Abstract
Background and objectives: Cool dialysate may ameliorate intradialytic hypotension (IDH). It is not known whether it is sufficient to prevent an increase in core temperature (CT) during hemodialysis (HD) or whether a mild decline in CT would yield superior results. The aim of this study was to compare both approaches with regard to IDH.
Design, setting, participants, & measurements: Fourteen HD patients with a history of IDH were studied. During three mid-week HD treatments, CT was set to decrease by 0.5°C (“cooling”) or to remain unchanged at the baseline level (“isothermic”). “Thermoneutral” HD (no energy is added to or removed from the patient) was used as a control. Central blood volume (CBV), BP, skin temperature, heart rate variability [low and high frequency] were recorded.
Results: CT increased during thermoneutral and remained respectively stable and decreased during isothermic and cooling. Skin temperature decreased significantly during isothermic and cooling, but not during thermoneutral. Nadir systolic BP (SBP) levels were lower during isothermic and thermoneutral compared with cooling. CBV tended to be higher during cooling compared with isothermic and thermoneutral. Three patients complained of shivering during cooling. Change in LF/HF was not different between cooling, isothermic, and thermoneutral.
Conclusions: IDH may be slightly improved by cooling compared with the isothermic approach, possibly because of improved maintenance of CBV. The hemodynamic effects of mild blood cooling should be balanced against a potentially higher risk of cold discomfort.
Despite technical improvements in dialysis therapy, intradialytic hypotension (IDH) remains common (1). IDH may not only yield discomfort for the patient, but is related to increased mortality (1,2).
The pathogenetic factors leading to symptomatic hypotension are complex, but three main factors are involved. The first is the decline in blood volume, which is resultant of the balance between ultrafiltration and refilling from the interstitial compartment. Second, cardiac factors such as left ventricular hypertrophy, systolic and diastolic dysfunction, and dilated cardiomyopathy may play an important role. The third factor contributing to IDH is an inadequate vascular response of both arterial and venous circulation during a decline in blood volume (3).
Various mechanisms have been implicated in the pathogenesis of the impaired vascular response during hemodialysis (HD) (4). However, there are strong indications that this phenomenon is mainly related to thermal factors (5–7). Others and we have observed that the core temperature (CT) increased during standard (37 to 37.5°C) dialysis (8,9) despite net energy loss from the patient to the extracorporeal system. The increase in internal heat production might very well be responsible for the impaired vascular response during HD, because this thermal response (leading to dilation of thermoregulatory vessels) will offset the vasoconstrictive response to hypovolemia (3,5,7). The fact that all differences in vascular reactivity between isolated ultrafiltration and HD disappear when both treatments are matched for the same extracorporeal heat flux is a strong argument for the overwhelming role of heat balance in the control of vascular reactivity during dialysis, although in individual patients other factors such as diabetes might also be involved (7).
Both arbitrary reduction in dialysate temperature (Td) and prescription of isothermic dialysis, during which the CT of the patient is held stable, improves hemodynamic stability during dialysis (5–7,10–14). In contrast to isothermic dialysis, CT may decrease during dialysis with a Td of 35 to 35.5°C (8,11), which may result in unpleasant effects such as shivering. However, it is not known whether a purposive slight lowering of CT has additional beneficial effects on hemodynamic stability compared with maintenance of CT during HD, possibly because of a further increase in sympathetic activity (15). In critically ill patients, mild lowering of CT by continuous veno-venous hemofiltration led to an increase in mean arterial pressure (16). However, this issue has not been addressed in chronic dialysis patients. The primary aim of this study was to test the hypothesis that a lowering of CT during cool dialysis will have additional hemodynamic benefits when compared with isothermic dialysis (i.e., maintenance of CT).
Materials and Methods
Study Design
Patients were studied during three consecutive mid-week dialysis sessions using a crossover design, each patient serving as his/her own control. Sessions were performed in random order. During the different treatment sessions, arterial temperature (Tart) was set to decrease by 0.5°C (“cooling”), or to remain unchanged at the individual patient's baseline level (“isothermic”), respectively [Fresenius Blood Temperature Monitor (BTM) Fresenius Medical Care®, Bad Homburg, Germany]. “Thermoneutral” dialysis (during which no energy is added to or removed from the patient) was used as a control modality (13). Treatments only differed with regard to the thermal prescription, in that ultrafiltration volume and hours of dialysis were the same.
Subjects
Seventeen patients, out of a population of 142 patients, on chronic HD treatment, who had experienced a drop in SBP of more than 25 mmHg in at least 75% of dialysis sessions in the preceding 6 mo were included. Other inclusion criteria were ages >18 yr but <85 yr, and the ability to read and understand English. Because not all of the patients who fulfilled this criteria were eligible for inclusion in the study because of exclusion criteria, and not all of the eligible patients were interested in participation, we ended up with these 17 patients. An exclusion criterion was a central venous catheter as vascular access for HD. All patients gave informed consent to participate in this study, which was approved by the Beth Israel Medical Center Institutional Review Board (New York).
Dialysis Prescription
HD was delivered by volumetric machines (2008H and 2008K; Fresenius Medical Care, Walnut Creek, California) and polysulfone dialyzers (Optiflux 160NR, 180NR, and 200NR; Fresenius Medical Care, Walnut Creek, California) using bicarbonate dialysate (Diasafe; Fresenius Medical Care, Walnut Creek, California). Blood flow was 400 ml/min and dialysate flow 800 ml/min. Endotoxin activity in dialysate was less than 0.06 endotoxin units/ml (limulus amoebocyte lysate test; Associates of Cape Cod, Falmouth, Massachusetts). Dialysate compositions were: sodium, 142 mEq/L; potassium, 2.0 mEq/L; calcium, 2.5 mEq/L; magnesium, 1 mEq/L; acetate, 4 mEq/L; bicarbonate, individualized (between 34 and 39 mEq/L); and glucose: 200 mg/dl.
Thermal Prescription and Assessment
Arterial (Tart) and venous (Tven) blood line temperatures and energy transfer between the extracorporeal circuit and the patient were noninvasively monitored using the BTM device, as described previously (7,13), at 60-s intervals (7,8,17,18). During isothermic, the change in patient temperature was set to zero. During cooling, the change in patient temperature was set to reach a decline in CT of 0.5°C versus the baseline temperature. During thermoneutral, the energy flow rate was set at 0 kJ/h, which means that no energy is fed to or withdrawn from the patient via the extracorporeal circuit.
Because the upright position may lead to heat accumulation all measurements were made in the supine position (19). Because eating and drinking may have an effect on thermogenesis, patients did not eat or drink during the study. Humidity in the room was approximately 60% and ambient temperature approximately 23°C.
Skin Temperature
Skin temperature (ST) was measured by a Hewlett Packard device (HP 78204C; Boeblingen, Germany) with a monitoring range of 15 to 45°C, a resolution of 0.1°C, and an accuracy of 0.2°C (0.1°C instrument, 0.1°C temperature probe). Two, unheated, attachable surface temperature probes (HP 21059A) were placed on the skin but not on a blood vessel of the arm contralateral to the vascular access. Measurements were made for 15 min before and at the end of dialysis.
Hemodynamic Measurements
BP and heart rate were measured every 10 min noninvasively with an oscillometric BP monitor. Also BP and heart rate were measured when patients experienced symptoms. Of these measurements for BP, the lowest value was used. Changes in relative blood volume (RBV) were monitored continuously and noninvasively with the Fresenius blood volume monitor (Fresenius Medical Care®, Bad Homburg, Germany), which measures changes in RBV by measuring ultrasonic changes on total protein concentration (20).
Central Blood Volume
Central blood volume (CBV), cardiac output, and systemic vascular resistance were measured by Transonic HD02 HD monitor (Transonic Systems, Ithaca, New York), as described previously (21). Measurements were performed at the start, in the middle, and at the end of the dialysis session.
Heart Rate Variability
Spectral analyses of heart rate variability (e.g., heart rate fluctuations around mean heart rate or beat-to-beat changes in heart rate) were performed (22,23). Using this method, the degree of variability and frequency-specific oscillations can be defined. The basis of this method has been described in detail elsewhere (23). For each study, continuous heart rate recordings (KCI Technology, Sewell, New Jersey) during HD were acquired digitally and analyzed in a blinded manner. Unsuitable heart rate tracings were edited and segments of normal sinus rhythm were used for fast Fourier transformation to derive: Low-frequency (LF) harmonic (0.05 to 0.15 Hz) and high-frequency (HF) harmonic (0.15 to 0.5 Hz) contributions to spectral power of heart rate. LF/LF + HF, HF/HF + LF and LF/HF were used to estimate sympathetic and vagal modulation of heart rate and their ratio, respectively.
Statistical and Power Analyses
The primary outcome parameter, according to the definition for IDH proposed by Santoro et al., was the differences in SBP between the treatments (24). Assuming a SD of 23 mmHg for the lowest SBP, 12 patients would be needed to show a difference of 20 mmHg between two different treatments with a α of 0.05 and a power of 0.8. To account for dropout and the comparison of three different treatments, 15 patients were recruited. For comparisons of normally distributed values between different treatments were analyzed by repeated measurements ANOVA. Nonparametric testing (Kruskal-Wallis, Mann-Whitney, and Wilcoxon, where appropriate) was performed in parameters without normal distribution. (SPSS version 12.0.1: SPSS, Inc., Chicago, Illinois). P < 0.05 was considered to be significant. Data are expressed as mean ± SD.
Results
Seventeen patients gave in first instance informed consent for participating in the study. In one patient, the BTM was not functioning adequately, yielding incomplete data for analysis. Two patients withdrew their informed consent. Finally, 14 patients (eight men; mean age 60.9 ± 10.4 yr; mean time on dialysis 227 ± 42 mo) completed the study. Mean dialysis time was 232 ± 37 min. Mean ultrafiltration volume in these patients was 3216 ± 1177 ml during isothermic, 3442 ± 1163 ml during cooling, and 3171 ± 1163 ml during thermoneutral (P = NS between treatments). Mean ultrafiltration rate was 843 ± 315 ml during isothermic, 891 ± 332 ml during cooling, and 820 ± 297 ml during thermoneutral (P = NS between treatments).
Thermal Effects
Predialytic CT was 36.5 ± 0.3°C before isothermic, 36.4 ± 0.4°C before cooling, and 36.4 ± 0.3°C before thermoneutral. CT remained stable during isothermic (0.0 ± 0.1°C), decreased during cooling (−0.3 ± 0.1°C), and increased during thermoneutral (0.3 ± 0.3°C; P < 0.001 between treatments).
Predialytic ST was 31.2 ± 1.0°C before isothermic, 31.4 ± 1.2°C before cooling, and 31.3 ± 0.9°C before thermoneutral. ST decreased significantly during isothermic (−1.1 ± 1.3°C; P < 0.05), and cooling (−1.9 ± 1.4°C; P < 0.05), but not significantly during thermoneutral (−0.5 ± 1.5°C; P = NS). The change in ST was significant between cooling and thermoneutral (P < 0.05), but not between any other combination of treatments.
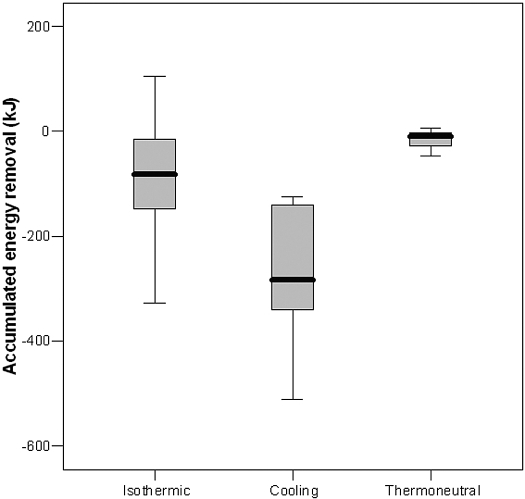
Accumulated energy removal from extracorporeal circuit to the patient during the entire treatment was −142 ± 119 kJ during isothermic, −300 ± 130 kJ during cooling, and 5 ± 21 kJ during thermoneutral (P < 0.001 between treatments; Figure 1). Mean thermal energy flow rate was −10 ± 8 W (−20 ± 53 kJ/h) during isothermic, −21 ± 8 W (−59 ± 33 kJ/h) during cooling, and 4 ± 4 W (23 ± 34 kJ/h) during thermoneutral (P < 0.001 between treatments).
Figure 1.
Accumulated energy removal. “Isothermic” is hemodialysis (HD) under core temperature (CT) control conditions (i.e., no change in CT). “Cooling” is HD under CT control conditions (i.e., decline in CT of 0.5°C versus baseline). “Thermoneutral” is HD under energy-neutral conditions (i.e., no change in energy flow). Boxes indicate 25 to 75% with median value; capped bars indicate the range of data.
The duration of dialysis treatment was significantly related to accumulated energy removal during cooling (r = −0.57; P < 0.001), but not to energy flow rate and changes in CT.
Hemodynamic Effects
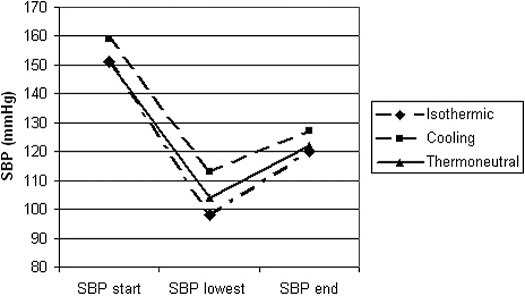
As shown in Table 1, predialytic SBP levels were not significantly different between isothermic, cooling, and thermoneutral (151 ± 28, 159 ± 35 and 151 ± 27 mmHg, respectively). The same held true for postdialytic SBP (120 ± 33, 127 ± 39, and 122 ± 28 mmHg; P = NS). However, during the treatment, lower nadir SBP levels were recorded during isothermic (98 ± 27 mmHg; P = 0.025) and thermoneutral (104 ± 27mmHg; P = 0.082), as compared with cooling (113 ± 30 mmHg; P < 0.05 between treatments) (Figure 2). The time to develop the lowest SBP was longer with cooling (188 ± 56 min; after 81 ± 23% of treatment time) as compared with isothermic (167 ± 68 min; after 72 ± 23% of treatment time) and thermoneutral (154 ± 53 min; after 66 ± 22% of treatment time). However, this was not significantly different between either one of the treatments.
Table 1.
Hemodynamic parameters during different stages of hemodialysisa
| Parameter | Isothermic
|
Cooling
|
Thermoneutral
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Start | Mid | End | Start | Mid | End | Start | Mid | End | |
| SBP | 151 (28) | 130 (29) | 120 (32)b | 159 (35) | 136 (19) | 127 (39)b | 151 (27) | 132 (33) | 122 (28)b |
| DBP | 80 (12) | 71 (11) | 63 (16)b | 82 (12) | 74 (7) | 70 (17)b | 82 (11) | 72 (12) | 65 (12)b |
| HR | 75 (11) | 74 (13) | 74 (15) | 72 (11) | 71 (12) | 72 (12) | 75 (11) | 73 (13) | 74 (17) |
| CO | 5.4 (1.7) | 4.4 (1.2) | 3.9 (1.1)b | 5.2 (1.4) | 4.5 (1.1) | 3.9 (1.1)b | 5.3 (1.4) | 4.4 (1.2) | 3.7 (0.9)b |
| CBV | 1.00 (0.31) | 0.91 (0.28) | 0.88 (0.31)b | 1.04 (0.32) | 0.97 (0.29) | 0.91 (0.29)b | 0.99 (0.31) | 0.87 (0.22) | 0.83 (0.21)b |
| LF/HF | 2.37 (2.22) | 3.25 (2.12) | 2.40 (2.05) | 2.75 (1.87) | 2.87 (2.19) | 3.75 (1.97) | |||
“Cooling” is hemodialysis under core temperature control conditions (i.e., decline in core temperature of 0.5°C versus baseline). “Thermoneutral” is hemodialysis under energy-neutral conditions (i.e., no change in energy flow). “Start” is at the start of dialysis, “Mid” is halfway through dialysis, and “End” is at the end of dialysis. SBP, systolic blood pressure in mmHg; DBP, diastolic blood pressure in mmHg; HR, heart rate in beats per minute; CO, cardiac output in liters per minute; CBV, central blood volume in liters; LF/HF, sympatho-vagal balance.
P < 0.05, end versus start of dialysis.
Figure 2.
Systolic BP (SBP) at various points during HD. “SBP start” is SBP at the start of dialysis, “SBP lowest” is lowest SBP during the treatment, and “SBP end” is SBP at the end of dialysis.
Pre- and postdialytic diastolic BP were not significantly different between treatments (Table 1).
Predialytic values for CBV were not significantly different between the different treatments. CBV declined during all dialysis sessions; however, CBV measured in the middle of the dialysis session tended to be higher during cooling (0.97 ± 0.30 L), compared with isothermic (0.91 ± 0.28 L) and thermoneutral (0.86 ± 0.21 L; P = 0.06) After dialysis, differences in CBV between cooling (0.91 ± 0.30 L), isothermic (0.88 ± 0.30 L), and thermoneutral treatments (0.83 ± 0.21 L) were NS (P = 0.09). The lowest RBV between cooling (87 ± 7%), isothermic (89 ± 7%), and thermoneutral treatments (89 ± 7%) were also NS. The same holds true for cardiac output between the different treatments (Table 1).
No relation was observed between predialytic CT and hemodynamic changes during the different treatments in this study.
Heart Rate Variability
As shown in Table 1, the change in LF/HF ratio between the treatment modalities was NS.
Side Effects
The number of hypotensive episodes with symptoms (cramps) were three with isothermic, three with thermoneutral, and one with cooling. During these episodes the ultrafiltration rate was slowed and saline was given (two patients isothermic, in total 500 ml saline; two patients thermoneutral, in total 500 ml saline), ultrafiltration rate was stopped, and saline was given (one patient isothermic, in total 150 ml saline; one patient thermoneutral, in total 150 ml saline), or ultrafiltration rate was only slowed (one patient cooling).
Three patients complained of shivering during cooling, which was significantly higher as compared with isothermic and thermoneutral. Three patients complained of feeling warm during thermoneutral.
Discussion
This randomized crossover study compared the effect of three different thermal prescriptions on the hemodynamic response during dialysis. The major finding of the study is the lower nadir SBP during isothermic, during which CT was maintained stable, and thermoneutral, during which no energy was removed or added from the extracorporeal circuit to the patient, as compared with cooling, in which a decrease in CT of 0.5°C was aimed at.
Previous studies have shown an increase in CT during thermoneutral treatments, whereas significant thermal energy had to be removed during isothermic (7,9,13). These earlier findings were confirmed in this study: CT increased by 0.3°C during the thermoneutral treatment, whereas a mean of 142 kJ had to be removed by the extracorporeal circuit to maintain CT unchanged during isothermic. The mechanism behind the internal heat accumulation during HD has still not been completely elucidated. It was initially suggested that cutaneous vasoconstriction in response to the decline in blood volume would impair heat transfer from the body core and the skin and thus result in a reduction in heat exchange between the patient and the environment (“skin insulation”) (17,25). The resulting increase in CT would then eventually evoke a reflex cutaneous vasodilation, resulting in a sudden decline in BP. However, in a recent controlled study, differences in the thermal response were not observed between dialysis treatments with or without concomitant ultrafiltration, which would appear to contradict the former hypothesis (18).
Various studies have shown vasodilation in the forearm or skin blood vessels during “standard” dialysis sessions with Td of 37°C or higher (26,27).
In contrast, “physiologic” vasoconstriction was observed during treatment in which the increase in CT is prevented, such as during cool isothermic dialysis, hemofiltration, or isolated ultrafiltration (7,26,28). In the study presented here, ST decreased during cool and isothermic dialysis, in contrast to thermoneutral treatments, which is an additional argument for skin vasoconstriction during the first treatments.
Given the relationship between the increase in CT during HD and cutaneous vasodilation, it would appear rational just to prevent this increase (isothermic treatment) to obtain a physiologic vascular response (13). However, the hemodynamic findings of this study suggest an additional hemodynamic benefit of a slight lowering of CT during dialysis. A possible explanation for this phenomenon is an increased mobilization of venous blood volume from the cutaneous blood vessels in response to body cooling, leading to an increase in venous return and better preservation of CBV. The tendency for CBV to remain higher during cooling might support this hypothesis, although statistical significance was not reached. CBV assessed by the saline dilution technique appears to be a relatively sensitive indicator for intradialytic hemodynamic changes due to thermal effects, because two studies have shown significant differences in CBV between cool and standard dialysis sessions (21,29).
To the best of our knowledge, our study is the first that has assessed heart rate variability in relation to thermal factors during dialysis. However, no significant differences in the heart rate variability spectrum were observed between the different treatments, although SD were observed. Previous studies have shown an increase in the LF/HF during stable dialysis treatments, whereas the HF component increased during treatments complicated by IDH (30,31). However, although the absence of differences in the heart rate variability response does not support a role for differences in the sympathetic tone as an explanation for the hemodynamic findings of this study, it should be stated that various authors have questioned the reliability of the LF/HF ratio as a synonym for sympatho-vagal balance (32).
Apart from the relatively small number of subjects, limitations of the study presented here are the observed relatively small hemodynamic differences between treatments. We therefore believe that it is by far too early to advocate body CT lowering during dialysis instead of isothermic treatments, especially because shivering complicated some cooling patients’ treatments. Our study should be primarily interpreted in relation to its pathophysiological findings and should not serve as an argument to change the dialysis prescription in clinical practice. A minor drawback in the performance of the study is that a decline of only 0.3°C was reached by the BTM instead of the prescribed decline of 0.5°C in CT during cooling. This is likely because we did not allow a decrease in Td below 35°C to prevent untoward symptoms and because dialysis time was relatively short in some of the patients. However, no relation was observed between the duration of the study and changes in CT during cooling. It is unlikely that this had a major effect on the assessment of findings, because differences in CT and energy transfer were significantly different between the three treatments. In agreement with others (33), we used the nadir SBP and not the incidence of IDH as the primary endpoint, because the sample size would have to be greatly increased if IDH had been chosen. Interestingly, the timing of the lowest recorded BP in our study does not appear to differ from some other studies in the literature; for example, in the study by Barnas et al., the timing of hypotension was 147 ± 29 min in patients treated for 240 min, therefore not basically different from the study presented here (31). These authors showed different trends for BP decline during dialysis; i.e., a gradual lowering of BP, with the lowest BP near the end of dialysis, and a more sudden drop of BP that generally occurs in the second part of the dialysis session because of a (paradoxical) reduction in sympathetic activity, but not necessarily at the end. The number of sessions included in our study, as well as the limited number of episodes of IDH, did not allow us to distinguish between these different BP trends with sufficient certainty.
Indeed, this study was not able to assess differences in IDH between treatments. However, our major aim was to study the pathophysiological response between different treatments, and not the effects on IDH per se.
A somewhat confusing finding is the lack of a significant difference in BP response between isothermic and thermoneutral, which is in contrast to previous observations (34). This is possibly also due to the small number of included patients. On the other hand, in agreement with expectations, the drop in CBV was the most pronounced during thermoneutral. Indeed, BP changes during dialysis are a resultant of various hemodynamic changes, whereas temperature changes basically only affect venous tone and systemic vascular resistance.
In the study presented here, we did not perform measurements on dialysis adequacy; however, a recent meta-analysis showed that cooling of the dialysate does not have adverse effects on dialysis adequacy (14).
In conclusion, intradialytic hemodynamic stability appears to be improved when a slight reduction in CT is prescribed compared with the isothermic approach, which may be related to increased venoconstriction leading to improved maintenance of CBV. However, the potential beneficial effects of mild blood cooling on hemodynamic stability should be balanced against a potentially higher risk of mild discomfort because of cold.
Disclosures
Renal Research Institute, LLC (RRI) was formed 10 years ago between Fresenius Medical Care, North America and Beth Israel Medical Center, New York, a teaching hospital of the Albert Einstein School of Medicine, New York. The research performed at RRI is independent and the authors are RRI research employees.
Acknowledgments
A best poster award was won at the annual meeting of the International Society of Blood Purification, September 15 through 17, 2007, Prague, Tsjech Republic. Some of the data were presented in oral form at the annual meeting of the American Society of Nephrology, October 31 through November 5, 2007, San Francisco, California.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Tisler A, Akocsi K, Borbas B, Fazakas L, Ferenczi S, Corogh S, Kulcsar I, Nagy L, Samik J, Szegedi J, Toth E, Wagner G, Kiss I: The effect of frequent or occasional dialysis-associated hypotension on survival of patients on maintenance haemodialysis. Nephrol Dial Transplant 18: 2601–2605, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Shoji T, Tsubakihara Y, Fujii M, Imai E: Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int 66: 1212–1220, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Kooman J, Basci A, Pizzarelli F, Canaud B, Haage P, Fouque D, Konner K, Martin-Malo A, Pedrini L, Tattersall J, Tordoir J, Vennegoor M, Wanner C, ter Wee P, Vanholder R: EBPG guideline on haemodynamic instability. Nephrol Dial Transplant 22[Suppl 2]: ii22–44, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Henderson LW: Hemodynamic instability during hemodialysis. Kidney Int 30: 605–612, 1986 [DOI] [PubMed] [Google Scholar]
- 5.Schneditz D, Levin NW: Keep your temper: How to avoid heat accumulation in haemodialysis. Nephrol Dial Transplant 16: 7–9, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Maggiore Q, Pizzarelli F, Sisca S, Zoccali C, Parlongo S, Nicolo F, Creazzo G: Blood temperature and vascular stability during hemodialysis and hemofiltration. Trans Am Soc Artif Intern Organs 28: 523–527, 1982 [PubMed] [Google Scholar]
- 7.van der Sande FM, Gladziwa U, Kooman JP, Bocker G, Leunissen KML: Energy transfer is the single most important factor for the difference in vascular response between isolated ultrafiltration and hemodialysis. J Am Soc Nephrol 11: 1512–1517, 2000 [DOI] [PubMed] [Google Scholar]
- 8.van der Sande FM, Kooman JP, Burema JH, Hameleers P, Kerkhofs AM, Barendregt JM, Leunissen KM: Effect of dialysate temperature on energy balance during hemodialysis: Quantification of extracorporeal energy transfer. Am J Kidney Dis 33: 1115–1121, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Provenzano R, Sawaya B, Frinak S, Polaschegg HD, Roy T, Zasuwa G, Dumler F, Levin NW: The effect of cooled dialysate on thermal energy balance in hemodialysis patients. ASAIO Trans 34: 515–518, 1988 [PubMed] [Google Scholar]
- 10.Sherman RA, Rubin MP, Cody RP, Eisinger RP: Amelioration of hemodialysis-associated hypotension by the use of cool dialysate. Am J Kidney Dis 5: 124–127, 1985 [DOI] [PubMed] [Google Scholar]
- 11.Jost CM, Agarwall R, Khair-el-din T, Grayburn PA, Victor RG, Henrich WL: Effects of cooler temperature dialysate on hemodynamic stability in “problem” dialysis patients. Kidney Int 44: 606–612, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Kaufman AM, Morris AT, Lavarias VA, Wang Y, Leung JF, Glabman MB, Yusuf SA, Levoci AL, Polaschegg HD, Levin NW: Effects of controlled blood cooling on hemodynamic stability and urea kinetics during high-efficiency hemodialysis. J Am Soc Nephrol 9: 877–883, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Maggiore Q, Pizzarelli F, Santoro A, Panzetta G, Bonforte G, Hannedouche T, Alvarezde Lara MA, Tsouras I, Loureiro A, Ponce P, Sulkova S, van Roost G, Brink H, Kwan JT. The effects of control of thermal balance on vascular stability in hemodialysis patients: results of the European randomized clinical trial. Am J Kidney Dis 40: 280–290, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Selby NM, McIntyre CW: A systematic review of the clinical effects of reducing dialysate fluid temperature. Nephrol Dial Transplant 21: 1883–1898, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Mahida BH, Dumler F, Zasuwa G, Fleig G, Levin NW: Effect of cooled dialysate on serum catecholamines and blood pressure stability. Trans Am Soc Artif Intern Organs 29: 384–389, 1983 [PubMed] [Google Scholar]
- 16.Rokyta R Jr, Matejovic M, Krouzecky A, Opatrny K Jr, Ruzicka J, Novak I: Effects of continuous venovenous haemofiltration-induced cooling on global haemodynamics, splanchnic oxygen and energy balance in critically ill patients. Nephrol Dial Transplant 19: 623–630, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Schneditz D, Martin K, Krämer M, Kenner T, Skrabal F: Effect of controlled extracorporeal blood cooling on ultrafiltration-induced blood volume changes during hemodialysis. J Am Soc Nephrol 8: 956–964, 1997 [DOI] [PubMed] [Google Scholar]
- 18.van der Sande FM, Rosales LM, Brener Z, Kooman JP, Kuhlmann M, Handelman G, Greenwood RN, Carter M, Schneditz D, Leunissen KM, Levin NW: Effect of ultrafiltration on thermal variables, skin temperature, skin blood flow, and energy expenditure during ultrapure hemodialysis. J Am Soc Nephrol 16: 1824–1831, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Schneditz D, Rosales L, Kaufman AM, Kaysen G, Levin NW: Heat accumulation with relative blood volume decrease. Am J Kidney Dis 40: 777–782, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Schneditz D, Pogglitsch H, Horina J, Binswanger U: A blood protein monitor for the continuous measurement of blood volume changes during hemodialysis. Kidney Int 38: 342–346, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Beerenhout C, Dejagere T, van der Sande FM, Bekers O, Leunissen KM, Kooman JP: Haemodynamics and electrolyte balance: A comparison between on-line pre-dilution haemofiltration and haemodialysis. Nephrol Dial Transplant 19: 2354–2359, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Malliani A, Lombardi F, Pagani M. Power spectrum analysis of heart rate variability: A tool to explore neural regulatory mechanisms. Br Heart J 71: 1–2, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagani M, Lombardi F, Guzzetti S, Rimaldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell'Orte S, Piccaluga E: Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 59: 178–193, 1986 [DOI] [PubMed] [Google Scholar]
- 24.Santoro A, Mancini E, Basile C, Amoroso L, Di Giulio S, Usberti M, Colasanti G, Verzetti G, Rocco A, Imbasciati E, Panzetta G, Bolzani R, Grandi F, Polacchini M: Blood volume controlled hemodialysis in hypotension-prone patients: A randomized, multicenter controlled trial. Kidney Int 62: 1034–1045, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Gotch FA, Keen ML, Yarian SR: An analysis of thermal regulation in hemodialysis with one and three compartment models. Trans Am Soc Artif Intern Organs 35: 622–624, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Kooman JP, Gladziwa U, Bocker G, van Bortel LM, van Hooff JP, Leunissen KM: Role of the venous system in hemodynamics during ultrafiltration and bicarbonate dialysis. Kidney Int 42: 718–726, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Van Kuijk WH, Luik AJ, de Leeuw PW, van Hooff JP, Nieman FH, Habets HM, Leunissen KM: Vascular reactivity during haemodialysis and isolated ultrafiltration: Thermal influences. Nephrol Dial Transplant 10: 1852–1858, 1995 [PubMed] [Google Scholar]
- 28.van Kuijk WH, Hillion D, Savoiu C, Leunissen KM: Critical role of the extracorporeal blood temperature in the hemodynamic response during hemofiltration. J Am Soc Nephrol 8: 949–955, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Hoeben H, Abu-Alfa AK, Mahnensmith R, Perazella MA: Hemodynamics in patients with intradialytic hypotension treated with cool dialysate or midodrine. Am J Kidney Dis 39: 102–107, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Cavalcanti S, Severi S, Chiari L, Avanzolini G, Enzmann G, Bianco G, Panzetta G: Autonomic nervous function during haemodialysis assessed by spectral analysis of heart-rate variability. Clin Sci (Lond) 92: 351–359, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Barnas MG, Boer WH, Koomans HA: Hemodynamic patterns and spectral analysis of heart rate variability during dialysis hypotension. J Am Soc Nephrol 10: 2577–2584, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Notarius CF, Floras JS: Limitations of the use of spectral analysis of heart rate variability for the estimation of cardiac sympathetic activity in heart failure. Europace 3: 29–38, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Cruz DN, Mahnensmith RL, Brickel HM, Perazella MA: Midodrine and cool dialysate are effective therapies for symptomatic intradialytic hypotension. Am J Kidney Dis 33: 920–926, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Pizzarelli F: From cold dialysis to isothermic dialysis: A twenty-five year voyage. Nephrol Dial Transplant 22: 1007–1012, 2007 [DOI] [PubMed] [Google Scholar]