Abstract
Background and objectives: Lanthanum carbonate (FOSRENOL®, Shire Pharmaceuticals) is an effective noncalcium, nonresin phosphate binder for the control of hyperphosphatemia in chronic kidney disease (CKD) stage 5 patients undergoing dialysis.
Design, setting, participants and measurements: A Phase 2, randomized, double-blind, placebo-controlled trial evaluating the efficacy and safety of lanthanum carbonate in CKD stage 3 and 4 patients. Of 281 patients screened, 121 were randomized (2:1) to lanthanum carbonate or placebo (80 versus 41). The modified intent-to-treat population included 90 patients (56 versus 34); 71 (43 versus 28) completed the study. After run-in, when any current phosphate binders were discontinued and dietary counseling reinforced, patients with serum phosphorus >4.6 mg/dl received lanthanum carbonate (titrated up to 3000 mg/d) or matching placebo for 8 wk.
Results: At the end of treatment, 25 (44.6%) versus nine (26.5%) patients had serum phosphorus ≤4.6 mg/dl (difference 18.1%, P = 0.12) in the lanthanum carbonate and placebo groups, respectively. Statistically significant differences were observed between groups in change from baseline to end of treatment for serum phosphorus (P = 0.02), intact parathyroid hormone (P = 0.02), and urinary phosphorus excretion (P = 0.04). The safety profile and tolerability of lanthanum carbonate were similar to that of placebo.
Conclusions: Because <1% of phosphorus is in the extracellular fluid, serum measurements may not accurately reflect total body burden in patients with CKD stages 3 and 4. However, lanthanum carbonate is an effective phosphate binder in this patient population, with a safety profile and tolerability similar to that of placebo.
The leading cause of death in patients with chronic kidney disease (CKD) is cardiovascular disease (accounting for approximately 50% of deaths) (1). Even patients with mild renal insufficiency demonstrate a prevalence of cardiovascular disease that is many times higher than that of the general population (2–4). Vascular calcification may contribute to cardiovascular mortality and is often present before patients proceed to dialysis, particularly in those with diabetes (5–7). Evidence suggests that hyperphosphatemia is actively involved in the pathogenesis of vascular calcification (8–10). In addition, evidence associating elevated serum phosphorus with morbidity and mortality is strong, including several large observational studies in patients with CKD who had not yet required dialysis (4,11–13). Kidney Disease Outcomes Quality Initiative guidelines recommend that serum phosphorus should be maintained within the reference range in patients with CKD stages 3 and 4 (14). Patients with CKD stages 3 and 4 who have elevated serum phosphorus despite dietary intervention may benefit from phosphate-binder therapy.
Lanthanum carbonate (FOSRENOL®, Shire Pharmaceuticals, Basingstoke, United Kingdom) is an effective noncalcium, nonresin phosphate binder for the control of hyperphosphatemia in patients with CKD stage 5 undergoing dialysis. Both short- and long-term clinical studies have demonstrated efficacy and an acceptable safety profile (15–18). This study was designed to evaluate the efficacy and safety of lanthanum carbonate for use in patients with CKD stages 3 and 4, a population in which randomized, controlled clinical trials of phosphate binders are sparse (19).
Materials and Methods
Study Design
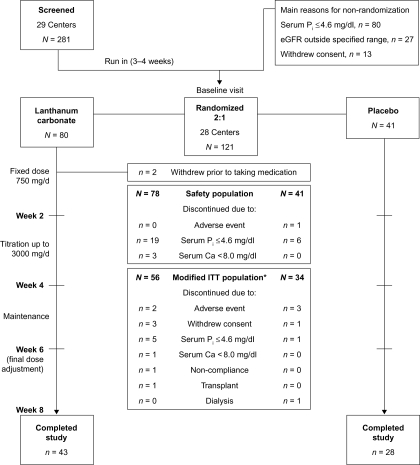
This was a randomized, double-blind, placebo-controlled trial conducted in the United States (clinicaltrials.gov identifier: NCT00234702). The study was conducted in accordance with the Declaration of Helsinki, and informed consent was obtained from all patients. The study design is shown in Figure 1. After being screened for eligibility, patients discontinued their current phosphate-binder therapy and entered a 3- to 4-wk run-in period (treatment-naïve patients were also included), during which dietary phosphorus counseling was reinforced and serum phosphorus concentrations were assessed.
Figure 1.
Study design and patient disposition. *Patients with serum phosphorus ≤4.6 mg/dl (1.49 mmol/L) or serum calcium <8.0 mg/dl (2.0 mmol/L) at the baseline visit were withdrawn from the study and excluded from the modified intent-to-treat (ITT) population. Serum Pi, serum phosphorus. To convert mg/dl to mmol/L, multiply by 0.323. Serum Ca, serum calcium. To convert mg/dl to mmol/L, multiply by 0.25. eGFR, estimated glomerular filtration rate.
Patients with serum phosphorus concentrations >4.6 mg/dl (1.49 mmol/L) after 2 to 3 wk of run-in were randomized 1 wk later to receive lanthanum carbonate or matching placebo (2:1 ratio). Patients with serum calcium concentrations <8.0 mg/dl (2.0 mmol/L) at the baseline visit were to be withdrawn from the study.
Study treatment was initiated at a dose of 750 mg/d; one tablet (for lanthanum carbonate, containing 250 mg elemental lanthanum) taken three times daily during or immediately after meals for 2 wk (weeks 1–2). The dose could then be titrated weekly (weeks 3–4) to a maximum of 3000 mg/d (two 500 mg tablets, taken three times daily) to achieve a target serum phosphorus level of <4.0 mg/dl (1.29 mmol/L). Patients continued to take the final titrated dose for an additional 4 wk (weeks 5–8). At week 6, the dose could be adjusted if serum phosphorus concentrations had increased to ≥4.0 mg/dl or decreased to <2.7 mg/dl (0.87 mmol/L).
Patients
Inclusion criteria included: ≥18 yr of age; an estimated glomerular filtration rate (eGFR) of 15 to 59 ml/min/1.73 m2 at screening; undergoing physician care for CKD for >2 mo; not expected to begin dialysis for ≥4 mo. Exclusion criteria included: requirement for treatment with cinacalcet HCl or compounds containing phosphorus, aluminum, magnesium, or calcium (calcium supplements were permitted); acute renal failure within 12 wk of screening; rapidly progressing glomerulonephritis; significant gastrointestinal (GI) surgery or disorders; and evidence of clinically significant liver disease (including alanine transaminase or aspartate transaminase > two times the upper limit of normal or bilirubin > one and a half times the upper limit of normal). Pregnant or lactating women and women of reproductive potential who did not agree to use effective contraception were also excluded.
Patients receiving a stable regimen (≥4 wk) of vitamin D compounds before screening could continue treatment during the study. Treatment could not be initiated during the study, and the dose could not be increased, but it could be decreased if a patient experienced hypercalcemia. Patients receiving calcium supplements for hypocalcemia could continue to receive a stable dose during the study, provided that the total daily elemental calcium dose did not exceed 1000 mg. Supplementation also could not be initiated during the study, and the dose could not be increased, but it could be decreased if a patient experienced hypercalcemia.
Assessments
The safety population comprised all patients who received at least one dose of lanthanum carbonate or placebo and had at least one safety measurement. The modified intent-to-treat (mITT) population comprised all patients who received at least one dose of the study drug and had at least one postdose serum phosphorus measurement.
The study's primary endpoint was the percentage of patients who had serum phosphorus concentrations ≤4.6 mg/dl after 8 wk of lanthanum carbonate or placebo treatment. Eighty-four patients (2:1 randomization) were required to complete the study to give 80% power (at α = 0.05) for a χ2 (two-sided) test for proportions, given the assumptions of 23% for placebo and 57% for lanthanum carbonate (34% difference).
Secondary endpoints included changes in serum phosphorus, intact parathyroid hormone (iPTH) and calcium–phosphorus product (Ca × P product) from baseline, and safety and tolerability, compared with placebo.
Biochemical and hematologic parameters were measured. Serum iPTH was measured using the Immulite 2000 immunoassay (Diagnostic Products Corporation, Los Angeles, CA). Urine specimens were collected at baseline, week 4, and week 8. Adverse events (AEs) and serious adverse events (SAEs) were recorded.
Statistics
For the primary efficacy analysis, the percentages of patients in each treatment group (mITT population) with phosphorus concentrations ≤4.6 mg/dl were compared using Fisher's exact test. For patients who discontinued treatment before the end of the study, the last available (i.e., at end of treatment) serum phosphorus value was used in the analysis. Observed-case data with 95% confidence intervals (CI) are presented along with end of treatment data in Figure 2.
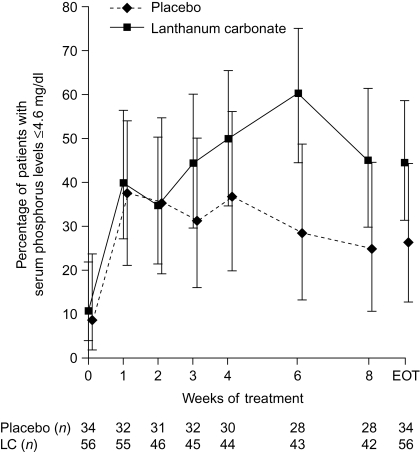
Figure 2.
Percentage of patients (95% CI) achieving serum phosphorus concentrations ≤4.6 mg/dl during 8 wk of double-blind treatment with lanthanum carbonate (LC) or placebo. Observed-case and end of treatment (EOT) data are shown. CI, confidence intervals. Nine patients had serum phosphorus concentrations ≤4.6 mg/dl (1.49 mmol/L) at baseline. Six of these patients (five lanthanum carbonate and one placebo) failed the baseline visit criteria of serum phosphorus (>4.6 mg/dl) but had a postdose efficacy assessment and were therefore included in the modified intent-to-treat population. Another three patients (one lanthanum carbonate and two placebo) failed the baseline visit criteria of serum phosphorus (>4.6 mg/dl) but were granted waivers to continue in the study.
Absolute changes in serum phosphorus concentrations from baseline to each visit were analyzed using an analysis of covariance (ANCOVA) model with treatment as a factor and baseline assessment as a covariate. Again, for patients who discontinued treatment before the end of the study, the last available serum phosphorus value was used in the analyses. Data are reported as mean ± standard error of the mean (SEM), although where statistical comparisons between groups are presented, data refer to the least squares mean ± SEM. Similar analyses were undertaken for other serum and urinary parameters. Observed-case data are presented along with end of treatment data in Figures 3–5.
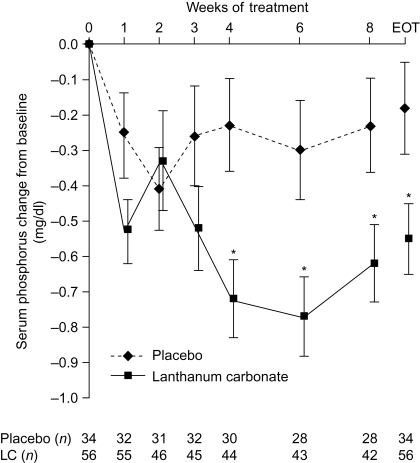
Figure 3.
Change from baseline in serum phosphorus concentrations during 8 wk of lanthanum carbonate (LC) or placebo treatment. Data are least squares mean ± SEM. Observed-case and end-of-treatment (EOT) data are shown. *P < 0.05 between treatments, analysis of covariance (ANCOVA) model. To convert mg/dl to mmol/L, multiply by 0.323.
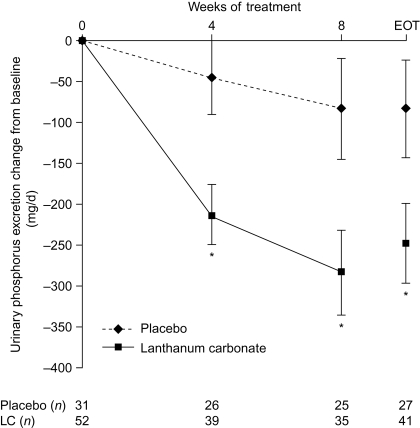
Figure 5.
Change from baseline in urinary phosphorus excretion during 8 wk of lanthanum carbonate (LC) or placebo treatment. Data are least squares mean ± SEM. Observed-case and end-of- treatment (EOT) data are shown. *P < 0.05 between treatments, analysis of covariance (ANCOVA) model. To convert mg/d to mmol/d, divide by 31.
Results
Patient Demographics
After screening, 121 patients met the entry criteria and were randomized (80 lanthanum carbonate, 41 placebo, Figure 1). The baseline characteristics of the safety population (N = 119) are shown in Table 1. Treatment groups were well matched. Twenty-nine patients were excluded from the mITT population because they did not have a postbaseline serum phosphorus value. The baseline characteristics of the mITT population (N = 90; 56 lanthanum carbonate, 34 placebo) were similar to those of the safety population. Seventy-one patients (58.7%) completed the study.
Table 1.
Baseline characteristics of the safety population
| Characteristic | Lanthanum carbonate (N = 78) | Placebo (N = 41) | P |
|---|---|---|---|
| Age (years) | 0.6557b | ||
| mean (SD) | 61.8 (12.9) | 63.0 (12.7) | |
| median | 63.0 | 61.0 | |
| range | 29–87 | 41–93 | |
| Sex, n (%) | 1.0000c | ||
| male | 40 (51.3) | 21 (51.2) | |
| female | 38 (48.7) | 20 (48.8) | |
| Race, n (%) | 0.8077c | ||
| white | 59 (75.6) | 33 (80.5) | |
| black or African American | 15 (19.2) | 7 (17.1) | |
| other | 4 (5.1) | 1 (2.4) | |
| Primary diagnosis, n (%) | 0.1905c | ||
| diabetes | 45 (57.7) | 24 (58.5) | |
| hypertension | 21 (26.9) | 9 (22.0) | |
| glomerulonephritis | 4 (5.1) | 1 (2.4) | |
| cystic kidney disease | 4 (5.1) | 0 (0.0) | |
| urologic disease | 0 (0.0) | 1 (2.4) | |
| other known causesa | 4 (5.1) | 6 (14.6) |
Other known causes included chronic reflux uropathy, systemic lupus erythematosus, 1-GA nephropathy and previous long-term lithium therapy in the lanthanum carbonate group, and systemic lupus erythematosus, nonsteroidal anti-inflammatory use, transplant, nephrosclerosis, membranous glomerulonephropathy and reflux nephropathy (FSGS 2 h/d) in the placebo group.
t test (lanthanum carbonate versus placebo);
Fisher's exact test (lanthanum carbonate versus placebo).
Mean eGFR values for the mITT population are shown in Table 2. When categorized by CKD stage, 75.0% and 79.4% of patients in the lanthanum carbonate and placebo groups, respectively, were classified as CKD stage 4 at screening. The difference between groups in the decrease in mean eGFR from screening to the end of treatment was not significantly different (P = 0.93). The percentage of patients who were naïve to phosphate-binder therapy was similar across groups (78.6% lanthanum carbonate, 79.4% placebo).
Table 2.
Chronic kidney disease status and prior calcium and vitamin D use in the modified intent-to-treat population
| Characteristic | Lanthanum carbonate (N = 56) | Placebo (N = 34) | P |
|---|---|---|---|
| Mean eGFR (ml/min/1.73 m2) | |||
| screening | 22.7 ± 0.9 | 24.0 ± 1.9 | 0.5225a |
| end of treatment | 21.4 ± 0.6 | 21.3 ± 0.8 | 0.9320b |
| KDOQI CKD classification (eGFR | 0.9305c | ||
| ml/min/1.73 m2), n (%) | |||
| stage 3 (30–59) | 9 (16.1) | 5 (14.7) | |
| stage 4 (15–29) | 42 (75.0) | 27 (79.4) | |
| stage 5 (<15) | 5 (8.9) | 2 (5.9) | |
| Prior calcium therapy, n (%) | |||
| overall | 6 (10.7) | 1 (2.9) | 0.2469c |
| calcium carbonate | 6 (10.7) | 1 (2.9) | |
| Prior vitamin D therapy, n (%) | |||
| overall | 17 (30.4) | 16 (47.1) | 0.1214c |
| calcitriol | 4 (7.1) | 5 (14.7) | |
| doxercalciferol | 4 (7.1) | 2 (5.9) | |
| ergocalciferol | 6 (10.7) | 7 (20.6) | |
| paricalcitol | 3 (5.4) | 2 (5.9) |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; KDOQI, Kidney Disease Outcomes Quality Initiative.
t test (lanthanum carbonate versus placebo);
ANCOVA model;
Fisher's exact test (lanthanum carbonate versus placebo).
Before entering the study, a greater proportion of patients were receiving supplemental calcium carbonate in the lanthanum carbonate group than in the placebo group (10.7% versus 2.9%; Table 2). During the study, 9/56 patients (16.1%) received concomitant calcium treatment in the lanthanum carbonate group. Of these, seven received calcium carbonate as a supplement, and three took calcium-based phosphate binders. In the placebo group, 3/34 patients (8.8%) received concomitant calcium treatment. Two patients took supplemental calcium carbonate, and one received calcium as a phosphate binder.
Before entering the study, a smaller proportion of patients were receiving vitamin D compounds in the lanthanum carbonate group than in the placebo group (30.4% versus 47.1%; Table 2). One patient in each group discontinued vitamin D use before the first dose of study drug, whereas in the placebo group, one patient increased their daily dose during the study.
At week 4, the majority of patients were receiving 2250 mg/d of lanthanum carbonate or matching placebo (62.8% and 71.9%, respectively); mean doses were 1930.2 ± 71.4 mg/d and 2085.9 ± 82.7 mg/d, respectively. At week 8, the majority of patients were receiving the maximum 3000 mg/d of lanthanum carbonate or matching placebo (74.4% and 85.7%, respectively); mean doses were 2645.3 ± 96.9 mg/d and 2785.7 ± 120.1 mg/d, respectively. Mean length of drug exposure was similar across groups (45.1 ± 2.6 d for lanthanum carbonate, 50.1 ± 2.2 d for placebo); at least 75% of patients in each group completed 6 wk of treatment. Compliance to treatment was >80% in both groups.
Efficacy
At baseline, mean serum phosphorus concentrations (mITT population) were similar in the lanthanum carbonate and placebo groups (5.28 ± 0.09 mg/dl [1.71 ± 0.03 mmol/L] versus 5.38 ± 0.12 mg/dl [1.74 ± 0.04 mmol/L]). At the end of treatment, 44.6% of patients in the lanthanum carbonate group and 26.5% in the placebo group had serum phosphorus concentrations ≤4.6 mg/dl; the difference between groups (18.1%) was not statistically significant (P = 0.12). The percentage of patients with serum phosphorus ≤4.6 mg/dl is shown by week in Figure 2. At the end of treatment, mean serum phosphorus concentrations had decreased from baseline by 0.55 ± 0.10 mg/dl (0.18 ± 0.03 mmol/L) and 0.18 ± 0.13 mg/dl (0.06 ± 0.04 mmol/L) in the lanthanum carbonate and placebo groups, respectively (P = 0.02 for difference between groups).
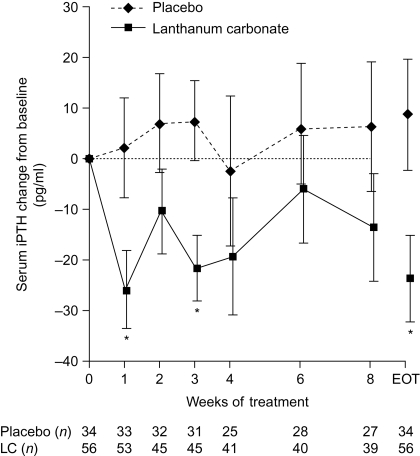
At baseline, mean serum iPTH concentrations were similar in the lanthanum carbonate and placebo groups (183.5 ± 19.5 pg/ml versus 179.3 ± 24.4 pg/ml). Changes from baseline are shown in Figure 4. At the end of treatment, the mean serum iPTH level had decreased by 23.8 ± 8.6 pg/ml in the lanthanum carbonate group and had increased by 8.8 ± 11.0 pg/ml in the placebo group (P = 0.02 for difference between groups).
Figure 4.
Change from baseline in intact parathyroid hormone (iPTH) concentrations during 8 wk of lanthanum carbonate (LC) or placebo treatment. Data are least squares mean ± SEM. Observed-case and end-of-treatment (EOT) data are shown. *P < 0.05 between treatments, analysis of covariance (ANCOVA) model. To convert pg/ml to ng/L, multiply by 1.0.
At baseline, mean serum calcium concentrations were similar in the lanthanum carbonate and placebo groups (8.86 ± 0.07 mg/dl [2.22 ± 0.02 mmol/L] versus 8.97 ± 0.09 mg/dl [2.24 ± 0.02 mmol/L]). From baseline to the end of treatment, there was a slight increase in mean serum calcium in the lanthanum carbonate group (0.12 ± 0.05 mg/dl [0.03 ± 0.01 mmol/L]), and a slight decrease in the placebo group (−0.09 ± 0.07 mg/dl [−0.02 ± 0.02 mmol/L]; P = 0.02 for difference between groups).
Mean Ca × P product decreased slightly from baseline in both the lanthanum carbonate and placebo groups. At the end of treatment, the difference in reduction from baseline between groups was not statistically significant.
At baseline, mean 24-h urinary excretion of phosphorus was similar in the lanthanum carbonate and placebo groups (836.35 ± 60.19 mg/d [26.98 ± 1.94 mmol/d] versus 783.58 ± 68.48 mg/d [25.28 ± 2.21 mmol/d]), and concentrations decreased only slightly in the placebo group during the study (Figure 5). In contrast, at the end of treatment, urinary phosphorus excretion had decreased by 247.70 ± 48.46 mg/d (7.99 ± 1.56 mmol/d) in the lanthanum carbonate group; the difference compared with the placebo group was statistically significant (P = 0.04).
Safety and Tolerability
AEs were experienced by 47.4% of patients in the lanthanum carbonate group compared with 61.0% in the placebo group. These were mainly gastrointestinal in nature, with nausea (lanthanum carbonate and placebo: 9.0% and 9.8%, respectively) and vomiting (6.4% and 2.4%, respectively) being the most common. In total, 19.3% of AEs experienced in the lanthanum carbonate group were considered related to treatment, compared with 16.7% in the placebo group.
Twelve treatment-emergent SAEs were experienced by seven patients in the lanthanum carbonate group. Acute pulmonary edema, exacerbated dyspnea, and myocardial infarction were experienced by one patient; anemia, congestive cardiac failure, and catheter-site pain were experienced by another. An additional patient experienced anemia and exacerbation of congestive cardiac failure, and the other four patients experienced single SAEs (bacterial arthritis, impaired gastric emptying, pneumothorax, and perinephric abscess). None of the SAEs were suspected to be related to treatment. In the placebo group, three SAEs were experienced by two patients (respiratory failure following chronic obstructive pulmonary disease in one patient, pneumonia in the other).
Two patients treated with lanthanum carbonate and four patients treated with placebo experienced AEs resulting in discontinuation of study participation. In the lanthanum carbonate group, nausea, loss of appetite, low-grade fever, and increased urinary frequency were experienced by one patient and were not suspected to be associated with treatment, whereas mild abdominal itching was suspected to be related to treatment in the other patient.
No clinically important differences were observed between groups with respect to mean concentrations of 1,25-dihydroxy vitamin D3 and 25-hydroxy vitamin D, or other laboratory parameters and vital signs, either at baseline or during treatment. At the end of treatment, the mean plasma lanthanum concentration of the lanthanum carbonate group (0.37 ± 0.05 ng/ml) was within the range observed in previous trials of similar duration involving patients with CKD stage 5 (20).
Discussion
In patients with CKD stages 3 and 4, lanthanum carbonate treatment resulted in a reduction in the mean serum phosphorus level (a difference of 0.37 mg/dl [0.12 mmol/L] compared with placebo, P = 0.02). A greater percentage of patients achieved the target serum phosphorus level of ≤4.6 mg/dl in the lanthanum carbonate group compared with placebo (44.6% versus 26.5%); however, this difference was not statistically significant.
The primary pharmacologic effect of lanthanum carbonate is to form insoluble complexes with dietary phosphorus within the GI tract (21). Therefore, the ability of lanthanum carbonate to bind dietary phosphorus is independent of CKD stage and dialysis status. In patients not on dialysis, serum phosphorus concentrations are influenced by residual renal function, dietary phosphorus burden, blood pH, vitamin D status, degree of hyperparathyroidism, the responsiveness of the skeleton to parathyroid hormone (PTH) (22), and the time after consumption at which phosphorus concentrations are assessed.
A 70-kg man has a total body phosphorus content of approximately 700 g. Approximately 85% is in the skeleton, and approximately 15% can be found in soft tissues (23). Only about 1% is present in the extracellular fluid. Patients with CKD stages 3 and 4 have a positive phosphorus balance; it is not known to what extent serum phosphorus concentrations accurately reflect total body phosphorus burden in this population. Dietary phosphorus restriction has been shown to prevent secondary hyperparathyroidism in patients with renal failure; experimental studies have demonstrated that this effect is independent of serum calcium and 1,25-dihydroxy vitamin D3 (24). The biologic effects of a reduction in phosphorus burden should also be considered in patients with CKD stages 3 and 4.
Unlike patients with CKD stage 5 undergoing dialysis, patients with CKD stages 3 and 4 maintain urine output and the ability to excrete a proportion of any phosphorus that is absorbed. In the “steady-state” condition, the amount excreted in the urine is proportional to the amount absorbed. As a result, measurement of urinary phosphorus excretion can be used as a marker of intestinal phosphorus absorption, and thus as an indicator of phosphorus-binding efficacy (19,25). Lanthanum carbonate treatment resulted in a substantial decrease in intestinal phosphorus absorption as demonstrated by the reduction in urinary phosphorus excretion compared with placebo (P = 0.04). Studies in healthy volunteers and patients with CKD stages 3 and 4 (with known dietary phosphorus intake) indicate that up to 75% of dietary phosphorus is absorbed (19,25). Thus with a daily phosphorus intake of 1200 mg, intestinal absorption may approach 900 mg (25). The decrease in urinary phosphorus excretion (approximately 300 mg) observed with <3000 mg/d lanthanum carbonate is equivalent to about a third of daily phosphorus absorption. This result confirms that lanthanum carbonate is an effective dietary phosphate binder in patients with CKD stages 3 and 4. Future studies should include estimates of dietary phosphorus intake and fasting serum phosphorus concentrations in addition to urinary phosphorus excretion.
Although there were differences in prior use of calcium and vitamin D compounds between treatment groups at the start of the study, these were controlled during the study and were not considered to be clinically significant. The decrease from baseline in mean iPTH observed in the lanthanum carbonate group was statistically significant (P = 0.02) compared with placebo. The slight increase in serum calcium observed with lanthanum carbonate treatment may be due to phosphorus reduction raising calcium levels via an increase in the calcemic action of PTH. In future studies designed to investigate the efficacy of phosphate binders, measurement and interpretation of these multiple markers of mineral metabolism, along with serum phosphorus and urinary phosphorus excretion, may be a more appropriate main efficacy variable than evaluation of serum phosphorus in isolation.
Analysis of 1,25-dihydroxy vitamin D3 and 25-hydroxy vitamin D concentrations revealed no differences between lanthanum carbonate and placebo groups. A further post hoc analysis in patients who received prior vitamin D therapy (data not shown) revealed that lanthanum carbonate did not reduce vitamin D concentrations in these patients, suggesting that it does not interfere with absorption of vitamin D. This is consistent with results from studies in patients with CKD stage 5 undergoing dialysis (21).
A study including patients with CKD and stable serum phosphorus concentrations treated with a low-phosphorus diet (<800 mg/d) and calcium carbonate (2000 mg/d) or sevelamer hydrochloride (1600 mg/d) showed that despite small but significant decreases in urinary phosphorus excretion (approximately 80 mg/d), neither binder had reduced serum phosphorus concentrations after an average of 2 yr of treatment (19). Notably, the decrease in phosphorus burden by a combination of dietary phosphorus restriction and reduced intestinal absorption was associated with a reduction in the progression of coronary artery calcification.
In this short-term study, the safety profile and tolerability of lanthanum carbonate was similar to that of placebo. When considering earlier intervention in patients with CKD stages 3 and 4, the long-term safety profile of a phosphate binder should also be considered. Data from animal studies (26,27) have demonstrated lanthanum deposition in bone and increased levels in the liver; the latter is consistent with its hepatic route of excretion (28,29). In patients with CKD stage 5 undergoing dialysis, data on the safety of lanthanum carbonate are published for up to 6 yr of treatment (18). In this long-term follow-up study, lanthanum carbonate was not associated with organ-specific toxicity in liver or bone. In addition, a detailed assessment over 2 yr showed no difference in changes in cognitive function between patients receiving lanthanum carbonate or alternative phosphate binders (30), providing clinical confirmation of animal experiments demonstrating that lanthanum does not cross the blood–brain barrier (31).
Results from this study also suggest that achieving target serum phosphorus concentrations may not accurately reflect changes in phosphorus load produced by phosphate-binder therapy in predialysis patients with CKD. Serum phosphorus levels should not be considered in isolation, but with other markers of disordered mineral metabolism. This study further demonstrates the utility of measuring urinary phosphorus excretion (19).
Overall, this study demonstrated that lanthanum carbonate is an effective phosphate binder in patients with CKD stages 3 and 4, with a safety profile and tolerability similar to that of placebo. It offers a potential treatment option for preventing phosphorus accumulation in this patient population. Further studies are required to assess the potential long-term benefits of earlier intervention with phosphate binders in patients with CKD.
Disclosures
This study was funded by Shire Pharmaceuticals. Shire Pharmaceuticals also provided assistance with analysis and interpretation of the data. Stuart Sprague, Hanna Abboud, and William Finn have received research funding from Shire Pharmaceuticals. Stuart Sprague and William Finn are consultants to Shire Pharmaceuticals. Ping Qiu, Matthew Dauphin, and Pinggao Zhang are employees of Shire Pharmaceuticals.
Acknowledgments
The following investigators contributed to the SPD405–206 study: Muralidhar Acharya, Hudson, FL; Michael Anger, Thornton, CO; M. Edwina Barnett, Torrance, CA; William Finn, Chapel Hill, NC; Marie Philipneri, St. Louis, MO; Leslie Steed, Portland, OR; Steven Zeig, Pembroke Pines, FL; Barton Levine, Los Angeles, CA; Wasae Tabibi, Houston, TX; Hanna Abboud, San Antonio, TX; Leland Garrett, Raleigh, NC; Harold Locay, Ocala, FL; N. Martin Lunde, Brooklyn Center, MN; Wolfgang Weise, Burlington, VT; Amy Barton Pai, Albuquerque, NM; John Robertson, Riverside, CA; Wei-Tzuoh Chen, Visalia, CA; Roger Haley, Visalia, CA; Aamir Jamal, Covina, CA; M. Francesca Egidi, Memphis, TN; John Reed, Columbus, MS; Mark Smith, Augusta, GA; Carlos Martinez, Macon, GA; Bruce Spinowitz, Flushing, NY; Arie Widerhorn, Long Beach, CA; Stuart Sprague, Evanston, IL; Greg Johnson, Fort Wayne, IN; Kerry Cooper, Phoenix, AZ.
We would also like to thank Oxford PharmaGenesis™ for providing editorial support in the development of this manuscript.
These data were presented in abstract form:
Sprague S, Finn WF, Abboud H et al. Am J Kidney Dis 2008; 51: A90
Sprague S, Finn WF, Abboud H et al. NDT Plus: 2008; 1(Suppl 2): ii58
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.US Renal Data System. Atlas of End-Stage Renal Disease in the United States. National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2000
- 2.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX: Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D: Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int 56: 2214–2219, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Russo D, Palmiero G, De Blasio AP, Balletta MM, Andreucci VE: Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis 44: 1024–1030, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Merjanian R, Budoff M, Adler S, Berman N, Mehrotra R: Coronary artery, aortic wall, and valvular calcification in nondialyzed individuals with type 2 diabetes and renal disease. Kidney Int 64: 263–271, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Mehrotra R, Budoff M, Christenson P, Ipp E, Takasu J, Gupta A, Norris K, Adler S: Determinants of coronary artery calcification in diabetics with and without nephropathy. Kidney Int 66: 2022–2031, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM: Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol 15: 2857–2867, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Nishizawa Y, Jono S, Ishimura E, Shioi A: Hyperphosphatemia and vascular calcification in end-stage renal disease. J Ren Nutr 15: 178–182, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Mathew S, Huskey M, Hruska KA: The role of hyperphosphataemia in vascular calcification (VC): Actions of lanthanum carbonate (LaCO3) [Abstract]. J Am Soc Nephrol 17: 357A, 2006 [Google Scholar]
- 11.Voormolen N, Noordzij M, Grootendorst DC, Beetz I, Sijpkens YW, van Manen JG, Boeschoten EW, Huisman RM, Krediet RT, Dekker FW: High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant 22: 2909–2916, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G: Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112: 2627–2633, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Eknoyan G, Levin A, Levin NW: Bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42: 1–201, 2003 [Google Scholar]
- 15.Chiang SS, Chen JB, Yang WC: Lanthanum carbonate (Fosrenol) efficacy and tolerability in the treatment of hyperphosphatemic patients with end-stage renal disease. Clin Nephrol 63: 461–470, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Hutchison AJ, Maes B, Vanwalleghem J, Asmus G, Mohamed E, Schmieder R, Backs W, Jamar R, Vosskuhler A: Long-term efficacy and tolerability of lanthanum carbonate: Results from a 3-year study. Nephron Clin Pract 102: c61–c71, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Finn WF: Lanthanum carbonate versus standard therapy for the treatment of hyperphosphatemia: Safety and efficacy in chronic maintenance hemodialysis patients. Clin Nephrol 65: 191–202, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Hutchison AJ, Barnett ME, Krause R, Kwan JTC, Siami GA. Long-term efficacy and safety profile of lanthanum carbonate: Results for up to 6 years of treatment. Nephron Clin Pract 110: c15–c23, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo D, Miranda I, Ruocco C, Battaglia Y, Buonanno E, Manzi S, Russo L, Scafarto A, Andreucci VE: The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int 72: 1255–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Finn WF, Joy MS, Hladik G: Efficacy and safety of lanthanum carbonate for reduction of serum phosphorus in patients with chronic renal failure receiving hemodialysis. Clin Nephrol 62: 193–201, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Shire Pharmaceutical Contracts Ltd: FOSRENOL - Summary of Product Characteristics, Hampshire, United Kingdom, Shire Pharmaceuticals, 2007
- 22.Levine MA: Normal mineral homeostasis. Interplay of parathyroid hormone and vitamin D. Endocr Dev 6: 14–33, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine: Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington DC, National Academy Press, 1997 [PubMed]
- 24.Slatopolsky E, Finch J, Denda M, Ritter C, Zhong M, Dusso A, MacDonald PN, Brown AJ: Phosphorus restriction prevents parathyroid gland growth. High phosphorus directly stimulates PTH secretion in vitro. J Clin Invest 97: 2534–2540, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burke SK, Slatopolsky EA, Goldberg DI: RenaGel, a novel calcium- and aluminium-free phosphate binder, inhibits phosphate absorption in normal volunteers. Nephrol Dial Transplant 12: 1640–1644, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Lacour B, Lucas A, Auchère D, Ruellan N, de Serre Patey NM, Drüeke TB: Chronic renal failure is associated with increased tissue deposition of lanthanum after 28-day oral administration. Kidney Int 67: 1062–1069, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Slatopolsky E, Liapis H, Finch J: Progressive accumulation of lanthanum in the liver of normal and uremic rats. Kidney Int 68: 2809–2813, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Damment SJ, Pennick M: Systemic lanthanum is excreted in the bile of rats. Toxicol Lett 171: 69–77, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Yang Z, Schryvers D, Roels F, D'Haese PC, De Broe ME: Demonstration of lanthanum in liver cells by energy-dispersive X-ray spectroscopy, electron energy loss spectroscopy and high-resolution transmission electron microscopy. J Microsc 223: 133–139, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Altmann P, Barnett ME, Finn WF: Cognitive function in Stage 5 chronic kidney disease patients on hemodialysis: No adverse effects of lanthanum carbonate compared with standard phosphate-binder therapy. Kidney Int 71: 252–259, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Ling EA: Studies of the ultrastructure and permeability of the blood-brain barrier in the developing corpus callosum in postnatal rat brain using electron dense tracers. J Anat 184: 227–237, 1994 [PMC free article] [PubMed] [Google Scholar]