Abstract
Background and objectives: Chronic kidney disease (CKD) is characterized by an exceptionally high mortality rate, primarily due to cardiovascular disease. Reduced soluble TNF-like weak inducer of apoptosis (sTWEAK) plasma levels have been reported both in patients with subclinical atherosclerosis and CKD.
Design, participants, & measurements: A cross-sectional study was conducted in 218 prevalent patients (121 men; 63 ± 14 yr) undergoing hemodialysis (HD). sTWEAK levels in relation with the patients’ outcome were studied.
Results: sTWEAK plasma levels were 208 [(165 to 272) pg/ml, median interquartile range], significantly lower than healthy controls (P < 0.0001). sTWEAK was negatively associated with inflammatory markers, such as C-reactive protein and IL-6. Overall mortality was assessed after an average follow-up of 31 mo, during which 81 patients died. After controlling for potential confounding variables, patients in the upper tertile of sTWEAK plasma levels had an increased risk of cardiovascular and all-cause mortality. A significant interaction effect between sTWEAK and IL-6 levels was found [synergy index: 2.19 (0.80, 5.93)]. Thus, the association of sTWEAK with mortality was strongest in patients with inflammation (defined as IL-6 > 7.0 pg/ml), in whom high sTWEAK strongly predicted cardiovascular and all-cause mortality. These results were confirmed in a second cohort of HD patients.
Conclusions: The concurrent presence of elevated sTWEAK plasma concentrations and an inflammatory environment have additive effects on mortality in HD patients. Further studies on the potential different role of sTWEAK in health and disease are warranted.
Cardiovascular disease (CVD) is the leading cause of death in patients with chronic kidney disease (CKD) (1,2). Because the lack of kidney function predisposes to a chronic inflammatory state (3) and an increase in both traditional and nontraditional risk factors (4), CKD may represent a clinical model of accelerated atherosclerosis (1,2). Because CKD patients seem to have a different risk factor profile than the general population (4), further understanding of these risk factors and the search of valid risk markers is needed to improve this poor survival (4,5). Better prognoses and early identification of patients at risk could in turn improve decision-making and clinical care.
TNF-like weak inducer of apoptosis (TWEAK, TNFSF12) is a member of the TNF superfamily of structurally related cytokines (6). The human TWEAK gene encodes a 249-amino acid type II transmembrane glycoprotein (30 kD). TWEAK may be expressed as a full-length, membrane-bound protein and as a 156-amino acid, 18-kD soluble protein, (sTWEAK) that results from proteolysis of TWEAK (7,8). TWEAK gene is expressed in many tissues, including brain, kidney, heart, arterial wall, monocytes and macrophages. In contrast, the expression of its receptor, fibroblast growth factor-inducible 14 (Fn14) is usually low in healthy tissues, including the normal vascular wall (9). Binding of TWEAK to Fn14 (8) mediates different biologic effects, such as induction of cellular growth and proliferation (10,11), osteoclastogenesis (12), angiogenesis (13), and, in an inflammatory microenvironment, stimulation of apoptosis (14). Moreover, TWEAK attenuates the transition from innate to adaptive immunity (15), activates nuclear factor kappa B signaling pathway, and induces the expression of different proinflammatory cytokines and cell adhesion molecules (16–18).
There is limited and contradictory information on the TWEAK-Fn14 signaling pathway. Although human atherosclerotic plaques release sTWEAK in lower amounts (19), increased TWEAK levels could have a role in the pathogenesis of inflammatory diseases (10,20,21). Thus, the existence of differential effects of TWEAK in diseased versus healthy tissue dependent on Fn14 expression levels has been proposed (7).
We demonstrated that TWEAK significantly activates the inflammatory response during kidney injury (22). However, patients with CKD exhibit lower levels of sTWEAK than healthy controls (23). Whether TWEAK plays a role in the inflammation-atherosclerosis axis present in CKD currently remains unknown. Thus, in this study we measured sTWEAK plasma levels in a well characterized cohort of prevalent patients undergoing hemodialysis (HD) and studied its effect on survival.
Materials and Methods
Patients
The study was performed at five dialysis units in Stockholm and one at Uppsala Academic Hospital. This is a post hoc analysis from a cross-sectional study including prevalent HD patients previously described in more detail (24). The only exclusion criteria were unwillingness to participate and infectious diseases. From 228 patients originally included in the study, ten patients had insufficient stored plasma available for sTWEAK analysis, leaving 218 patients. The Ethics Committee of Karolinska Institutet and Uppsala University Hospital approved the study protocols.
Each patient's medical chart was reviewed, extracting data pertaining to underlying kidney disease, history of CVD, other comorbid conditions, and survival. Clinical signs of CVD were found in 139 patients (63%). Of these, 47 patients had one or more myocardial infarctions, 42 angina pectoris, seven percutaneous transluminal coronary angiography, 23 coronary artery bypass surgery, and 47 left ventricular dysfunction. Forty-three patients suffered cerebrovascular disease, 39 had signs of peripheral atherothrombotic vascular disease, and four had an aortic aneurysm.
Survival was determined from the day of examination, with a mean follow-up period of 31 (range 3 to 41) months. There was no loss of follow-up of any patient. Cardiovascular mortality was defined as death as a result of coronary heart disease, sudden death, stroke, or complicated peripheral vascular disease. Causes of death were registered by nephrologists blind to other clinical or biochemical data of the patients.
Healthy Controls
Age- and sex-matched blood donors {n = 40, median age 60 [interquartile range (IQR) 58 to 63] yr; 60% men} were recruited in the Fundación Jiménez-Díaz (Madrid) as controls. Control subjects did not present hypertension, hypercholesterolemia, diabetes, wasting, metabolic syndrome, or history of CVD at the time of blood extraction.
Independent Cohort
To confirm our findings, sTWEAK levels were measured in a cohort of CKD stage 5 patients from the Karolinska University Hospital at Huddinge, Stockholm, whose original protocol was described elsewhere (25). This cohort consisted of 79 patients [65% men, median age 58 (IQR 43 to 64) yr] who were examined approximately after 1 yr of start of HD therapy (median time after commencement of HD 12 (11 to 13) mo). Patients <18 or >70 yr, with HIV, hepatitis B, and signs of acute infection or unwilling to participate were excluded from the protocol. Presence of diabetes mellitus or CVD was documented in 30 and 29% of the patients, respectively. The patients were followed-up a median of 39 (21 to 64) mo from the time of blood extraction. Follow-up was censored on transplantation and no patient was lost to follow-up.
Laboratory Analyses
Blood samples were collected before the HD session. Plasma and serum were kept frozen at −70°C if not analyzed immediately. In both cohorts, serum concentrations of IGF-1, IL-6, and TNF-α were quantified on the Immulite automatic analyzer (Diagnostic Products Corporation, Los Angeles, California, USA). High-sensitivity C-reactive protein (CRP), fibrinogen, and albumin concentrations were analyzed using routine methods at the Karolinska University Hospital. Serum cholesterol and triglycerides were analyzed by standard enzymatic procedures (Roche Diagnostics GmbH, Mannheim, Germany).
Plasma concentrations of sTWEAK were determined in duplicate with commercially available enzyme-linked immunosorbent assay kits (BMS2006INST; Bender MedSystems). The minimum detectable level of sTWEAK was 10 pg/ml. Intra- and interassay coefficients of variation were 6.4 and 8.1%, respectively.
Nutritional Status
Body mass index and dynamometric measurements were determined on a dialysis day. Handgrip strength was measured in the dominant hand with a Harpenden Handgrip Dynamometer. The highest of three measurements was noted, and values were then normalized for that of age- and gender-matched healthy Swedish individuals (26). Subjective global assessment was used to evaluate the overall protein-energy wasting (PEW) (27).
Statistical Analyses
All variables were expressed as mean ± SD or as median (IQR), unless otherwise indicated. Comparisons between two groups were assessed with the Student's unpaired t test and Mann–Whitney test or χ2 test, as appropriate. Differences between more than two groups were analyzed by ANOVA using one-way ANOVA or Kruskal–Wallis test, as appropriate, followed by a post-hoc test if ANOVA was significant. Spearman's rank correlation (ρ) was used for univariate analysis. We performed a receiver operator characteristic curve analysis to determine the IL-6 cutoff point in our patient material of maximum sensitivity and highest specificity for prognosis of all-cause mortality. Such cutoff point was set at a level of 7.0 pg/ml, and this value was used in these analyses to define inflammation. Survival analyses were made with the Kaplan–Meier survival curve and the Cox proportional hazard model, presenting data as hazard ratio [HR; 95% confidence intervals (CI)].
Finally, we examined the presence of biologic interactions between inflammation and elevated sTWEAK levels on mortality. Crude and adjusted HR with 95% CI were estimated for each category using noninflamed patients with low sTWEAK values as the reference category. An interaction effect was defined as departure from causal additivity of effects (28). Following this concept, we calculated the relative excess risk due to interaction (RERI), the synergy index (S) and the attributable proportion (AP) due to interaction, according to Andersson et al. (29). All statistical analyses were performed using SAS version 9.1.3 (SAS Campus Drive, Cary, North Carolina, USA).
Results
sTWEAK Plasma Levels in HD Patients
General characteristics are summarized in Table 1 and compared with values of healthy controls. Patients were treated with HD three times a week (4 to 5 h per session) using bicarbonate dialysate and high-flux (24%) or low-flux (76%) dialysis membranes. The average Kt/V was 1.58 ± 0.35. Most patients were on antihypertensive medications [β-blockers, n = 108; calcium-channel blockers, n = 55; and angiotensin converting enzyme inhibitors/angiotensin receptor blockers, n = 71], as well as other commonly used drugs (such as phosphate and potassium binders) and vitamins B, C, and D. Seventy-one patients were on statins, and 210 patients were receiving erythropoiesis stimulating agents (ESA) at time of evaluation. The median ESA dose was equivalent to 10.000 (6.000 to 14.750) U/wk.
Table 1.
General characteristics of the 218 prevalent hemodialysis patients and 40 healthy controls included in the studya
| Parameters | Healthy Controls (n = 40) | Hemodialysis Patients (n = 218) | P |
|---|---|---|---|
| Age (yr) | 60 (58 to 63) | 66 (51 to 74) | NS |
| Men (%) | 60 | 55 | NS |
| Dialysis vintage time (mo) | Not applicable | 29 (15 to 57) | - |
| Cardiovascular disease (%) | 0 | 64 | - |
| Diabetes (%) | 0 | 26 | - |
| Protein-energy wastingb (%) | 0 | 46 | - |
| Albumin (g/L) | 42 ± 2 | 34 ± 5 | <0.0001 |
| sTWEAKc (pg/ml) | 461 (389 to 542) | 208 (165 to 272) | <0.0001 |
Values expressed as mean ± SD, median (interquartile range) or percent of patients.
Protein-energy wasting was assessed by subjective global assessment.
sTWEAK, soluble TNF-like weak inducer of apoptosis.
sTWEAK plasma levels were significantly decreased in the HD population as compared with the healthy controls [median (IQR) sTWEAK 208 (165 to 272) versus 461 (389 to 542) pg/ml, respectively; P < 0.0001]. Whereas 139 patients with clinical CVD showed even lower sTWEAK concentrations [198 (150 to 266) versus 225 (175 to 274) pg/ml; P < 0.05], no difference was observed in wasted or diabetic patients (not shown). No difference was found according to the prescription of statins, angiotensin converting enzyme inhibitors/angiotensin receptor blockers, ESA, β-blockers, or calcium-channel blockers. However, patients on acetyl-salicylic acid or derivates (ASA; n = 152) presented higher sTWEAK levels [226 (174 to 304) versus 203 (152 to 255) pg/ml, P < 0.01].
Univariate Correlates for sTWEAK Levels
sTWEAK levels were negatively associated with inflammatory markers including CRP (ρ = −0.16, P = 0.01), IL-6 (ρ = −0.17, P = 0.009), fibrinogen (ρ = −0.20, P = 0.001) and white blood cell count (ρ = −0.14, P = 0.04), but not with TNF-α. A negative association was observed for IGF-1 (ρ = −0.19, P = 0.004), whereas a positive association was seen for triglycerides (ρ = 0.15, P = 0.02). A nonsignificant positive trend with preceding time on maintenance dialysis therapy (dialysis vintage) was observed (ρ = 0.12, P = 0.06).
Conditions Associated with sTWEAK Plasma Concentrations
To study conditions associated with increased sTWEAK levels in the HD population range, we defined high sTWEAK as those levels above the 66th percentile (upper tertile) of distribution (>244 pg/ml). We should remind the reader that, for a correct interpretation of our results, our definitions of low and high sTWEAK levels correspond to the HD patients’ range. General characteristics according to this division are listed in Table 2, Panel A. Patients with low sTWEAK presented shorter dialysis vintage and a nonsignificant trend toward a higher prevalence of a clinical history of CVD (P = 0.06). Lower IGF-1 levels and lower handgrip strength were observed in the patients with high sTWEAK. Although levels of CRP or TNF-α remained unchanged (not shown), patients with low sTWEAK values presented higher IL-6 values [9.2 (5.5 to 16.0) versus 7.2 (4.2 to 12.1) pg/ml; P = 0.01]. Among those who died, a significantly shorter time to death was observed for the patients with high sTWEAK levels.
Table 2.
General characteristics of 218 prevalent HD patients according to their sTWEAK levels (Panel A) or the interaction IL-6/sTWEAK levels (Panel B)a
| Variable | Panel A, sTWEAK levels
|
Panel B, Interaction sTWEAK-Inflammation (IL-6)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Low IL-6 (≤7.0 pg/ml)
|
High IL-6 (>7.0 pg/ml)
|
P | ||||||
| Low sTWEAK ≤244 pg/ml (n = 144) | High sTWEAK >244 pg/ml (n = 74) | P | Low sTWEAK ≤244 pg/ml (n = 50) | High sTWEAK >244 pg/ml (n = 36) | Low sTWEAK ≤244 pg/ml (n = 94) | High sTWEAK >244 pg/ml (n = 38) | ||
| Age (yr) | 66 (52 to 74) | 66 (47 to 75) | NS | 58 (48 to 71) | 56 (41 to 74) | 68 (57 to 76) | 68 (59 to 76) | 0.005 |
| Dialysis vintage (mo) | 27 (13 to 48) | 35 (17 to 75) | 0.01 | 27 (12 to 51) | 29 (16 to 70) | 27 (14 to 46) | 34 (18 to 86) | NS |
| Sex, n (% men) | 80 (55%) | 71 (55%) | NS | 30 (60%) | 19 (53%) | 50 (53%) | 22 (58%) | NS |
| Diabetes, n (%) | 37 (25%) | 19 (25%) | NS | 9 (18%) | 8 (22%) | 28 (30%) | 11 (29%) | NS |
| CVD, n (%) | 98 (68%) | 41 (55%) | NS | 31 (62%) | 13 (36%) | 67 (71%) | 28 (73%) | 0.001 |
| Wasting (SGA>1), n (%)b | 65 (45%) | 34 (47%) | NS | 14 (28%) | 11 (30%) | 51 (55%) | 23 (64%) | 0.0006 |
| BMI, Kg/m2 | 24.4 ± 5.2 | 24.1 ± 4.9 | NS | 23.4 ± 3.5 | 24.4 ± 5.2 | 24.9 ± 5.9 | 23.9 ± 4.7 | NS |
| s-Albumin, g/L | 34 ± 4 | 35 ± 5 | NS | 37 ± 4 | 37 ± 3 | 33 ± 4 | 33 ± 5 | <0.0001 |
| Handgrip strength (%) | 61.2 | 55.5 | 0.03 | 72 ± 22 | 64 ± 27 | 58 ± 19 | 50 ± 20 | <0.0001 |
| IGF-1, ng/ml | 173 (125 to 242) | 137 (96 to 203) | 0.01 | 181 (135 to 256) | 182 (125 to 257) | 172 (115 to 225) | 106 (77 to 160) | <0.0001 |
| Time to death (mo) | 19 ± 8 | 14 ± 9 | 0.01 | 18 ± 6 | 19 ± 9 | 18 ± 8 | 13 ± 8 | <0.05 |
Data are expressed as mean ± SD, median (interquartile range), or number of patients (%).
SGA, subjective global assessment; BMI, body mass index; CVD, cardiovascular disease.
Effect of sTWEAK on Survival and Relative Risks
During the follow-up period (median 31 (20 to 38) months) 81 patients died, 34 (44%) of which were due to CVD-related diseases, such as myocardial infarction (n = 12), sudden death (n = 9); thrombotic stroke (n = 3), hemorrhagic stroke (n = 2), aneurysm (n = 1), mesenteric infarction (n = 1), or other CVD-related causes (n = 6). Kaplan–Meier curves showed a trend toward high sTWEAK levels being associated with worse survival. Although this difference did not attain statistical significance, high sTWEAK levels conferred a somewhat increased mortality risk (Table 3). To eliminate the effect of traditional confounding factors in HD patients, a Cox-proportional Hazards model was performed. After adjustment for age, sex, IL-6, comorbidities, intake of ASA and dialysis vintage, high sTWEAK levels were associated with an increased mortality risk. The same adjustment was performed using different definitions of inflammation, mainly CRP > 10 mg/L or fibrinogen > 4.8 μmol/L (>66th percentile), results being the same (data not shown). When only cardiovascular death was assessed, a similar pattern was observed but with relatively higher HR (Table 3).
Table 3.
Crude and adjusted relative risk of all-cause and cardiovascular-related mortality in 218 prevalent patients undergoing hemodialysisa
| Variable | All-Cause Mortality (Crude)
|
All-Cause Mortality (Adjusted)
|
Cardiovascular Disease Mortality (Crude)
|
Cardiovascular Disease Mortality (Adjusted)
|
||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | P | Hazard Ratio | P | Hazard Ratio | P | Hazard Ratio | P | |
| sTWEAK levels | ||||||||
| low sTWEAK (n = 144) | 1.00 | 1.00b | 1.00 | 1.00b | ||||
| high sTWEAK (n = 74) | 1.22 (0.78 to 1.90) | NS | 1.94 (1.13 to 2.99) | 0.01 | 1.52 (0.76 to 3.01) | NS | 2.66 (1.24 to 5.62) | 0.01 |
| Interaction sTWEAK-inflammation | ||||||||
| low IL-6, low sTWEAK (n = 50) | 1.00 | 1.00c | 1.00 | 1.00c | ||||
| low IL-6, high sTWEAK (n = 36) | 0.99 (0.37 to 2.61) | NS | 1.35 (0.50 to 3.61) | NS | 0.93 (0.15 to 5.59) | NS | 1.32 (0.21 to 8.15) | NS |
| high IL-6, low sTWEAK (n = 94) | 2.70 (1.36 to 5.38) | 0.004 | 1.92 (0.95 to 3.87) | NS | 3.40 (1.10 to 11.83) | 0.05 | 2.43 (0.70 to 8.44) | NS |
| high IL-6, high sTWEAK (n = 38) | 4.72 (2.24 to 9.94) | <0.0001 | 3.91 (1.80 to 8.45) | 0.0005 | 7.85 (2.21 to 27.89) | 0.001 | 7.45 (1.98 to 27.9) | 0.002 |
Data are presented as hazard ratios (95% confidence intervals) and P values. High sTWEAK was defined as above the 66th percentile (see results). For interaction analyses only (Interaction sTWEAK-inflammation), IL-6 groups were defined by receiver operating characteristic cutoff points (see results).
Adjusted for age, sex, Davies comorbidity index, acetylsalicylic acid intake, dialysis vintage, and IL-6.
Adjusted for age, sex, Davies comorbidity index, acetylsalicylic acid intake, and dialysis vintage.
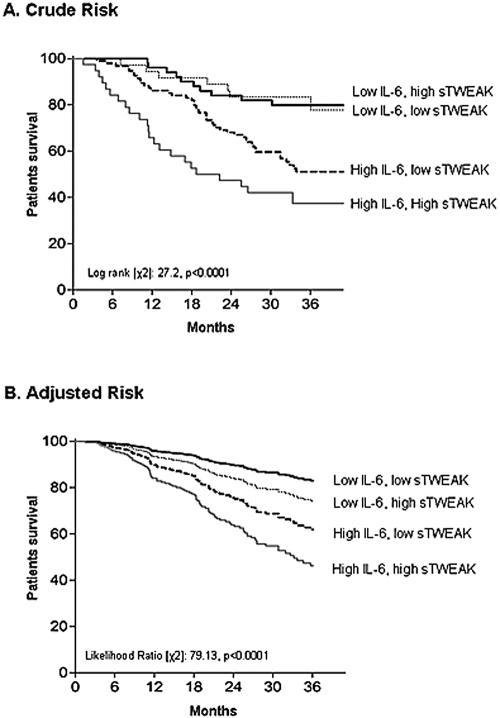
Recent reports have highlighted IL-6 levels as the best predictor of mortality in the renal population (30,31). Because sTWEAK induces cytokine response, and in particular IL-6 expression (22,32), we studied the potential additive effects of inflammation (defined as IL-6 > 7.0 pg/ml; cutoff value derived from receiver operator characteristic curve analysis) and sTWEAK levels in relation to outcome. In other words, we tested whether sTWEAK levels may add any prognostic gain to that of IL-6 alone. Thus, we divided the patients into four groups on the basis of the sTWEAK groups (> and ≤244 pg/ml) and IL-6 levels (> and ≤7.0 pg/ml) as follows: Group I (n = 50) included patients who had no signs of inflammation and low sTWEAK, group II (n = 36) included patients with no signs of inflammation and high sTWEAK, group III (n = 94) included patients who had signs of inflammation and low sTWEAK, and group IV (n = 38) included patients with both signs of inflammation and high sTWEAK. As expected, in the nonadjusted analysis (Table 3) patients from groups III and IV had a significantly higher all-cause mortality (Figure 1, panel A). However, this relationship only remained significant in group IV after adjustment for age, sex, Davies comorbidity score, intake of ASA, and dialysis vintage (Figure 1, panel B). The same analysis was performed using different definitions of inflammation [i.e., IL-6 > 11.4 pg/ml (>66th percentile), CRP >10 mg/L, and fibrinogen >4.8 μmol/L (>66th percentile)], results being the same (not shown). When only cardiovascular death was assessed, a similar behavior was observed but with higher HR (Table 3).
Figure 1.
(A) Crude and (B) adjusted mortality risk in patients undergoing hemodialysis according to their levels of IL-6 and soluble TNF-like weak inducer of apoptosis (sTWEAK). High sTWEAK was defined as sTWEAK concentration above the 66th percentile (>244 pg/ml). High IL-6 was defined as IL-6 > 7.0 pg/ml (determined by receiver operator characteristic curves). Adjustment of the Cox was done for age, sex, Davies comorbidity score, intake of acetylsalicylic acid, and dialysis vintage.
Biologic interaction effects were calculated for crude and adjusted all-cause HR. In crude mortality, elevated sTWEAK levels showed a significant interaction effect [S = 2.19 (0.80, 5.93)] with inflammation. The RERI was 2.02 (−0.29, 4.33) and the AP due to interaction was 43%. Thus, the HR for patients with both inflammation and elevated sTWEAK was 2.19 times higher than the sum of the independent relative risk of each effect separated. After adjustment for confounding factors (age, sex, Davies comorbidity score, and dialysis vintage), the S was 2.27 (0.70, 7.29), the RERI was 1.64 (−0.21, 3.50), and the AP due to interaction was 42%.
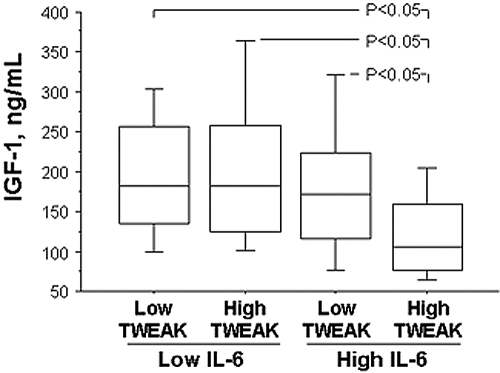
A more detailed analysis revealed that there were no differences in gender, dialysis vintage, or the presence of diabetes among these four IL-6/sTWEAK groups (Table 2, Panel B). As expected, the inflamed patient groups were older and had a higher prevalence of PEW. Among those who died during the follow-up period, a significantly shorter time to death was limited to the high sTWEAK and inflamed group. Of interest, decreased IGF-1 levels were observed in that same group (Figure 2). A similar observation was made for handgrip strength, a surrogate marker for muscle mass.
Figure 2.
Serum IGF-1 concentration in different sTWEAK/IL-6 groups. Sidak–Ansari post hoc analysis of pair differences at the level of α = 0.05.
Replication of the Results
The effect of inflammation and elevated sTWEAK on survival was studied in an independent cohort of 79 patients undergoing HD for about 12 mo. sTWEAK levels [median 217 (143–285) pg/ml] were similar to the previous cohort of patients and significantly lower (P < 0.0001) than the healthy controls. Although the low sample size prevented statistical significance, elevated sTWEAK levels per se were associated with increased nonsignificant HR for both all-cause and CVD-related mortality (Table 4) in a magnitude similar to the previous cohort (Table 3). The interaction sTWEAK-inflammation was studied by creating four groups according to median IL-6 (>4.8 pg/ml) and median sTWEAK (>217 pg/ml) levels (Table 4). Even with this low sample size, our previous results were replicated. Although inflamed groups presented increased HR, the group with both elevated IL-6 levels and elevated sTWEAK exhibited the highest and significant HR for all-cause and CVD-related mortality. Because of the limited sample size, further adjustments of the Cox were only performed for age and sex. The same analysis was performed using a different definition for inflammation; that is, CRP > 4.0 mg/L (median), results being the same (not shown).
Table 4.
Crude and adjusted relative risk of all-cause and cardiovascular-related mortality in an independent cohort of 79 hemodialysis patientsa
| Variable | All-Cause Mortality (Crude)
|
All-Cause Mortality (Adjusted)b
|
Cardiovascular Disease Mortality (Crude)
|
Cardiovascular Disease Mortality (Adjusted)b
|
||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | P | Hazard Ratio | P | Hazard Ratio | P | Hazard Ratio | P | |
| sTWEAK levels | ||||||||
| low sTWEAK (n = 40) | 1.00 | 1.00b | 1.00 | 1.00b | ||||
| high sTWEAK (n = 39) | 1.21 (0.53 to 2.75) | NS | 1.91 (0.82 to 4.43) | NS | 1.78 (0.53 to 5.97) | NS | 2.91 (0.86 to 10.10) | NS |
| Interaction sTWEAK-inflammation | ||||||||
| low IL-6, low sTWEAK (n = 22) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| low IL-6, high sTWEAK (n = 18) | 0.43 (0.07 to 2.61) | NS | 1.62 (0.34 to 19.80) | NS | 0.62 (0.03 to 7.96) | NS | 1.42 (0.07 to 37.48) | NS |
| high IL-6, low sTWEAK (n = 24) | 2.64 (0.68 to 10.25) | NS | 3.77 (0.84 to 16.85) | NS | 3.32 (0.44 to 32.06) | NS | 5.07 (0.41 to 61.80) | NS |
| high IL-6, high sTWEAK (n = 15) | 5.16 (1.46 to 18.25) | 0.01 | 4.68 (1.23 to 17.70) | 0.02 | 9.14 (1.13 to 73.70) | 0.03 | 9.24 (0.99 to 82.10) | 0.05 |
Data are presented as hazard ratios (95% confidence intervals) and P values. sTWEAK and IL-6 values were defined according to the median values (see results).
Adjusted for age and sex.
Discussion
Ours is the first report in the literature investigating the association between sTWEAK and mortality in any patient population. The results showed, in two independent cohorts, that sTWEAK may be an additive, but not a primary marker, of the high mortality rate seen in HD patients with systemic inflammation.
In agreement with Kralisch et al. (23), sTWEAK levels were much lower in HD patients as compared with healthy individuals with similar age, indicating that the reference range for sTWEAK may differ between HD patients and individuals with normal renal function. However, in contrast to that report, no difference in sTWEAK values was observed between diabetic and nondiabetic patients. The finding that HD subjects with history of CVD have even lower circulating sTWEAK is in agreement with previous reports in subjects with carotid atherosclerosis (19). Moreover, in our study sTWEAK was inversely associated with several inflammatory biomarkers such as CRP, IL-6, and fibrinogen, concurrent with a previous report (33). However, within the HD patients’ range, higher sTWEAK per se was associated with a shorter time to death in our study and, after adjustment for traditional confounding factors, the HD patients with high sTWEAK were at increased cardiovascular and all-cause mortality risk. This was much more evident in patients with both increased IL-6 and sTWEAK levels, in which a biologic interaction effect between both risk factors was observed, even after adjusting for confounders.
There is an apparent contradiction between the fact that sTWEAK levels are negatively related to inflammatory cytokines and the association found in our study of high sTWEAK/high IL-6 levels with mortality. TWEAK research is in its infancy and the mechanism(s) leading to lower sTWEAK levels in some animal models of inflammation (33), in patients with subclinical atherosclerosis (19), or in our patients remains poorly understood. However, a pathogenic role of TWEAK has recently been demonstrated in animal models of inflammatory kidney disease characterized by a marked upregulation of the TWEAK receptor (Fn14) (20,22). In healthy conditions, TWEAK is expressed in most tissues, but Fn14 expression is relatively low. However, Fn14 gene induction has been reported in various models of tissue injury (7). In cultured cells, induction of Fn14 expression resulted in increased responsiveness to the putative injurious functions of TWEAK, such as apoptosis in tubular cells (14) and proinflammatory actions in vascular smooth muscle cells (9). Indeed, Fn14 is undetectable in the healthy arterial wall, but it is increased in human atherosclerotic plaques (9,14). Thus, our findings are consistent with the hypothesis that low levels of sTWEAK in diseases with an inflammatory component may be a compensating mechanism to protect from the adverse consequences of Fn14 engagement (7). Under inflammatory conditions any minor upward change in sTWEAK might engage overexpressed Fn14 receptors (7).
Finally, nothing is yet known about the clinical implications of elevated sTWEAK in HD patients. Recently, it has been demonstrated that sTWEAK acts a strong muscle-wasting inducing agent through activation of the ubiquitin-proteasome and nuclear factor kappa B pathways (34). Because several proinflammatory cytokines, including IL-6, also play a key role in the loss of muscle mass and function (35), it is confirmatory that a condition with both increased IL-6 and sTWEAK was associated with significant reductions in two surrogate markers of PEW and muscle mass (i.e., IGF-1 and handgrip strength). Also, in the context of prolonged tissue injury TWEAK may promote chronic inflammation and tissue degeneration through production of cytokines, chemokines, and pathologic angiogenesis (36).
Some limitations of this study should be considered. First, our cross-sectional design precludes from causality. Second, although confirmed in two independent cohorts, the relatively low number of patients and deaths in each of those may limit our findings, which would need to be verified and should be understood as a hypothesis-generating study. Third, the classification of CVD included patients with clinically significant vascular disease, which may underestimate the true prevalence of CVD. Fourth, the prevalent nature of our cohort may represent a selection of patients who have survived from CVD or survived despite presence of factors potentially contributing to increased cardiovascular risk. However, adjustment for dialysis vintage was considered in our statistical analyses. Finally, the relative contribution of altered synthesis versus clearance (receptor-mediated, renal, dialysis) of sTWEAK to the sTWEAK concentrations or the variability of sTWEAK over time has not been explored and warrants further study.
Conclusions
Our study suggests that HD patients have lower sTWEAK levels and a lower range than controls with normal renal function. Within the HD population range, high sTWEAK plasma levels have additive effects to the high cardiovascular and all-cause mortality of HD patients with systemic inflammation through pathways that may relate to increased muscle wasting. Thus, the combined use of IL-6 and sTWEAK may help to identify a subpopulation of HD patients at particularly increased mortality risk. Further prospective studies confirming these results and targeting the potential different role of sTWEAK in health and disease are warranted.
Disclosures
Bengt Lindholm and Belén Marrón are employed by Baxter Healthcare.
Acknowledgments
We would like to thank the patients and personnel involved in the MIMICK study. Also, we are indebted to our research staff at KBC (Ann Dreiman-Lif, Annika Nilsson and Anki Emmot) and KFC (Björn Anderstam, Monica Ericsson and Anki Bragfors-Helin). We also thank Drs de Mutsert, Verduijn, and Dekker from the Department of Clinical Epidemiology at the Leiden University Medical Centre for their teaching on interaction analyses.
This cohort was supported by an unrestricted grant from Amgen Inc. Further support was given by the ERA-EDTA, KI Research Funds, Swedish Medical Research Council, Scandinavian Clinical Nutrition AB, Swedish Export Association, FIS (CP04-00060, 06-0046), MEC (SAF2007-60896; SAF2007-63648, EX2006-1670), CAM (S2006-GEN-0247, S-BIO-0283-2006), MSC, Instituto de Salud Carlos III (RD06-0014-0035, RETICS06-0016), Mutua Madrileña, ISCIII/Agencia Laín-Entralgo/CM (Programa Intensificación Actividad Investigadora), and the Heart and Lung Foundation.
Published online ahead of print. Publication date available at www.cjasn.org.
P.S. and L.M.B.-C. share equal responsibility as senior authors.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Tonelli M, Pfeffer MA: Kidney disease and cardiovascular risk. Ann Rev Med 58: 123–139, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Stenvinkel P, Wanner C, Metzger T, Heimburger O, Mallamaci F, Tripepi G, Malatino L, Zoccali C: Inflammation and outcome in end-stage renal failure: Does female gender constitute a survival advantage? Kidney Int 62: 1791–1798, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimburger O, Massy Z: Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 3: 505–521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, Klag MJ, Mailloux LU, Manske CL, Meyer KB, Parfrey PS, Pfeffer MA, Wenger NK, Wilson PW, Wright, JT Jr: Controlling the epidemic of cardiovascular disease in chronic renal disease: What do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis 32: 853–906, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, Garcia I, Browning JL: TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem 272: 32401–32410, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Winkles, JA: The TWEAK-Fn14 cytokine-receptor axis: Discovery, biology and therapeutic targeting. Nat Rev Drug Discov 7: 411–425, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiley SR, Winkles JA: TWEAK, a member of the TNF superfamily, is a multifunctional cytokine that binds the TweakR/Fn14 receptor. Cytokine Growth Factor Rev 14: 241–249, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Munoz-Garcia B, Martin-Ventura JL, Martinez E, Sanchez S, Hernandez G, Ortega L, Ortiz A, Egido J, Blanco-Colio LM: Fn14 is upregulated in cytokine-stimulated vascular smooth muscle cells and is expressed in human carotid atherosclerotic plaques: Modulation by atorvastatin. Stroke 37: 2044–2053, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Desplat-Jego S, Varriale S, Creidy R, Terra R, Bernard D, Khrestchatisky M, Izui S, Chicheportiche Y, Boucraut J: TWEAK is expressed by glial cells, induces astrocyte proliferation and increases EAE severity. J Neuroimmunol 133: 116–123, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Lynch CN, Wang YC, Lund JK, Chen YW, Leal JA, Wiley SR: TWEAK induces angiogenesis and proliferation of endothelial cells. J Biol Chem 274: 8455–8459, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Polek TC, Talpaz M, Darnay BG, Spivak-Kroizman T: TWEAK mediates signal transduction and differentiation of RAW264.7 cells in the absence of Fn14/TweakR. Evidence for a second TWEAK receptor. J Biol Chem 278: 32317–32323, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Ho DH, Vu H, Brown SA, Donohue PJ, Hanscom HN, Winkles JA: Soluble tumor necrosis factor-like weak inducer of apoptosis overexpression in HEK293 cells promotes tumor growth and angiogenesis in athymic nude mice. Cancer Res 64: 8968–8972, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Justo P, Sanz AB, Sanchez-Nino MD, Winkles JA, Lorz C, Egido J, Ortiz A: Cytokine cooperation in renal tubular cell injury: The role of TWEAK. Kidney Int 70: 1750–1758, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Maecker H, Varfolomeev E, Kischkel F, Lawrence D, LeBlanc H, Lee W, Hurst S, Danilenko D, Li J, Filvaroff E, Yang B, Daniel D, Ashkenazi A: TWEAK attenuates the transition from innate to adaptive immunity. Cell 123: 931–944, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Kang YJ, Kim WJ, Woo DK, Lee Y, Kim DI, Park YB, Kwon BS, Park JE, Lee WH: TWEAK can induce pro-inflammatory cytokines and matrix metalloproteinase-9 in macrophages. Circ J 68: 396–399, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Saas P, Boucraut J, Walker PR, Quiquerez AL, Billot M, Desplat-Jego S, Chicheportiche Y, Dietrich PY: TWEAK stimulation of astrocytes and the proinflammatory consequences. Glia 32: 102–107, 2000 [PubMed] [Google Scholar]
- 18.Saitoh T, Nakayama M, Nakano H, Yagita H, Yamamoto N, Yamaoka S: TWEAK induces NF-kappaB2 p100 processing and long lasting NF-kappaB activation. J Biol Chem 278: 36005–36012, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Blanco-Colio LM, Martin-Ventura JL, Munoz-Garcia B, Orbe J, Paramo JA, Michel JB, Ortiz A, Meilhac O, Egido J: Identification of soluble tumor necrosis factor-like weak inducer of apoptosis (sTWEAK) as a possible biomarker of subclinical atherosclerosis. Arterioscler Thromb Vasc Biol 27: 916–922, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Zhao Z, Burkly LC, Campbell S, Schwartz N, Molano A, Choudhury A, Eisenberg RA, Michaelson JS, Putterman C: TWEAK/Fn14 interactions are instrumental in the pathogenesis of nephritis in the chronic graft-versus-host model of systemic lupus erythematosus. J Immunol 179: 7949–7958, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Iocca HA, Plant SR, Wang Y, Runkel L, O'Connor BP, Lundsmith ET, Hahm K, van Deventer HW, Burkly LC, Ting JP: TNF superfamily member TWEAK exacerbates inflammation and demyelination in the cuprizone-induced model. J Neuroimmunol 194: 97–106, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Sanz AB, Justo P, Sanchez-Nino MD, Blanco-Colio LM, Winkles JA, Kreztler M, Jakubowski A, Blanco J, Egido J, Ruiz-Ortega M, Ortiz A: The cytokine TWEAK modulates renal tubulointerstitial inflammation. J Am Soc Nephrol 19: 695–703, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kralisch S, Ziegelmeier M, Bachmann A, Seeger J, Lossner U, Bluher M, Stumvoll M, Fasshauer, M: Serum levels of the atherosclerosis biomarker sTWEAK are decreased in type 2 diabetes and end-stage renal disease. Atherosclerosis 199: 440–444, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Carrero JJ, Qureshi AR, Axelsson J, Avesani CM, Suliman ME, Kato S, Barany P, Snaedal-Jonsdottir S, Alvestrand A, Heimburger O, Lindholm B, Stenvinkel P: Comparison of nutritional and inflammatory markers in dialysis patients with reduced appetite. Am J Clin Nutr 85: 695–701, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Stenvinkel P, Heimburger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T: Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55: 1899–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Qureshi AR, Alvestrand A, Divino-Filho JC, Gutierrez A, Heimburger O, Lindholm B, Bergstrom J: Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J Am Soc Nephrol 13[Suppl 1]: S28–S36, 2002 [PubMed] [Google Scholar]
- 27.Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN: What is subjective global assessment of nutritional status? J Parenter Enteral Nutr 11: 8–13, 1987 [DOI] [PubMed] [Google Scholar]
- 28.Rothman, KJ: Measuring interactions. In: Epidemiology: An Introduction. New York, Oxford University Press, 2002, pp 168–180
- 29.Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A: Calculating measures of biological interaction. Eur J Epidemiol 20: 575–579, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Honda H, Qureshi AR, Heimburger O, Barany P, Wang K, Pecoits-Filho R, Stenvinkel P, Lindholm B: Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis 47: 139–148, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Tripepi G, Mallamaci F, Zoccali C: Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: Searching for the best risk marker by multivariate modeling. J Am Soc Nephrol 16[Suppl 1]: S83–S88, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Kamijo S, Nakajima A, Kamata K, Kurosawa H, Yagita H, Okumura K: Involvement of TWEAK/Fn14 interaction in the synovial inflammation of RA. Rheumatology (Oxford) 47: 442–450, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Chicheportiche Y, Fossati-Jimack L, Moll S, Ibnou-Zekri N, Izui S: Down-regulated expression of TWEAK mRNA in acute and chronic inflammatory pathologies. Biochem Biophys Res Commun 279: 162–165, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Dogra C, Changotra H, Wedhas N, Qin X, Wergedal JE, Kumar A: TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB J 21: 1857–1869, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haddad F, Zaldivar F, Cooper DM, Adams GR: IL-6-induced skeletal muscle atrophy. J Appl Physiol 98: 911–917, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Burkly LC, Michaelson JS, Hahm K, Jakubowski A, Zheng TS: TWEAKing tissue remodeling by a multifunctional cytokine: Role of TWEAK/Fn14 pathway in health and disease. Cytokine 40: 1–16, 2007 [DOI] [PubMed] [Google Scholar]