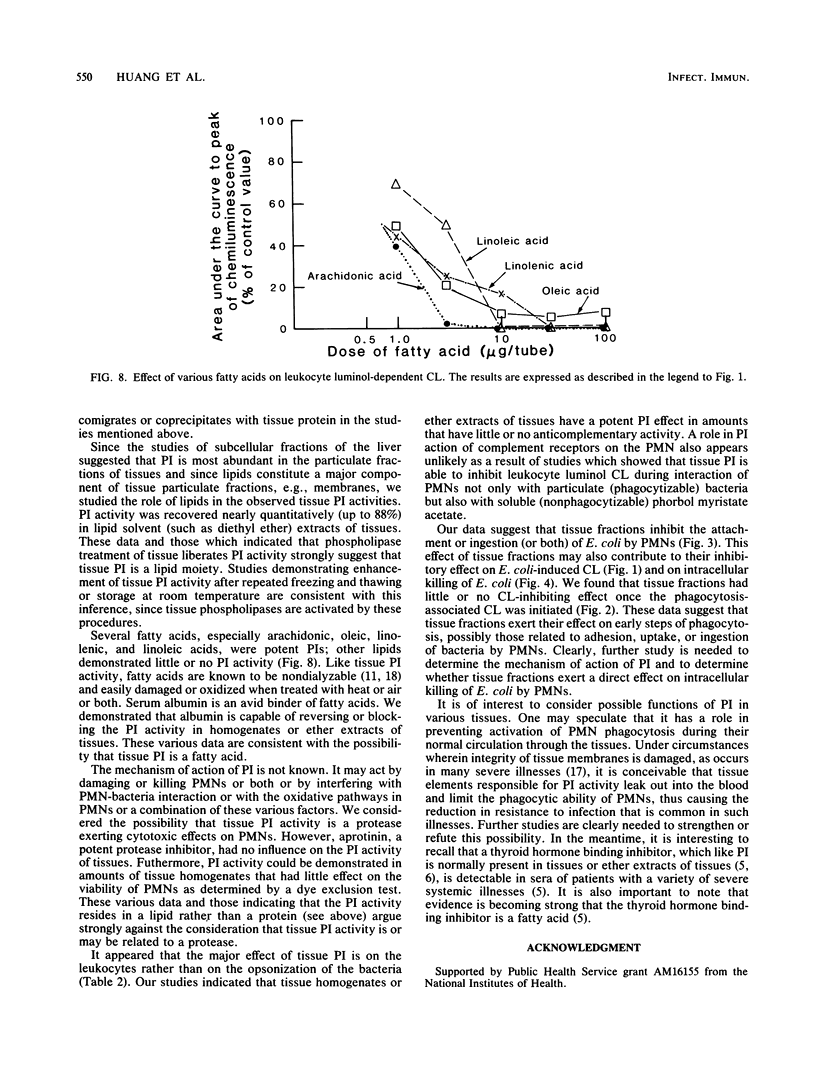
Abstract
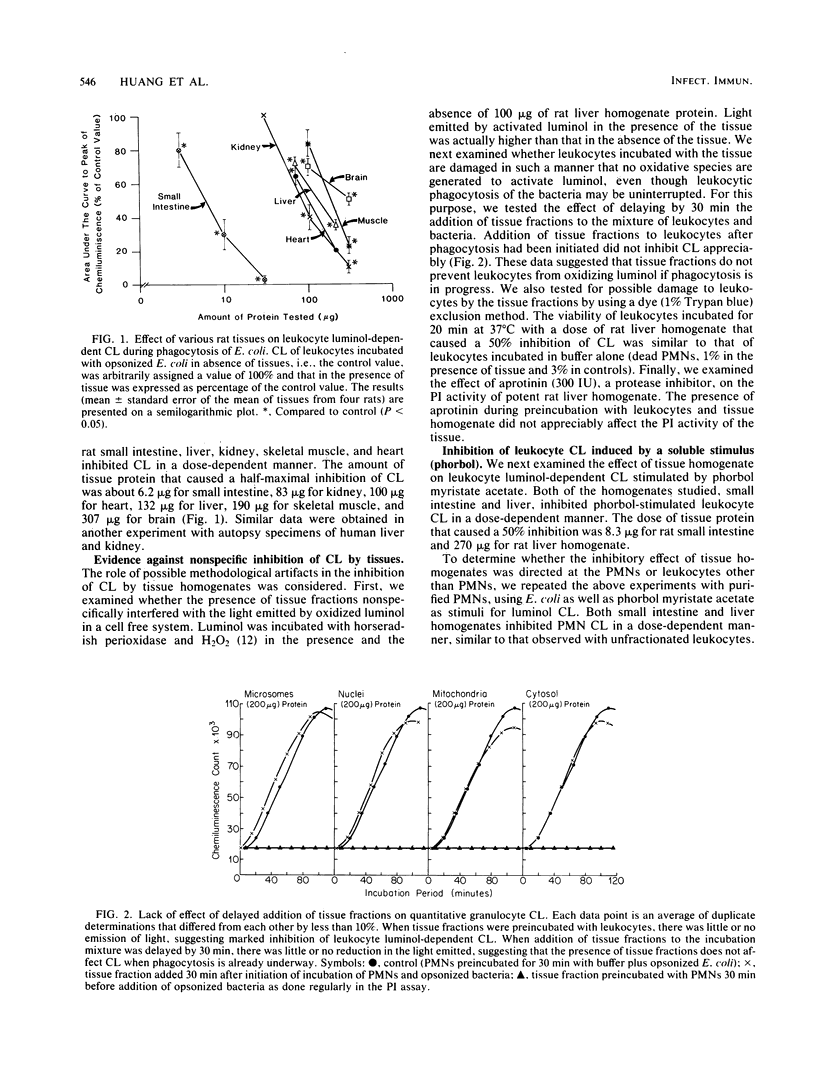
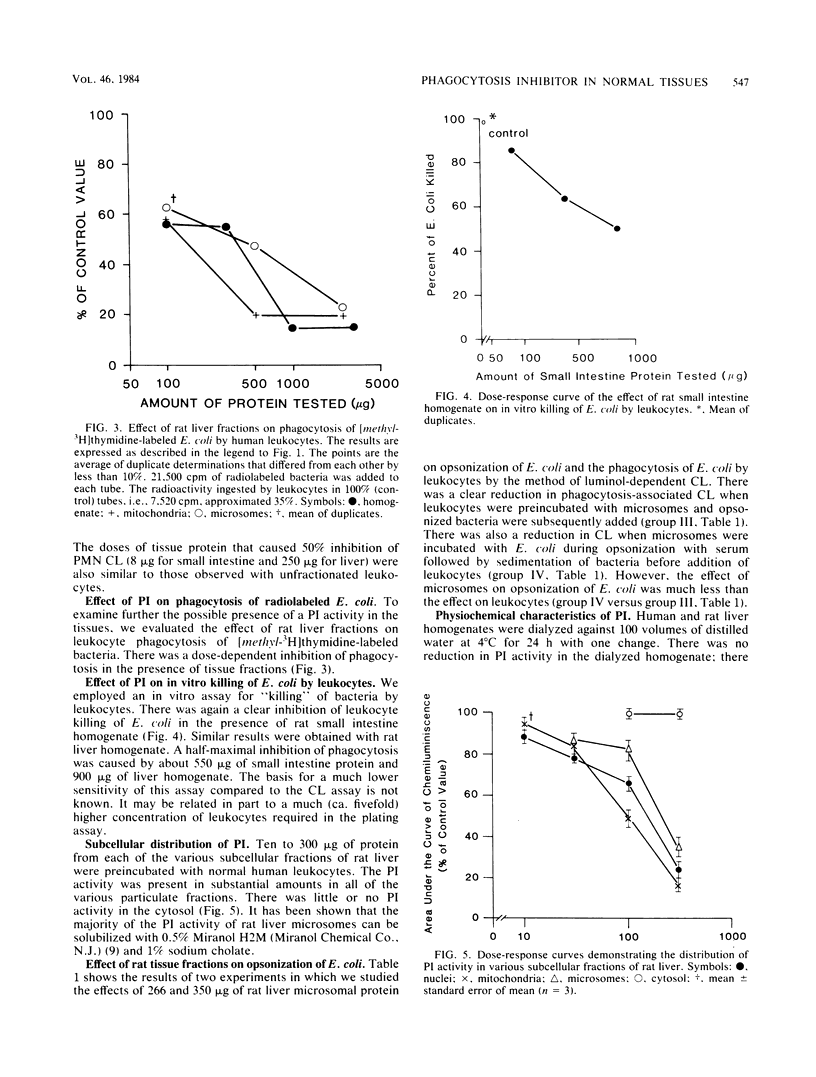
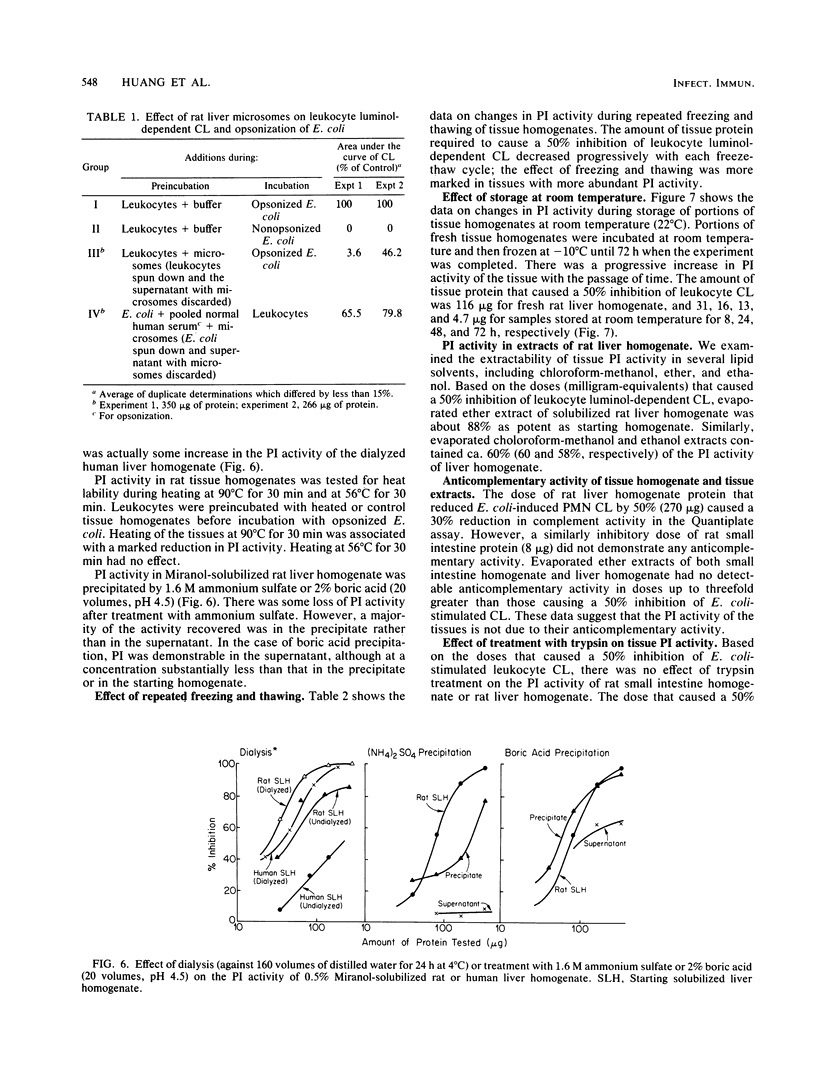
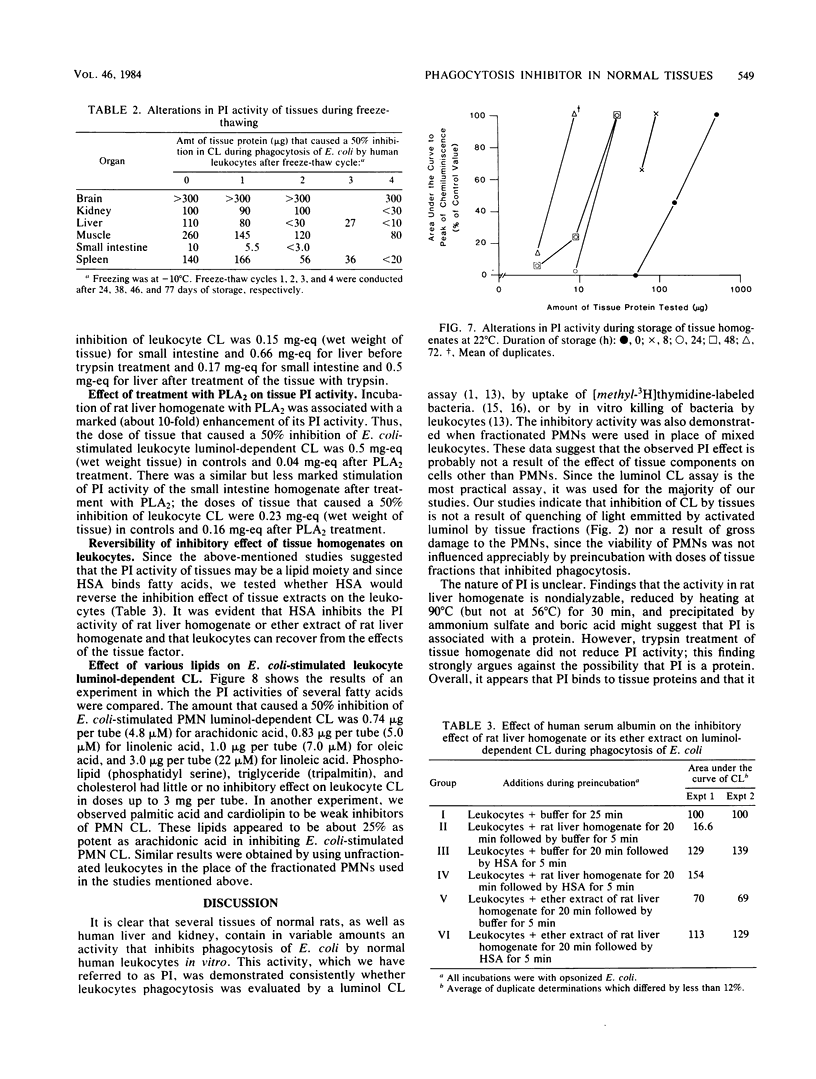
Homogenates of normal rat tissues inhibited several functional parameters of normal human peripheral blood leukocytes, including luminol-dependent chemiluminescence induced by both soluble (phorbol myristate acetate) and particulate (Escherichia coli) stimuli; in vitro uptake of radiolabeled E. coli; and in vitro phagocytosis and killing of E. coli. The doses of rat tissue protein that caused a 50% inhibition of leukocyte chemiluminescence were ca. 6.2 micrograms for small intestine, 83 micrograms for kidney; 100 micrograms for heart; 132 micrograms for liver, 190 micrograms for skeletal muscle, and 307 micrograms for brain. The putative phagocytosis inhibitor (PI) in rat liver was more plentiful in particulate fractions than in the cytosol. The PI activity in the original or Miranol-solubilized rat liver homogenate was nondialyzable, and it was reduced substantially by heating at 90 degrees C for 30 min but not at 56 degrees C for 30 min. It was unaffected by aprotinin, a potent inhibitor of proteolytic activity. Treatment of tissues with trypsin did not reduce PI activity, whereas treatment with phospholipase A2 clearly increased it. The bulk (up to 88%) of PI in rat liver or small intestine could be extracted by lipid solvents, e.g., diethyl ether. Purified fatty acids were potent inhibitors of leukocyte chemiluminescence; other lipids had little or no inhibiting activity. The various data suggest that (i) normal tissues contain a potent PI and (ii) that the PI is a lipid moiety.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Bjornson A. B., Bjornson H. S., Altemeier W. A. Serum-mediated inhibition of polymorphonuclear leukocyte function following burn injury. Ann Surg. 1981 Nov;194(5):568–575. doi: 10.1097/00000658-198111000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyum A. Separation of blood leucocytes, granulocytes and lymphocytes. Tissue Antigens. 1974;4(4):269–274. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Huang T. S., Hurd R. E., Beredo A., Solomon D. H. A competitive ligand binding assay for measurement of thyroid hormone-binding inhibitor in serum and tissues. J Clin Endocrinol Metab. 1984 Apr;58(4):619–628. doi: 10.1210/jcem-58-4-619. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Solomon D. H., Teco G. N., Eisenberg J. B. An inhibitor of the binding of thyroid hormones to serum proteins is present in extrathyroidal tissues. Science. 1982 Jan 22;215(4531):407–409. doi: 10.1126/science.7058324. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Wright D. G., Malech H. L., Davis J. M., Klempner M. S., Kirkpatrick C. H. Disorders of phagocyte chemotaxis. Ann Intern Med. 1980 Apr;92(4):520–538. doi: 10.7326/0003-4819-92-4-520. [DOI] [PubMed] [Google Scholar]
- Iwamori M., Moser H. W., Kishimoto Y. Solubilization and partial purification of steroid sulfatase from rat liver: characterization of estrone sulfatase. Arch Biochem Biophys. 1976 May;174(1):199–208. doi: 10.1016/0003-9861(76)90339-8. [DOI] [PubMed] [Google Scholar]
- Spector A. A. Fatty acid binding to plasma albumin. J Lipid Res. 1975 May;16(3):165–179. [PubMed] [Google Scholar]
- Stevens P., Winston D. J., Van Dyke K. In vitro evaluation of opsonic and cellular granulocyte function by luminol-dependent chemiluminescence: utility in patients with severe neutropenia and cellular deficiency states. Infect Immun. 1978 Oct;22(1):41–51. doi: 10.1128/iai.22.1.41-51.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens P., Young L. S. Quantitative granulocyte chemiluminescence in the rapid detection of impaired opsonization of Escherichia coli. Infect Immun. 1977 Jun;16(3):796–804. doi: 10.1128/iai.16.3.796-804.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk W. C., Verbrugh H. A., van der Tol M. E., Peters R., Verhoef J. Role of Escherichia coli K capsular antigens during complement activation, C3 fixation, and opsonization. Infect Immun. 1979 Aug;25(2):603–609. doi: 10.1128/iai.25.2.603-609.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]


