Abstract
Background and objectives: An upper arm vascular access is often placed in patients with a failed forearm fistula or with vessels unsuitable for a forearm fistula. The aim of this study was to compare the outcomes of three upper arm access types: brachiocephalic fistulas, transposed brachiobasilic fistulas, and grafts.
Design, setting, participants, & measurements: A prospective, computerized access database was queried retrospectively to identify the clinical outcomes of upper arm accesses placed in 678 patients at a large dialysis center, including 322 brachiocephalic fistulas, 67 brachiobasilic fistulas, and 289 grafts.
Results: Primary access failures were less common for brachiobasilic fistulas and grafts compared with brachiocephalic fistulas (18%, 15%, and 38%; hazard ratio of brachiocephalic fistulas versus brachiobasilic fistulas 2.76; 95% confidence interval 1.41 to 5.38; P < 0.003). For the subset of patients receiving a brachiocephalic fistula, a multiple variable logistic regression analysis including age, sex, race, diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, prior access, surgeon, arterial diameter, and venous diameter found that only vascular diameters predicted primary failure (P < 0.001). When primary failures were excluded, cumulative access survival was similar for brachiobasilic and brachiocephalic fistulas, but superior to that of grafts. Total access interventions per year were lower for brachiobasilic and brachiocephalic fistulas than for grafts (0.84, 0.82, and 1.87, respectively, P < 0.001).
Conclusions: Transposed brachiobasilic fistulas may be preferred, due to (1) a lower primary failure rate (similar to grafts), and (2) a lower intervention rate (similar to brachiocephalic fistulas). However, this advantage must be balanced against the more complex surgery.
An upper arm vascular access is typically placed in patients whose vessels are unsuitable for a forearm fistula or in those with a failed forearm fistula. There are two major considerations in selecting the type of vascular access to place. First, the new access must mature adequately to be suitable for dialysis. That is, it must be cannulated reproducibly and achieve a dialysis blood flow sufficient to provide adequate dialysis. Second, once maturation has been achieved, the access should maintain long-term patency with a minimum of percutaneous or surgical interventions (1). The two major types of upper arm access placed are the brachiocephalic fistula and the upper arm graft. Once suitability for dialysis has been attained, the brachiocephalic fistula is superior to an upper arm graft, because it requires substantially fewer interventions to maintain long-term patency (2). This advantage of fistulas is counterbalanced by their higher suitability failure, which occurs in 20% to 60% of new fistulas in recent series, as compared with 10% to 20% of new grafts (1,3).
A third type of upper arm access is the transposed brachiobasilic fistula. This fistula is placed less commonly than the brachiocephalic fistula, as it is more technically challenging and time-consuming to create. Four series reporting on the outcomes of 34 to 59 brachiobasilic fistulas observed a primary failure (nonmaturation) rate ranging from 0% to 21% (4–7). Only two of these publications provided a direct comparison of the outcomes of brachiobasilic and brachiocephalic fistulas. These studies observed a lower suitability failure rate of brachiobasilic fistulas as compared with brachiocephalic fistulas, but a similar cumulative access survival (5,7). In both series, the access outcomes were ascertained retrospectively, and the fistulas were placed without the benefit of preoperative vascular mapping, an important factor that could affect vascular access outcomes.
The goal of the present study was to evaluate the relative advantages and disadvantages of brachiocephalic fistulas, transposed brachiobasilic fistulas, and upper arm grafts when routine preoperative vascular mapping is used to plan the access surgery. We retrospectively analyzed a prospective, computerized access database to evaluate the outcomes of a large cohort of patients receiving an upper arm access at a single large dialysis center. The primary clinical outcomes analyzed were: (1) failure to achieve suitability for dialysis (primary failure) and (2) cumulative access survival (i.e. the time from access creation to its permanent failure, regardless of number of salvage procedures). Major secondary outcomes were the frequency of interventions required to maintain access patency and the duration of catheter dependence.
Materials and Methods
Vascular Access Management
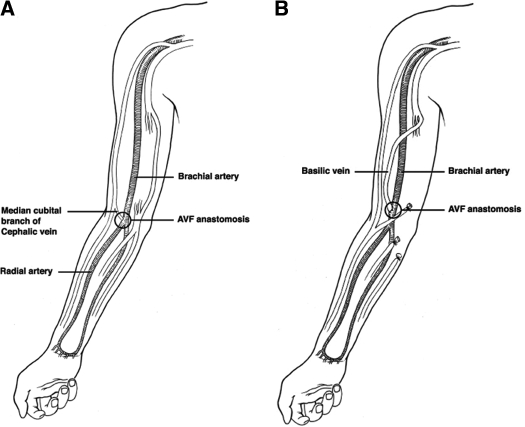
All patients were under the care of nephrologists at University of Alabama at Birmingham (UAB). Patients underwent preoperative sonographic vascular mapping (1,8,9). The minimum criteria for creation of a vascular access were an arterial diameter ≥ 2 mm, venous diameter ≥ 2.5 mm for fistulas and ≥ 4 mm for grafts, and absence of stenosis or thrombosis in the draining vein. The venous diameters were measured after application of a tourniquet. All vascular accesses were performed directly by one of five experienced surgeons (>100 vascular accesses placed) or by a surgical trainee supervised by the staff surgeon. In patients with a failed forearm fistula or those with vessels unsuitable for creation of a forearm fistula, the surgeon placed a vascular access in the upper arm. Brachiocephalic fistulas were placed in patients whose preoperative mapping indicated a suitable brachial artery and cephalic vein in the antecubital space (Figure 1A). If the cephalic vein was unsuitable, but the basilic vein was suitable, the surgeon created a transposed brachiobasilic fistula. After identifying the basilic vein in the axilla, and verifying that the brachial vein was present, the surgeon made an incision from the axilla to the elbow. When possible, the basilic vein was traced to the forearm for additional length. Once the entire vein had been dissected, side branches were ligated, and the basilic vein was removed from its bed. It was flushed with heparinized saline and occluded with a vascular clamp. A counter-incision was made over the bicep, and a subcutaneous tunnel was created, such that the basilic vein could be pulled through in a semicircular fashion. The brachial artery was then dissected free above the elbow, the patient was heparinized, and the vein was spatulated to facilitate better inflow. The artery was incised after clamping, and an end-to-side anastomosis between the basilic vein and the brachial artery was completed. Once done, clamps were removed and bleeding was controlled. The fistula could then be easily palpated through the skin (Figure 1B). Grafts were placed in patients with vessels unsuitable for a fistula or in patients with a previous primary failure of a fistula, at the surgeon's discretion.
Figure 1.
Illustration of (A) brachiocephalic and (B) transposed brachiobasilic fistulas. Note that creation of a brachiobasilic fistula requires a longitudinal incision from the axilla to the antecubital space to dissect out the basilic vein.
Grafts were cannulated 2 to 3 wk after their construction. Fistulas were cannulated 6 to 8 wk after their placement if the nurses deemed them mature. Patients with fistulas judged to be clinically immature underwent a postoperative ultrasound to assess for potentially remediable anatomic lesions, such as stenosis, accessory veins, or excessively deep fistulas (10,11). Appropriate percutaneous or surgical interventions were attempted to promote maturation (10,11). These could include angioplasty or surgical revision for stenosis; ligation of large accessory veins; or superficialization of excessively deep fistulas. The initial cannulation of a fistula was performed with 17 gauge needles and a dialysis blood flow of 250 ml/min. The needle size and dialysis blood flow were gradually increased at the discretion of the dialysis nurses. Thrombectomy was not attempted in fistulas that clotted before their successful cannulation for dialysis.
Patients without a mature fistula or graft underwent dialysis with a tunneled dialysis catheter, most commonly placed in the internal jugular vein. Catheter-related bacteremia was diagnosed in patients with fever or rigors, positive blood cultures, and absence of an alternative source of infection (12,13). Catheters were scheduled for removal after three consecutive successful uses of the fistula or graft for dialysis.
Management of vascular Access Complications
The dialysis nurses monitored all vascular accesses (fistulas and grafts) for clinical evidence of dysfunction. A fistulogram was performed by a UAB interventional radiologist or nephrologist when there was a clinical suspicion of stenosis (14,15). The clinical indication for a fistulogram could include abnormalities of physical examination (absent thrill, discontinuous bruit, or distal edema); an unexplained decrease in Kt/V on a constant dialysis prescription; or abnormalities of dialysis (difficult cannulation, aspiration of clots, or prolonged bleeding from needle sites) (15,16). Angioplasty was done in patients with ≥50% stenosis. An elective surgical revision was performed if the angioplasty was unsuccessful.
Thrombectomy was attempted in fistulas and grafts that clotted after successful cannulation for dialysis. Clotted grafts and fistulas underwent percutaneous thrombectomy, along with angioplasty of the underlying stenotic lesion. If thrombectomy was unsuccessful, a surgical revision was performed in selected cases (17). If this was not feasible or was unsuccessful, the access was abandoned, and a new permanent access was placed.
Graft or fistula infection was suspected if the access site was red, swollen, and tender (18); infection was confirmed by positive wound or blood cultures. The infection was initially treated with intravenous antibiotics. If the infection failed to respond to antibiotics, the access was excised surgically.
Data Analysis
Two full-time access coordinators scheduled all access procedures; communicated regularly with the nephrologists, access surgeons, radiologists, and dialysis staff; and maintained a prospective, computerized database of all access-related procedures and complications (19). Permission was obtained from the local institutional review board to review each patient's medical records for research purposes. The access database was queried retrospectively to identify 1007 new upper arm fistulas and grafts placed during the 6-yr period from 10/1/00 to 9/30/06. If a patient received more than one access during the study period, only the first access was included in the analysis. After excluding 283 subsequent accesses, we were left with 724 patients who received a new access during the study period. The vascular access outcome was considered indeterminate if the access had not failed at the time of analysis but the patient died, received a kidney transplant, transferred to an outside dialysis unit before the access could be cannulated, or had not yet started dialysis. After 46 patients with indeterminate access outcomes were excluded, the remaining 678 patients were the focus of the current analysis. These included 322 patients with a brachiocephalic fistula, 67 with a transposed brachiobasilic fistula, and 289 with an upper arm arteriovenous graft. Baseline demographic and clinical characteristics of the study patients were obtained from the UAB Hospital electronic medical records.
Definition of Access Outcomes
The access database was used to determine the clinical outcomes and complications of each vascular access. The access was considered mature if it could be cannulated reproducibly for dialysis with two needles with a blood flow ≥ 300 ml/min for at least 1 month. A primary access failure was defined as the inability to use it successfully for dialysis, due to either early thrombosis or failure to mature. Cumulative access survival was calculated as the time from access placement to permanent failure, regardless of the number of interventions required to maintain patency. Because fistulas and grafts with a primary failure had no useful life expectancy, their cumulative survival was considered “0 d” for the purpose of this analysis. The database was also used to quantify the number of access angioplasties, thrombectomies, elective surgical revisions, total access interventions, access infections, and episodes of catheter-related bacteremia.
Statistical Analysis
The clinical characteristics were compared among the three patient groups with analysis of variance (ANOVA) or nonparametric statistics for continuous variables, and χ2 analysis for categorical variables. A P value < 0.05 was considered statistically significant. Multiple variable logistic regression analysis was used to evaluate which factors were associated with primary access failure. Survival analysis techniques were used to model access survival time, and the log rank test used to compare the survival of patient subgroups. Hazard ratios and their 95% confidence intervals (CI) were computed. Finally, multiple variable Cox proportional survival analysis was used to model the association between the clinical variables and access survival. All analyses were performed using the Statistical Analysis Software (SAS) version 9.0.
Results
Patient Population
The three patient groups were similar in terms of age, sex, race, diabetes, and vascular disease (Table 1). Hypertension was slightly less common among patients who received a transposed brachiobasilic fistula. Approximately half of the study population had had a previous permanent vascular access, but the proportion was higher among patients receiving a graft than among those receiving a fistula. Routine preoperative vascular measurements were obtained and used by the surgeons to plan their access placement. The mean preoperative brachial arterial diameter was 4.8 ± 1.1 mm in the patients receiving a brachiocephalic fistula and 4.7 ± 1.1 mm in those receiving a transposed brachiobasilic fistula (P = 0.60). The mean preoperative vein diameter was 4.3 ± 1.3 mm in the patients receiving a brachiocephalic fistula and 4.3 ± 1.4 mm in those receiving a transposed brachiobasilic fistula (P = 0.95).
Table 1.
Baseline characteristics of the study patients
| Transposed Brachiobasilic Fistula | Brachiocephalic Fistula | Arteriovenous Graft | P | |
|---|---|---|---|---|
| N pts | 67 | 322 | 289 | |
| Age, years | 56 ± 15 | 56 ± 14 | 56 ± 14 | 0.98 |
| Sex, N (%) female | 32 (48%) | 167 (52%) | 167 (58%) | 0.19 |
| Race, N (%) black | 56 (84%) | 249 (77%) | 240 (83%) | 0.16 |
| Diabetes, N (%) | 39 (58%) | 172 (53%) | 143 (49%) | 0.36 |
| Hypertension, N (%) | 55 (82%)a | 299 (93%) | 259 (90%) | 0.02 |
| Coronary artery disease, N (%) | 22 (33%) | 95 (30%) | 80 (28%) | 0.68 |
| Peripheral vascular disease, N (%) | 8 (12%) | 51 (16%) | 55 (19%) | 0.30 |
| Cerebrovascular disease, N (%) | 9 (13%) | 30 (9%) | 31 (11%) | 0.58 |
| Previous access, N (%) | 31 (46%) | 146 (45%) | 163 (56%)a | 0.02 |
Indicates which group differs from the other two groups.
Primary Access Failure
Primary access failure (suitability failure) was relatively low (15% to 18%) in patients receiving a graft or a transposed brachiobasilic fistula, but at least twice as frequent (38%) in patients receiving a brachiocephalic fistula (Table 2). In multiple variable logistic regression analysis including access type, patient age, sex, diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, and prior access, only patient sex and access type predicted primary access failure. Specifically, primary access failure was lower in males than in females (HR 0.54; 95% CI, 0.38 to 0.78, P = 0.001). Primary access failure was higher for brachiocephalic fistula versus brachiobasilic fistula (HR 2.76; 95% CI, 1.41 to 5.38, P < 0.003). Primary access failure was not different between grafts and brachiobasilic fistulas (HR 0.78; 95% CI 0.38 to 1.57, P = 0.48).
Table 2.
Survival of upper arm accesses
| Transposed Brachiobasilic Fistula | Brachiocephalic Fistula | Arteriovenous Graft | P | |
|---|---|---|---|---|
| N patients | 67 | 322 | 289 | |
| Primary access failure | 18% | 38%a | 15% | <0.001 |
| Median cumulative survival, days (including primary failure) | 994 | 386 | 401 | 0.17 |
| Median cumulative survival, days (excluding primary failure) | 1494 | 1254 | 595a | <0.001 |
Indicates which group differs from the other 2 groups.
Primary failure, inability to use a new access reproducibly for dialysis.
Cumulative survival, time from access placement to permanent access failure.
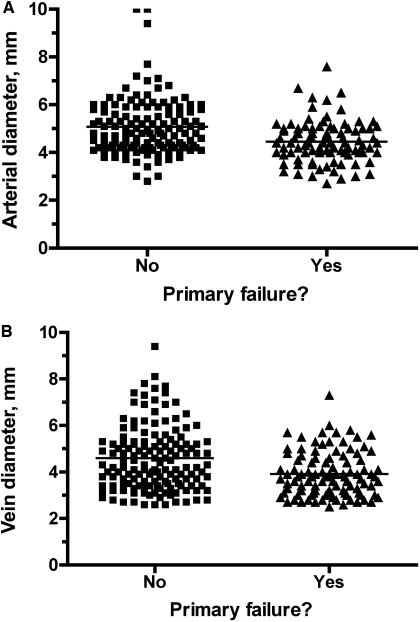
Because the patients receiving a brachiocephalic fistula had a much higher primary failure rate, we performed a separate multiple variable logistic regression analysis for this subgoup, including age, sex, race, diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, prior access, surgeon, arterial diameter, and venous diameter. On this analysis, only arterial and venous diameters predicted primary failure (P < 0.001). The mean arterial diameter was significantly lower for brachiocephalic fistulas with a primary failure than those without a failure (4.4 ± 0.9 mm versus 5.1 ± 1.2 mm, P < 0.001). Similarly, the mean venous diameter was significantly lower for brachiocephalic fistulas with a primary failure than those without a failure (3.9 ± 1.0 mm versus 4.6 ± 1.5 mm, P < 0.001). However, there was a substantial overlap in the vascular diameters between the two patient groups (Figure 2).
Figure 2.
Scatter plot of the distribution of the (A) arterial diameters and (B) venous diameters of vessels used to create brachiocephalic fistulas. Although the mean arterial and venous diameters were significantly lower in fistulas with a primary failure than in those successfully used for dialysis, there was substantial overlap in the values between the two patient groups.
Cumulative Access Survival
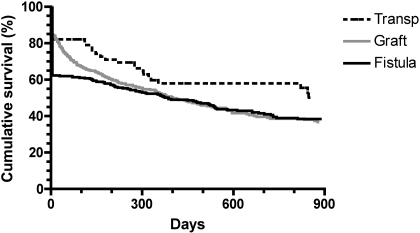
When primary access failures were included, the median cumulative access survival (from creation to permanent failure) was approximately 1 yr for grafts and brachiocephalic fistulas, as compared with 2.7 yr for transposed brachiobasilic fistulas. The HR for cumulative failure of transposed brachiobasilic fistulas versus brachiocephalic fistulas was 0.74 (95% CI 0.50 to 1.03, P = 0.07) (Figure 3).
Figure 3.
Cumulative access survival (time from access creation to access failure), including primary access failures. The differences in survival among the three access types were not statistically significant (P = 0.17).
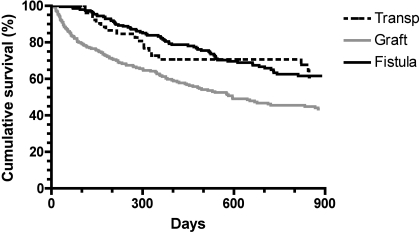
When primary access failures were excluded, the median cumulative survival was similar for transposed brachiobasilic fistulas and brachiocephalic fistulas (4.1 and 3.4 yr, respectively), but significantly longer than for grafts (1.6 yr) (Table 2). The HR for cumulative failure of transposed fistulas versus grafts was 0.58 (95% CI 0.43 to 0.90, P = 0.01) (Figure 4).
Figure 4.
Cumulative access survival (time from access creation to access failure), excluding primary access failures. P = 0.01 for transposed brachiobasilic fistulas versus grafts.
Multiple variable survival analysis was performed to evaluate which clinical factors were associated with cumulative access survival, with access type, patient age, sex, diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, and prior access included in the model. When primary failures were included, sex and prior access were the only significant factors associated with cumulative access failure (P = 0.01 for the overall model) (Table 3). When primary failures were excluded, access type was the only significant factor associated with cumulative access failure (P < 0.001 for the overall model) (Table 3).
Table 3.
Multiple variable survival analysis of factors associated with access survival
| Type of access survival | Clinical variable | HR (95% CI) | P |
|---|---|---|---|
| Cumulative survival including primary failures | Males vs female | 0.80 (0.65 to 0.97) | 0.02 |
| Prior access | 1.26 (1.04 to 1.54) | 0.02 | |
| Cumulative survival excluding primary failures | BC fistula vs graft | 0.53 (0.40 to 0.70) | <0.001 |
| BB fistula vs BC fistula | 1.07 (0.67 to 1.70) | 0.78 |
BB, brachiobasilic; BC, brachiocephalic.
Cox proportional survival analysis was modeled with the following clinical variables: access type, patient age, sex, diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, and prior access.
Access Interventions and Complications
The duration of catheter dependence was calculated from access creation to catheter removal due to access maturity, for the subset of patients who did not begin dialysis with a mature access. Catheter dependence was at least 2 mo longer for patients receiving a transposed brachiobasilic or brachiocephalic fistula, as compared with those receiving a graft (Table 4). Dependence tended to be longer for brachiocephalic fistulas, as compared with transposed brachiobasilic fistulas, but this difference did not achieve statistical significance (P = 0.06). The likelihood of catheter-related bacteremia before use of a permanent access was about twice as high in patients receiving a transposed brachiobasilic or brachiocephalic fistula, as compared with those receiving a graft.
Table 4.
Complications of upper arm accesses
| Transposed Brachiobasilic Fistula | Brachiocephalic Fistula | Graft | P | |
|---|---|---|---|---|
| Catheter-dependence until mature access in use, days | 96 | 126 | 32a | <0.001 |
| CRB before perm acc use? (%) | 46% | 44% | 24%a | <0.001 |
| PTA/yr | 0.67 | 0.48 | 0.66 | 0.49 |
| Thrombectomy/yrb | 0.04 | 0.08 | 1.01a | <0.001 |
| Surgical revisions/yr | 0.13c | 0.25 | 0.20 | 0.01 |
| Total interventions/yr | 0.84 | 0.82 | 1.87a | <0.001 |
| Access infections, %/yr | 0% | 0.7% | 9.7%a | <0.001 |
All complications were standardized for the duration of access follow-up. CRB, catheter-related bacteremia; PTA, percutaneous transluminal angioplasty.
Indicates which group differs from the other two groups.
Thrombectomy was not attempted in fistulas that clotted prior to their first cannulation.
Significantly lower than brachiocephalic fistulas.
The frequency of access interventions, standardized per access year, was calculated for the three patient groups (Table 4). The frequency of angioplasty was similar among the three groups. Thrombectomy frequency was low for both types of fistulas, and much higher for grafts. Surgical revisions occurred less commonly in transposed brachiobasilic fistulas than in brachiocephalic fistulas. The rate of total access interventions was similar for brachiobasilic and brachiocephalic fistulas, and less than half of that for grafts. Finally, the annual rate of access infection was <1% for either type of fistula, as compared with almost 10% for grafts.
Discussion
This study evaluated the clinical outcomes of the three major types of upper arm vascular access. Transposed brachiobasilic upper arm fistulas had a substantially lower primary failure rate (suitability failure) than brachiocephalic fistulas, similar to the rate observed with grafts. These findings are qualitatively similar to those of a previous study that observed primary failure rates of 32%, 21%, and 15% for brachiocephalic fistulas, transposed brachiobasilic fistulas, and upper arm grafts, respectively (7). Likewise, a small study in diabetic dialysis patients reported a 0% nonmaturation rate of transposed fistulas, as compared with 27% with brachiocephalic fistulas in the upper arm (5). Finally, Silva et al. reported a markedly lower primary failure rate of transposed fistulas, as compared with nontransposed fistulas, in the forearm (8% versus 38%) (20,21). It is not entirely clear why transposed brachiobasilic fistulas have a lower primary failure rate than brachiocephalic fistulas. The differences in outcomes of the two fistula types were not likely due to clinical differences among the patient populations (Table 1), nor could they be attributed to differences in vascular diameter. One possible explanation is that transposed brachiobasilic fistulas are more superficial, and thus easier to cannulate. This may lower the risk for needle infiltration, a complication associated with both fistula thrombosis and prolonged catheter dependence (22). A second possibility is that tying off multiple collaterals of the transposed vein enhances fistula maturation by ensuring that the entire arterial inflow is channeled into a single draining vein.
The relative cumulative survival of the three types of upper arm vascular access differed dramatically, depending on whether failure to mature (primary failure) was included in the analysis (Table 2). If primary failures were excluded, the cumulative survival of both types of fistulas was virtually identical (Figure 4). However, when primary failures were included, the cumulative survival tended to be superior (P = 0.07) for brachiobasilic fistulas than for brachiocephalic fistulas (Table 2, Figure 3). The reason for the discrepancy between the two analyses was that the primary failure rate of brachiobasilic fistulas was substantially lower than that of brachiocephalic fistulas (Table 2).
The long-term risk of access infection was substantially lower in fistulas (<1%) than in grafts (approximately 10% per year) (Table 4). On the other hand, the duration of catheter dependence was substantially longer in patients receiving fistulas, translating into a significantly higher short-term risk of catheter-related bacteremia. Thus, it is imperative for nephrologists to monitor new fistulas closely and intervene early to facilitate their maturation, so as to minimize the period of catheter dependence and its associated risk of bacteremia.
The present study has a number of limitations. First, it was retrospective. Because we queried a prospective, electronic access database, and because the vast majority of access procedures were performed at a single hospital, we are confident that we captured all of the access events. Second, this was not a randomized clinical trial, and there may have been an indication bias in patients receiving a transposed brachiobasilic fistula. However, any patient with vessels unsuitable for a brachiocephalic fistula was considered for a brachiobasilic fistula, including very obese patients. Moreover, none of the measured demographic or clinical characteristics differed between patients receiving the two types of fistula (Table 1). Third, this study represents the outcomes from a single dialysis center and may not generalize to all centers. For example, in a center with fewer primary failures of brachiocephalic fistulas, the superiority of brachiobasilic fistulas may not be evident. In addition, brachiobasilic fistulas may be less successful in the hands of surgeons with limited experience in their creation. Obtaining a definitive answer about the relative merits of the three access types would require a randomized, multicenter clinical trial, in which patients have an equal chance of being allocated to each access type.
Transposed brachiobasilic fistulas may be preferred to brachiocephalic fistulas because of their lower primary failure rate. The decision about placing a brachiobasilic fistula in a given patient must be tempered, however, by the greater technical challenge of this surgical procedure. For the majority (62%) of patients in whom the brachiocephalic fistula is used successfully for dialysis, one would prefer to forego the more extensive brachiobasilic fistula surgery. The challenge is identifying preoperatively those patients in whom the brachiocephalic fistula is unlikely to succeed. Unfortunately, none of the clinical or demographic factors predicted that subset of patients. The mean arterial and venous diameters were significantly lower in brachiocephalic fistulas that failed to mature than in those that were used successfully for dialysis. Unfortunately, there was substantial overlap in vessel diameters between the two groups, such that there was no satisfactory threshold diameter that would both maximize fistula placement and minimize fistula nonmaturation (Figure 2). If, for example, the minimum arterial diameter were set at 5 mm, 30% of brachiocephalic fistulas created would still have a primary failure, whereas 48% of brachiocephalic fistulas that were not placed due to an arterial diameter of <5 mm would have actually matured. What is desperately needed is a better understanding of those factors that predict nonmaturation of brachiocephalic fistulas (23).
Disclosures
None.
Acknowledgments
This research was supported in part by grant number 1 K24 DK59818–01 from the NIDDK to Dr. Allon. Portions of this manuscript were presented at the Clinical Meeting of the National Kidney Foundation; April 2 to 6, 2008, Dallas, TX.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 2.KDOQI clinical practice guidelines and clinical practice recommendations for vascular access 2006. Am J Kidney Dis 48(Suppl 1): S176–S322, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, Himmelfarb J, Vazquez MA, Gassman JJ, Greene T, Radeva MK, Braden GL, Ikizler TA, Rocco MV, Davidson IJ, Kaufman JS, Meyers CM, Kusek JW, Feldman HI: Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis. JAMA 299: 2164–2171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chemla E, Morsy MA: Is basilic vein transposition a real alternative to an arteriovenous bypass graft? A prospective study. Semin Dial DOI:10.1111/j. 1525–1139X. 2008.00449.x, 2008 [DOI] [PubMed]
- 5.Hakaim AG, Nalbandian M, Scott T: Superior maturation and patency of primary brachiocephalic and transposed basilic vein arteriovenous fistulae in patients with diabetes. J Vasc Surg 27: 154–157, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Moossavi S, Tuttle AB, Vachharajani TJ, Plonk G, Bettmann MA, Majekodunmi O, Russell GB, Regan JD, Freedman BI: Long-term outcomes of transposed basilic vein arteriovenous fistulae. Hemodial Int 12: 80–84, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Oliver MJ, McCann RL, Indridason OS, Butterly DW, Schwab SJ: Comparison of transposed brachiobasilic fistulas to upper arm grafts and brachiocephalic fistulas. Kidney Int 60: 1532–1539, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, Deierhoi MH, Robbin ML: Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int 60: 2013–2020, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Robbin ML, Gallichio ML, Deierhoi MH, Young CJ, Weber TM, Allon M: US vascular mapping before hemodialysis access placement. Radiology 217: 83–88, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Robbin ML, Chamberlain NE, Lockhart ME, Gallichio MH, Young CJ, Deierhoi MH, Allon M: Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology 225: 59–64, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Singh P, Robbin ML, Lockhart ME, Allon M: Clinically immature arteriovenous hemodialysis fistulas: Effect of US on salvage. Radiology 246: 299–305, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Krishnasami Z, Carlton D, Bimbo L, Taylor ME, Balkovetz DF, Barker J, Allon M: Management of hemodialysis catheter related bacteremia with an adjunctive antibiotic lock solution. Kidney Int 61: 1136–1142, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Poole CV, Carlton D, Bimbo L, Allon M: Treatment of catheter-related bacteremia with an antibiotic lock protocol: Effect of bacterial pathogen. Nephrol Dial Transplant 19: 1237–1244, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Lilly RZ, Carlton D, Barker J, Oser R, Hamrick K, Saddekni S, Westfall AO, Allon M: Clinical predictors of A-V graft patency following radiologic intervention in hemodialysis patients. Am J Kidney Dis 37: 945–953, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Maya ID, Oser R, Saddekni S, Barker J, Allon M: Vascular access stenosis: Comparison of arteriovenous grafts and fistulas. Am J Kidney Dis 44: 859–865, 2004 [PubMed] [Google Scholar]
- 16.Robbin ML, Oser RF, Lee JY, Heudebert GR, Mennemeyer ST, Allon M: Randomized comparison of ultrasound surveillance and clinical monitoring on arteriovenous graft outcomes. Kidney Int 69: 730–735, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Maya ID, Smith TL, Young CJ, Allon M: Is surgical salvage of arteriovenous grafts feasible after unsuccessful percutaneous mechanical thrombectomy? Semin Dial 21: 174–177, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Minga TE, Flanagan KH, Allon M: Clinical consequences of infected arteriovenous grafts in hemodialysis patients. Am J Kidney Dis 38: 975–978, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Allon M, Bailey R, Ballard R, Deierhoi MH, Hamrick K, Oser R, Rhynes VK, Robbin ML, Saddekni S, Zeigler ST: A multidisciplinary approach to hemodialysis access: Prospective evaluation. Kidney Int 53: 473–479, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Silva MB, Hobson RW, Pappas PJ, Araki CT, Goldberg MC, Jamil Z, Padberg FT: Vein transposition in the forearm for autogenous hemodialysis access. J Vasc Surg 26: 981–988, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Silva MB, Hobson RW, Pappas PJ, Jamil Z, Araki CT, Goldberg MC, Gwertzman G, Padberg FT: A strategy for increasing use of autogenous hemodialysis access procedures: Impact of preoperative noninvasive evaluation. J Vasc Surg 27: 302–308, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Lee T, Barker J, Allon M: Needle infiltration of arteriovenous fistulas in hemodialysis: Risk factors and consequences. Am J Kidney Dis 47: 1020–1026, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Dixon BS: Why don't fistulas mature? Kidney Int 70: 1413–1422, 2006 [DOI] [PubMed] [Google Scholar]