Abstract
Background and objectives: In kidney disease, the concept of loss is widely discussed but minimally researched. It appears that dialysis patients who grieve a range of losses suffer increased depression and reduced quality of life (QoL). Limited research is partly due to the lack of a relevant loss measure. The study presented here developed a measure and tested the criterion validity of loss in relation to depression and QoL.
Design, setting, participants, & measurements: In a cross-sectional observational study, 151 long-term dialysis patients were interviewed using standardized psychometric measures and the Kidney Disease Loss Scale (KDLS), developed for the study. Factor, path and multigroup analyses were conducted.
Results: The factor structure and reliability of KDLS were supported. The path analyses supported the criterion validity of loss. It was a stronger contributor to depression than other clinical variables. Its effect on QoL was fully mediated by depression and positive affect (coping). The magnitude of the paths from loss to QoL through depression and positive affect was larger in home-based dialysis patients than in hospital-based patients.
Conclusions: KDLS is a promising measure of loss. Patient-defined losses may contribute to the high level of depression and in turn a reduction in patients’ coping and QoL. These findings suggest several points of intervention to improve long-term dialysis patients’ QoL.
The concept of Kidney-Disease-Related Loss (KDRL) in ESRD has been clinically and theoretically significant for decades (1–4). ESRD patients experience multiple losses, both tangible and symbolic; for example, loss of physical strengths, freedom, employment, and social life, resulting in grief throughout the course of ESRD and dialysis (1,2,4). Resolving loss successfully is identified as one of the adaptational factors in ESRD (3). Although widely discussed, there exists no clear definition or measure of KDRL.
Loss, traditionally discussed in the context of death, has recently been broadened to comprise loss of significant elements in a person's life due to chronic illness (5,6). In chronic-illness-caused losses, individual grief responses, rather than actual losses, are of conceptual importance (6). These grief responses, which can persist for many years and cause significant dysfunctions, are shown to be empirically and conceptually different from depression and anxiety in several studies (7–10). The grief symptomatology is characterized by cognitive preoccupation or rumination, yearning, disbelief, stunned responses, and nonacceptance of losses (7,9,11), which could be summarized as cognitive and affective grief responses (6). Thus, the study presented here defines KDRL as the cognitive and affective grief responses after tangible and symbolic losses due to kidney disease and its treatments. This definition will guide the development of a measure for KDRL.
KDRL has traditionally been seen as one of the causes of depression in ESRD patients. In the psychodynamic perspective, depression may be an extension of patients’ grief responses (1) or KDRL may increase patients’ vulnerability to developing depression (4). In the cognitive-behavioral perspective, patients’ cognitive rumination over their losses may lead to depression, as negative rumination has been found to be associated with depression (12,13).
There exists a strong inverse relationship between depression and quality of life (QoL) in ESRD patients (14,15). If KDRL is a significant cause of depression, it may have a mediated effect on QoL through depression or KDRL may also independently affect both depression and QoL. Although the literature suggests a direct relationship between depression and QoL, positive affect as a way of coping (16,17) may mediate the effect of depression and loss (18). Positive affect may have adaptive values in dealing with chronic stress (16,17) and thus moderate the effect of depression and KDRL on QoL.
The clinical validity of KDRL can be understood not only by measuring it, but also by examining its relations to clinical variables, including medical comorbidity, length of time on dialysis, and level of hemoglobin and its effects on hospital- and home-based dialysis patients. It is hypothesized that patients’ KDRL will covary with these clinical variables influencing QoL through depression and positive affect (15,19–23). Moreover, the clinical observation suggests that hospital-based patients may experience more losses and become accustomed to their effects, whereas home-based patients may regain or minimize some losses through the advantages of home treatments. Thus, it is assumed that each additional loss for home-based patients will have a greater effect on their well being and QoL, and thus the clinical validity of the KDRL can be demonstrated.
The aim of this research is to show the criterion-related validity of the KDRL construct by developing a measure for KDRL, to examine its relationship with relevant psychologic and clinical variables, and its effect on home-based dialysis patients. It is hypothesized that (1) the proposed scale of KDRL will consist of cognitive and affective grief responses and that (2a) loss will lead to depression, (2b) covary with other clinical variables to influence QoL through depression and positive affect, and (2c) influence the home-based patients more.
Materials and Methods
Participants
Participants were recruited from two major university teaching hospitals in Sydney South West Area Health Service (SSWAHS) and South Eastern Sydney & Illawarra Area Health Service (SESIAHS) Sydney, Australia. The inclusion criteria were a diagnosis of ESRD, receiving dialysis treatment for approximately 2 yr or more and aged 18 or above. The main exclusion criterion was incapacity to complete questionnaires and/or the semistructured interview with reasonable assistance. Of 209 eligible patients, 31 (14.8%) refused, 7 (3.4%) withdrew during the interview as being too sick to continue, 16 (7.7%) did not return the questionnaires, and 4 (1.9%) did not complete the data collection because of death, transplant, acute medical problems, or cognitive impairment. Thus, 151 (72.2%) patients participated in the interview and returned the questionnaires. The sample consisted of 90 (60%) men and 61 women (40%) with the mean age 58 ± 14.28 (SD) yr, the mean duration of dialysis was 67.97 ± 42.84 (SD) mo (range 22 to 248 mo). Approximately half (n = 68, 45%) of the participants were doing home-based dialysis, including home hemodialysis (n = 26, 17%) and peritoneal dialysis (n = 42, 28%), and the others (n = 83, 55%) were on hospital-based dialysis including satellite (n = 56, 37%) and in-center hemodialysis (n = 27, 18%).
Procedures
This study was approved by the SSWAHS and SESIAHS Ethics Committees. After being informed of the study details and having signed a consent form, participants completed the semistructured interview and returned completed questionnaires by mail. One reminder letter and spare questionnaire were sent to those who did not return the questionnaires. Two interviewers were trained in the application of measures before the initiation of the study to ensure the quality of the data collection.
Measures
Seven variables were examined in the study: QoL, depression, KDRL, positive affect (a key component of coping), medical comorbidity, length of time on dialysis, and hemoglobin. In addition to developing a scale to measure KDRL, standardized measures were used in the study—the Schedule for Evaluation of Individual Quality of Life—Direct Weighting (SEIQoL-DW) (24), the Depression Scale of Depression Anxiety Stress Scale 21 (DASS21) (25), the Positive Affect Scale (PAS) (18), and the Comorbidity Index (CMI) (26,27). Additional questions were included to collect participants’ demographic and dialysis related information including length of time on dialysis (the total number of months). Hemoglobin level was an average over 3 mo, including the month of the research interview.
The Kidney Disease Loss Scale
The measurement context in which an individual considers their losses will in part determine the validity of the measurement. The context can be determined from individual-defined losses or from a standard set of losses. The type and importance of losses varies widely between patients and thus a predefined set of losses may only be partially relevant to any individual's experience (6,24). The Kidney Disease Loss Scale (KDLS) was constructed to first elicit the five most important individual KDRL and second to obtain cognitive and affective grief responses. A second-order factor structure of KDLS was hypothesized with four statements measuring cognitive rumination and four measuring the affective response to loss.
The SEIQoL-DW
The SEIQoL-DW is a patient-centered semistructured interview to measure individual QoL (24). It was chosen because of known limitations in multidimensional measures of QoL (28) and its ability to produce both qualitative and quantitative data. Of particular importance for renal patients are that preselected life domains may not be relevant, and equal weighting of life domains is inconsistent with patients’ own values (29,30).
In the SEIQoL-DW, the five most important areas of a respondent's life are elicited, then the level of satisfaction and relative importance of each area is determined. Its global index score, ranging from 0 to 100, is the sum of a product of the ratings and weightings of each nominated area. Its reliability, validity, and utility in the medical and ESRD populations are excellent (24,30–33).
The DASS21
The DASS21 is a 21-item scale, comprising of three subscales: depression, anxiety, and stress (seven items each), a total score for each subscale ranging from 0 to 21. Only scores on the depression subscale were used in the analyses. The DASS21 focuses on the cognitive and affective aspects of depression, limiting the methods bias of using a measure that contains somatic elements common to depression and kidney disease (2,34,35). Additionally, it was developed in the Australian population (the site of this study); it has strong psychometric properties in both general and clinical populations (25,36,37); it is relatively short, reducing administrative burden on patients; and differentiates depression, anxiety, and stress.
PAS
The PAS is a four-item scale on a Likert scale ranging from 0 (not at all) to 10 (very much), with a total score ranging from 0 to 40 to measure individual positive feelings. Its reliability and discriminant and convergent validities have been well established (18).
CMI
The CMI was compiled on the basis of Friedman's Index (26) and Charlson's Index (27). It is in a checklist format, with scores ranging from 0 to 15, higher scores indicating more comorbid conditions.
Data Analyses
Analyses were performed with SPSS 15 (38) and LISREL 8.72 (39). Missing data were rare and nonsystematic and thus replaced using EM (expectation-maximization) method (38,40). The first set of analyses examined and validated the factor structure of the KDLS using confirmatory factor analysis (CFA) and exploratory factor analysis (EFA). Standard techniques of correlation, ANOVA, and t test were used with a critical value of alpha set to 0.05. The final set of analyses used structural equation modeling (SEM) to test, develop, and examine alternative models. Multigroup analyses were used to compare hospital and home dialysis.
The two-step approach was used with SEM (41). Composite scores of the observed variables, using the unit-weighted addition method, were calculated and error variances fixed in the measurement model (42,43) before the structural model was tested. Because the distributions of the observed variables were skewed, SEM was undertaken using “asymptomatic distribution free covariance” matrices using the robust maximum likelihood and the Satorra-Bentler statistic for parameter estimation (44–46). Several goodness-of-fit (GFI) indices were chosen to evaluate the overall model fit (40): Satorra-Bentler χ2 test (P > 0.05, in SEM nonsignificant χ2 indicates a good model fit), the GFI (≥0.90), adjusted GFI (AGFI) (>0.90), the root mean square residual (RMR; close to 0), the root mean square error of approximation (RMSEA; ≤0.05, 90% confidence interval <0.08), and the comparative fit index (CFI; ≥0.90).
Results
Development of KDLS
The hypothesized factor structure of KDLS was not supported using CFA [χ2 = 73.44, degrees of freedom (df) = 19, P = 0.00, RMSEA = 0.09, GFI = 0.89, AGFI = 0.80, CFI = 0.99, RMR = 0.05; LISREL 8.72 39]. Re-examining the data, EFA using SPSS (38) indicated two factors with 72.24% of the total variance explained. There were two items loading onto both factors and therefore excluded (42). The first factor consisted of four items and resembled cognitive responses and the second factor consisted of two items reflecting affective responses. The second factor was retained for its theoretical importance and the items’ correlation was greater than 0.7 (47). The two factors were strongly correlated (r = 0.71, P < 0.01), suggesting the previously hypothesized second-order factor. The new factor structure and possible second-order factor model were examined using CFA (χ2 = 12.63, df = 8, P = 0.128, RMSEA = 0.00, GFI 0.97, AGFI 0.93, CFI 1.00, RMR 0.03) (LISREL 8.72 39). The first-order factors measure cognitive and affective responses and a second-order factor reflects an overall sense of loss regarding self-defined KDRLs. Therefore, the KDLS comprises an overall loss scale by summing the six items (48) and two subscales (see Table 1). High scores are indicative of greater sense of loss, with more rumination and stronger affect. The internal consistence of KDLS was good (α = 0.88 for both the cognitive and affective subscales).
Table 1.
The final version of the Kidney Disease Loss Scale (KDLS)a
| When you consider life as it is now with kidney disease and dialysis treatments, it is clearly different from what your life used to be or would have been. You might find yourself having lost many things. Please consider LOSSES: things that you used to do before kidney disease/dialysis and now you cannot do anymore or things that you would have been doing if you did not have kidney disease/dialysis. Please list below the five most important things you have lost because of the kidney disease/dialysis. | ||||
| 1: | ||||
| 2: | ||||
| 3: | ||||
| 4: | ||||
| 5: | ||||
| In regard to the five losses described above, please read each of the following statements carefully and circle a number 0, 1, 2 or 3 that indicates how much the statement applied to you | ||||
| The rating scale is as follows: | ||||
| 0 = Did not apply to me at all | ||||
| 1 = Applied to me to some degree, or some of the time | ||||
| 2 = Applied to me to a considerable degree, or a good part of time | ||||
| 3 = Applied to me very much, or most of the time | ||||
| 1. I think about these losses so much that it is hard for me to do the everyday things I normally do | 0 | 1 | 2 | 3 |
| 2. Memories of the losses upset me | 0 | 1 | 2 | 3 |
| 3. I am preoccupied with thoughts of the losses | 0 | 1 | 2 | 3 |
| 4. I feel myself longing for regaining what I have lost | 0 | 1 | 2 | 3 |
| 5. I feel disbelief over what happened | 0 | 1 | 2 | 3 |
| 6. I feel stunned or dazed over what happened | 0 | 1 | 2 | 3 |
The first part of the KDLS that requires respondents to nominate the five most important losses must be retained when the KDLS is used, because it sets the context for rating the six items.
The convergent and discriminant validity of KDLS were supported by its significant positive correlation with depression (r = 0.60, P < 0.01), and negative correlation with QoL (r = −0.20, P < 0.05) and positive affect (r = −0.36, P < 0.01). It was unrelated to clinical variables (hemoglobin r = 0.07, comorbidity r = 0.04, length of time since dialysis r = −0.15). Construct validity was examined by conducting a two-factor EFA of the six KDLS and the seven DASS21 depression items. The depression items loaded onto one factor and KDRL items loaded onto another without cross-loading items.
Patients’ nominated losses on KDLS were codified by two independent coders by iteratively examining the data. The intercoder agreement was 95% and the differences in the remaining 5% were resolved by consensus. In total, 109 of 151 respondents nominated 431 losses. Of the 23 types of losses identified, the seven most common were travel (18.56% of the total nominated losses), leisure activities (12.06%), physical functioning (10.90%), employment/work (9.74%), family/personal relationships (6.50%), freedom (perception of being free from constraints; 4.87%) and social life (4.87%). The mean score difference on KDLS between those who did not nominate losses (x̄ 5.41) and those who did (x̄ 5.84) was nonsignificant (t = 0.49, df = 149, P > 0.05).
Univariate Analyses
There was no gender differences in the variables used in the study. An examination of differences between the two hospitals identified a significant difference in hemoglobin levels (SSWSAH x̄ = 123.84 (13.88), SESIAHS x̄ = 117.13 (14.05), P < 0.01); there were no differences on other variables. There were no treatment modality (in-center hemodialysis, home hemodialysis, peritoneal dialysis, satellite hemodialysis) differences on any variable.
Path Analyses
Model Testing and Modification.
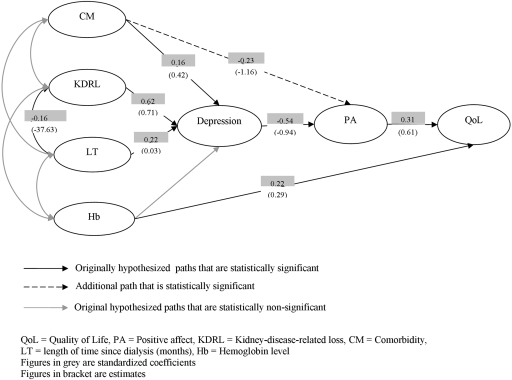
The structural relationships between length of time on dialysis, hemoglobin, comorbidity, loss, depression, positive affect, and individual QoL were examined using SEM. The hypothesized path model was not supported (χ2 = 16.57, df = 8, P = 0.04, RMSEA = 0.09; for all fit statistics see Table 2). Therefore, the model modification process was conducted to improve the model fit by examining the t-values of path coefficients, standardized residual values, and modification indexes. The path from hemoglobin to depression was insignificant and thus deleted, and an additional path from comorbidity to positive affect added (χ2 = 7.91, df = 13, P = 0.85, RMSEA = 0.00; see Table 2 and Figure 1), indicating a plausible model for the observed data.
Table 2.
The goodness-of-fit statistics of the hypothesized, modified, and alternative modelsa
| Path Models | Goodness-of-Fit Statistics | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hypothesized path model | χ2 | df | P | GFI | AGFI | RMR | RMSEA | 90%CI | CFI |
| 16.57 | 8 | 0.04 | 0.97 | 0.89 | 16.25 | 0.09 | 0.02 to 0.14 | 0.95 | |
| Modified path model | χ2 | df | P | GFI | AGFI | RMR | RMSEA | 90%CI | CFI |
| 7.91 | 13 | 0.85 | 0.99 | 0.97 | 15.81 | 0.00 | 0.00 to 0.05 | 1.00 | |
| Alternative model 1b | χ2 | df | P | GFI | AGFI | RMR | RMSEA | 90%CI | CFI |
| 6.06 | 12 | 0.91 | 0.99 | 0.98 | 17.42 | 0.00 | 0.00 to 0.03 | 1.00 | |
| Alternative model 2c | χ2 | df | P | GFI | AGFI | RMR | RMSEA | 90%CI | CFI |
| 41.20 | 13 | 0.00 | 0.93 | 0.84 | 13.33 | 0.12 | 0.08 to 0.16 | 0.84 | |
χ2, Satorra-Bentler scaled chi-square; df, degrees of freedom; GFI, goodness-of-fit index; AGFI, adjusted GFI; RMR, root mean square residual; RMSEA, root mean square error of approximation; NNFI, non-normed fit index; CFI, comparative fit index.
Additional path from depression to quality of life was added in the model.
Kidney-disease-related loss precedes depression, given all other parameters being constant.
Figure 1.
Path diagram of the final modified model of KDRL. QoL, quality of life; PA, positive affect; KDRL, kidney-disease-related loss; CM, comorbidity; LT, length of time since dialysis (months); Hb, hemoglobin level. Figures in gray are standardized coefficients. Figures in brackets are estimates.
As shown in Table 3, each variable explained a small proportion of variance in QoL with positive affect (9.6%) and hemoglobin (4.8%) having the highest direct effect. The effects on positive affect from comorbidity and length of time since dialysis were moderate whereas depression explained the largest proportion (29.2%) of variance in positive affect. Loss explained 38.4% of variances in depression, larger than comorbidity (2.3%) and length of time since dialysis (5.3%), and only had a small effect on QoL fully mediated by depression and positive affect.
Table 3.
Standardized direct, indirect, total effects of the variablesa
| CMI | Loss | Hemoglobin | Length of Time Since Dialysis | Positive Affect | Depression | |
|---|---|---|---|---|---|---|
| Direct effects | ||||||
| QoLb | - | - | 0.22 | - | 0.31 | - |
| Positive affect | −0.23 | - | - | - | - | −0.54 |
| Depression | 0.16 | 0.62 | - | 0.23 | - | - |
| Indirect effects | ||||||
| QoL | −0.10 | −0.11 | - | −0.04 | - | −0.17 |
| Positive affect | −0.08 | −0.34 | - | −0.12 | - | - |
| Depression | - | - | - | - | - | - |
| Total effects | ||||||
| QoL | −0.10 | −0.11 | 0.22 | −0.04 | 0.31 | −0.17 |
| Positive affect | −0.31 | −0.34 | - | −0.12 | - | −0.54 |
| Depression | 0.16 | 0.62 | - | 0.23 | - | - |
Standardized total effect is the sum of direct and indirect effects of one variable on another variable. Its value ranges from 0 to 1 with either negative or positive direction. The larger the value is, the stronger the effect is.
QoL, quality of life.
Alternative Models
In covariance structure modeling, several equivalent models may exist (40). Two theoretically based alternative models were also tested. Alternative model 1 was to examine if depression has a direct effect on QoL, and alternative model 2 was to investigate the ordinal relations between KDRL and depression. The χ2 difference statistics showed that alternative model 1 was not a better model (χ2D = 1.85, dfD = 1, nonsignificant, P < 0.05; also see Table 2). Alternative model 2 was rejected by the fit statistics (Table 2); depression does not precede KDRL.
KDRL in Relation to the Treatment Location Effect
To further demonstrate the criterion validity of KDRL, a multigroup analysis on the hospital- and home-based dialysis patients was conducted. The poor global fit statistics (χ2 = 135.66, df = 38, P = 0.00, RMESA = 0.19) suggested differences between the two groups. A reasonable fit (χ2 = 43.87, df = 34, P = 0.12, RMSEA = 0.06) of the re-specified model suggested that the main difference was a greater strength of the relations between KDRL, depression, positive affect, and QoL in home-based dialysis patients, although mean score differences on these variables between the groups were nonsignificant.
Discussion
The study presented here provides empirical support for KDLS and the hypotheses that loss leads to depression, covaries with length of time since dialysis to influence QoL through depression and positive affect, and has greater effect on home-based patients. Moreover, the moderate direct effect that hemoglobin has on QoL is consistent with previous clinical trials showing erythropoietin treatment enhances ESRD patients’ QoL (49).
The concept of KDRL is operationalized by KDLS, which has encouraging psychometric properties. Consistent with previous research, depressive and loss symptoms loaded onto separate factors, and the path analyses demonstrated that KDRL precedes depression (7–10). Thus the KDRL is empirically distinct from depression. The cognitive rumination, yearning, disbelief, and stunned responses remain the key symptomatology that contributes to depression.
KDRL as a strong contributor to depression is consistent with the previous theories that loss is a significant cause of depression. Although the findings presented here may not support depression as being an extension of KDRL, whether depression is due to increased vulnerability or cognitive rumination over negative contents of loss remains equivocal. Perhaps, more cognitive rumination items contributing to the total score of KDLS may suggest that cognitive rumination is the underlying mechanism between KDRL and depression. Nevertheless, the findings that travel, leisure activities, and physical functioning are the most nominated types of losses may not be surprising clinically, because many dialysis patients commonly describe their dialysis experiences as being restrictive. Therefore, these results may suggest that to improve patients’ depression and QoL, psychosocial interventions could target loss; for example, helping patients to regain losses by helping them to travel or perform activities more, or to process their related thoughts and feelings by reducing their cognitive ruminations. Encouragingly, these findings have already changed the educational practice of one of the renal units where the study was conducted.
Another interesting finding of the study is that the effect of both KDRL and clinical variables on QoL is mediated by depression and positive affect. This shows that disease-specific variables may influence QoL indirectly through psychologic states (50). However, the type of psychologic states has not been explicitly specified, but rather implicitly assumed to be depression in the ESRD literature. The findings presented here suggest that both positive and negative psychologic states could act as mediators. Positive affect may be a distinct factor and has adaptive values buffering the effect of loss and depression on QoL (17,18).
The criterion validity of the KDRL construct is also illustrated by the multigroup analyses showing that a stronger magnitude of the relations between KDRL and other psychologic variables exists for home-dialysis patients. For a small increase in their sense of loss, home-based patients experience more depression and greater decline in positive affect and QoL than do the hospital-based. This may mean that psychosocial interventions for home-based dialysis patients, especially on the KDLS and depression, could result in greater improvement in their QoL and potentially delay the need for hospital-based treatment.
The study presented here has several limitations. The KDLS is a newly developed scale and awaits further research to crossvalidate its validity and reliability. Future research may also focus on item generation for the affective subscale of KDLS to improve its construct validity. Because the main purpose of the path analyses results was to examine the construct validity of the KDLS, the substantive meaning of the model requires replication in different samples. The sample size for the multigroup analyses is considered small in SEM, and although consistent with preliminary expectations, the interpretation of these results should be cautious.
Overall, the study presented here demonstrated the construct and criterion validity of KDRL and the promising psychometric properties of KDLS. KDRL may lead to depression and through it reduces QoL. The effect of depression is mediated by the coping mechanism of positive affect. The development of any new scale requires multiple studies to fully understand its properties. Studies in different populations (e.g., pre- and early-dialysis patients) will also add to the construct validity.
Disclosures
None.
Acknowledgments
The results of this paper were presented at the American Society of Nephrology 40th Annual Scientific Conference, San Francisco, California, 2007 and the 43rd Annual Scientific Meeting of the Australian and New Zealand Society of Nephrology, Gold Coast, Australia, 2007. The abstract of this paper has been published in Nephrology (Chan, R, Brooks, R, Erlich, J, Chow, J, Suranyi, M: The role of kidney disease related loss in dialysis patients’ quality of life: depression and positive affect as mediating factors [Abstract], Nephrology 12[Suppl 2]: A6, 2007). The authors thank all staff and patients at both participating hospitals for their support and Ms. Ruth Orchison for reviewing this paper.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Kimmel PL, Weihs K, Peterson RA: Survival in hemodialysis patients: The role of depression. J Am Soc Nephrol 4: 12–27, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Kimmel PL: Psychosocial factors in adult end-stage renal disease patients treated with hemodialysis: Correlates and outcomes. Am J Kidney Dis 35: S132–S140, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Wright S, Kirby A: Deconstructing conceptualizations of ′Adjustment’ to chronic illness: A proposed integrative framework. J Health Psych 4: 259–272, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Israel M: Depression in dialysis patients: A review of psychological factors. Can J Psychiatry 31: 445–451, 1986 [DOI] [PubMed] [Google Scholar]
- 5.Harvey JH, Miller ED: Toward a psychology of loss. Psychol Sci 9: 429–434, 1998 [Google Scholar]
- 6.Niemeier JP, Kennedy RE, McKinley WO, Cifu DX: The Loss Inventory: Preliminary reliability and validity data for a new measure of emotional and cognitive responses to disability. Disabil Rehabil 26: 614–623, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Prigerson HG, Bierhals AJ, Kasl SV, Reynolds CF III, Shear MK, Newsom JT, Jacobs S: Complicated grief as a disorder distinct from bereavement-related depression and anxiety: A replication study. Am J Psychiatry 153: 1484–1486, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Boelen PA, van den Bout J: Complicated grief, depression, and anxiety as distinct postloss syndromes: A confirmatory factor analysis study. Am J Psychiatry 162: 2175–2177, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Prigerson HG, Frank E, Kasl SV, Reynolds CF III, Anderson B, Zubenko GS, Houck PR, George CJ, Kupfer DJ: Complicated grief and bereavement-related depression as distinct disorders: Preliminary empirical validation in elderly bereaved spouses. Am J Psychiatry 152: 22–30, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Ogrodniczuk JS, Piper WE, Joyce AS, Weideman R, McCallum M, Azim HR, Rosie JS: Differentiating symptoms of complicated grief and depression among psychiatric outpatients. Can J Psychiatry 48: 87–93, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Prigerson HG, Maciejewski PK, Reynolds CF III, Bierhals AJ, Newsom JT, Fasiczka A, Frank E, Doman J, Miller M: Inventory of Complicated Grief: A scale to measure maladaptive symptoms of loss. Psychiatry Res 59: 65–79, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Thomsen DK: The association between rumination and negative affect: A review. Cogn Emot 20: 1216–1235, 2006 [Google Scholar]
- 13.Ito T, Takenaka K, Tomita T, Agari I: Comparison of ruminative responses with negative rumination as a vulnerability factor for depression. Psychol Rep 99: 763–772, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Berlim MT, Mattevi BS, Duarte APG, Thome FS, Barros EJG, Fleck MP: Quality of life and depressive symptoms in patients with major depression and end-stage renal disease: A matched-pair study. J Psychosom Res 61: 731–734, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Vazquez I, Valderrabano F, Fort J, Jofre R, Lopez-Gomez JM,, Moreno F, Sanz-Guajardo D, Spanish Cooperative Renal Patients Quality of Life Study Group: Psychosocial factors and health-related quality of life in hemodialysis patients. QualLife Res 14: 179–190, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Folkman S, Moskowitz JT: Positive affect and the other side of coping. Am Psychol 55: 647–654, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Folkman S, Moskowitz JT: Coping: Pitfalls and promise. Ann Rev Psychol 55: 745–774, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Brooks R: Health related quality of life of intensive care patients: Development of the Sydney quality of life questionnaire. School of Community Medicine. Sydney, University of New South Wales, 1999
- 19.Martin CR, Thompson DR: Prediction of quality of life in patients with end-stage renal disease. Brit J Health Psychol 5: 41–55, 2000 [Google Scholar]
- 20.Steele TE, Baltimore D, Finkelstein SH, Juergensen PPA, Kliger AS, Finkelstein FO: Quality of life in peritoneal dialysis patients. J Nerv Ment Dis 184: 368–374, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Bommer J, Muller-Buhl E, Ritz E, Eifert J: Recombinant human erythropoietin in anaemic patients on haemodialysis. Lancet 1: 392, 1987 [DOI] [PubMed] [Google Scholar]
- 22.Evans RW, Rader B, Manninen DL: The quality of life of hemodialysis recipients treated with recombinant human erythropoietin. J Am Med Assoc 263: 825–830, 1990 [PubMed] [Google Scholar]
- 23.Winearls CG, Oliver DO, Pippard MJ, Reid C, Downing MR, Cotes PM: Effect of human erythropoietin derived from recombinant DNA on the anaemia of patients maintained by chronic haemodialysis. Lancet 2: 1175–1178, 1986 [DOI] [PubMed] [Google Scholar]
- 24.O'Boyle CA, McGee HM, Hickey A, Joyce CRB, Browne J, O'Malley K, Hiltbrunner B: The Schedule for the Evaluation of Individual Quality of Life (SEIQoL): a direct weighting procedure for quality of life domains (SEIQoL-DW) administration manual. Dublin, Department of Psychology, Royal College of Surgeons in Ireland, 1993
- 25.Lovibond PF, Lovibond SH: The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther 33: 335–343, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Friedman EA: Diabetic nephropathy. In: Therapy of Renal Diseases and Related Disorders, 2nd Ed., edited by Suki WN, Massry SG. Boston, Kluwer Academic Publishers, 1991, pp 533–542
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 28.Kalantar-Zadeh K, Unruh M: Health related quality of life in patients with chronic kidney disease. Int Urol Nephrol 37: 367–378, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Addington-Hall J, Kalra L: Who should measure quality of life? BMJ 322: 1417–1420, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tovbin D, Gidron Y, Jean T, Granovsky R, Schnieder A: Relative importance and interrelations between psychosocial factors and individualized quality of life of hemodialysis patients. Qual Life Res 12: 709–717, 2003 [DOI] [PubMed] [Google Scholar]
- 31.O'Boyle CA: Assessment of quality of life in surgery. Br J Surg 79: 395–398, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Joyce CRB, Hickey A, McGee HM, O'Boyle CA: A theory-based method for the evaluation of individual quality of life: The SEIQoL. Qual Life Res 12: 275–280, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Hickey AM, Bury G, O'Boyle CA, Bradley F, O'Kelly FD, Shannon W: A new short form individual quality of life measure (SEIQoL-DW): Application in a cohort of individuals with HIV/AIDS. BMJ 313: 29–33, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimmel PL, Peterson RA: Depression in end-stage renal disease patients treated with hemodialysis: Tools, correlates, outcomes, and needs. Semin Dial 18: 91–97, 2005 [DOI] [PubMed] [Google Scholar]
- 35.O'Donnell K, Chung JY: The diagnosis of major depression in end-stage renal disease. Psychother Psychosom 66: 38–43, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Clara IP, Cox BJ, Enns MW: Confirmatory factor analysis of the depression-anxiety-stress scales in depressed and anxious patients. J Psychopathol Behav Assess 23: 61–67, 2001 [Google Scholar]
- 37.Antony MM, Bieling PJ, Cox BJ, Enns MW, Swinson RP: Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychol Assess 10: 176–181, 1998 [Google Scholar]
- 38.SPSS for Windows: SPSS for Windows, 15th Ed., Chicago, SPSS Inc., 2006
- 39.Joreskog K, Sorbom D: LISREL 8.72: User's Reference Guide. Chicago, Scientific Software International, 2004
- 40.Kline RB: Principles and Practice of Structural Equation Modeling. New York, The Guilford Press, 2005
- 41.Anderson JC, Gerbing DW: Structural equation modeling in practice: A review and recommended two-step approach. Psychol Bull 103: 411–423, 1988 [Google Scholar]
- 42.Comrey AL: Factor-analytic methods of scale development in personality and clinical psychology. J Consult Clin Psychol 56: 754–761, 1988 [DOI] [PubMed] [Google Scholar]
- 43.Munck IME: Model building in comparative education: Applications of the LISREL method to cross-national survey data.: International Association for the Evaluation of Education Achievement Monograph Series No 10. Stockholm, Almqvist & Wiksell, 1979
- 44.Satorra A, Bentler PM: Corrections to test statistics and standard errors in covariance structure analysis. In: Latent Variable Analysis: Applications for Developmental Research, edited by von Eye A, Clogg CC, Thousand Oaks CA, Sage, 1994, pp 399–419
- 45.Chou C-P, Bentler PM: Estimates and tests in structural equation modeling. In: Structural Equation Modeling: Concepts, Issues, and Applications, edited by Hoyle RH, Thousand Oaks CA, Sage Publications, Inc., 1995, pp 37–55
- 46.Chou CP, Bentler PM, Satorra A: Scaled test statistics and robust standard errors for non-normal data in covariance structure analysis: A Monte Carlo study. Br J Math StatPsychol 44: 347–357, 1991 [DOI] [PubMed] [Google Scholar]
- 47.Worthington RL, Whittaker TA: Scale development research: A content analysis and recommendations for best practices. Couns Psychol 34: 806–838, 2006 [Google Scholar]
- 48.Gerbing DW, Ahadi SA, Patton JH: Toward a conceptualization of impulsivity: Components across the behavioral and self-report domains. Multivariate Behav Res 22: 357–379, 1987 [DOI] [PubMed] [Google Scholar]
- 49.Beusterien KM, Nissenson AR, Port FK, Kelly M, Steinwald B, Ware JE Jr: The effects of recombinant human erythropoietin on functional health and well-being in chronic dialysis patients. J Am Soc Nephrol 7: 763–773, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Brenner MH, Curbow B, Legro MW: The proximal-distal continuum of multiple health outcome measures: The case of cataract surgery. Med Care 33: AS236–AS244, 1995 [PubMed] [Google Scholar]