Abstract
Background and objectives: A male infant with a family history of thrombotic microangiopathy developed atypical hemolytic uremic syndrome (aHUS).
Design, setting, participants, & measurements: Case report.
Results: Genetic analysis demonstrated a heterozygous mutation (S1191L) of CFH, the gene coding complement factor H (CFH). The child suffered many episodes of HUS, each treated with plasma exchange. In time, despite initiation of a prophylactic regimen of plasma exchange, his renal function declined significantly. At the age of 4 yr he received a (split liver) combined liver-kidney transplant (LKT) with preoperative plasma exchange and enoxaparin anticoagulation. Initial function of both grafts was excellent and is maintained for nearly 2 yr.
Conclusions: This report adds to the small but growing number of individuals in whom LKT has provided a favorable outcome for aHUS associated with CFH mutation, expands the technique of using a split liver graft, and describes the unique histologic features of subclinical liver disease in HUS.
Individuals who suffer ESRD due to atypical hemolytic uremic syndrome (aHUS) have poor outcomes after kidney transplant because of high rates of disease recurrence (1–3). In the case of carriers of a CFH [complement factor H (CFH)] mutation, the recurrence risk is over 80%, usually accompanied by graft loss (2,4,5). Patients with aHUS associated with CFI [complement factor I (CFI)] mutation also appear to have extremely high rates of recurrence after isolated kidney transplantation (6–9). In contrast, most patients known to have only a MCP [membrane cofactor protein (MCP)] mutation have enjoyed favorable outcomes with isolated renal grafts (2). The difference seems to relate to the site of production of these different factors. CFH and CFI are circulating factors synthesized by the liver, whereas MCP is generated and expressed by nearly all cell types (including in a normal renal graft), where it functions locally, membrane-bound, to limit complement activity (10–13).
Following this logic, liver-kidney transplant (LKT) was explored as a way to both restore renal function and prevent recurrence of aHUS related to CFH mutation. The first patient who underwent full orthotopic LKT with native hepatectomy had acute hepatic failure shortly after the procedure. Although he suffered severe neurologic damage that led to his death several years later, the fact that he was free of HUS during those years proved the principle that liver transplant corrects the CFH defect (14). Fatal liver failure also developed just after transplant of the subsequent patient (15). With these disappointments, LKT was avoided despite its theoretical appeal.
In 2006 we reported a modified approach to LKT that was successful for a child with ESRD due to aHUS and a compound heterozygous CFH mutation (16). The key modifications were to provide large quantities of plasma before and during the transplant and to introduce anticoagulation prophylaxis. That child (patient 1) continues to enjoy a good long-term outcome now over 4 yr posttransplant. With minor modification, the technique was later utilized by Jalanko et al. to successfully treat two children with CFH mutations (17). In this report, we describe another child who remains stable 21 mo after LKT. We highlight that the donor liver was a split organ, that the child was transplanted before suffering ESRD, and describe, we believe for the first time, the histologic features of subclinical liver disease related to HUS.
Case Reports
A male infant born to nonconsanguineous parents was healthy until age 9 mo, when he presented to another facility with renal insufficiency (creatinine 2.7 mg/dl); hemolytic anemia; and thrombocytopenia without diarrhea, sepsis, or signs of infection. At that time, the maternal family history included two female second cousins with ESRD secondary to thrombotic microangiopathy (TMA). Subsequent to this child's presentation, a third female maternal-side second cousin also developed aHUS that precipitated ESRD. Of these relatives, one failed isolated kidney transplantation due to aHUS recurrence, one is dialysis-dependent, and one is deceased because of complications of ESRD.
Renal biopsy confirmed TMA, with acute tubular necrosis and moderate chronic interstitial nephritis with focal fibrosis. Several weeks of plasmapheresis resulted in resolution of hemolysis and improvement in plasma creatinine to 0.6 mg/dl.
The child then experienced multiple recurrences of HUS, each treated with a series of nine plasma exchanges given 3 to 5 times per week. His longest period free of HUS was from age 19 to 26 mo. At age 3 he was noted to have ongoing HUS activity after a series of nine plasma exchanges and so a regimen of chronic maintenance plasma exchange was commenced. The frequency of treatment ranged from once per month to twice per week, depending on hematologic parameters. Most episodes of HUS recurrence were preceded by viral infections or catheter-related bacterial infections. These catheter-related infections were numerous, requiring six catheter changes before age 4 yr.
The child was evaluated at age 4 at our institution when his estimated (18) creatinine clearance was 40 ml/min/1.73m2. Renal sonography demonstrated echogenic but nearly normal-length (6.7 cm) kidneys bilaterally. He required three antihypertensive medications to achieve normal blood pressure, and also received paricalcitol, phosphorous binders, and twice-weekly erythropoietin. Twice-weekly plasma exchanges were performed with approximately 1.5 volumes fresh frozen plasma (FFP), packed red blood cell priming, and partial rinseback. He required administration of several doses of oral calcium, acetaminophen, diphenydramine, and granisetron to tolerate the treatments. Although he had no signs or symptoms of liver disease, preoperative sonography suggested mild hepatosplenomegaly with a diffuse increase in liver echotexture.
Plasma and Genetic Analysis
A blood sample had been analyzed at the Mario Negri Institute for Pharmacologic Research in Bergamo, Italy. The C3 and C4 concentrations were normal and CFH was 449.17 mg/dl (normal 350 to 750), but these samples were affected by preceding plasma therapy. Genotyping of CFH showed c3645C>T corresponding to Ser 1191 Leu and a second normal allele. The reference nucleotide sequence for CFH is taken from Genbank RefSeq-file NM_000186.1 and the nucleotide numbering uses the A of the ATG translation initiation start site as nucleotide + 1. The CFH amino acid numbering includes the 18-residue signal peptide. The same heterozygous mutation in CFH was present in the maternal relatives affected by TMA. MCP sequence analysis demonstrated two normal alleles. Follow-up testing for additional mutations in genes that have subsequently been associated with aHUS, including CFB and C3, was not done. CFI analysis, ongoing but pending comparative investigation within the extended family, remains indeterminate in terms of potential linkage to affected individuals.
Pretransplant Risk-Benefit Considerations
The genotyping results raised the possibility of LKT. Unlike the first patient we reported, this child's life was not immediately in danger. However, it was felt that the ongoing decline in kidney function despite twice-weekly plasma exchange represented inexorable progression to ESRD. The child faced the cumulative complications of plasma exchange therapy, infectious complications of vascular access, prolonged renal insufficiency, and chronic dialysis. The decision to proceed with LKT was shared by the medical team and the parents in hopes of avoiding those complications.
Combined LKT and Subsequent Course
At age 4 yr, the child underwent a combined deceased-donor transplant with a split liver from a 43-yr-old donor. The child's serology, positive for IgG for both Epstein-Barr Virus (EBV) and Cytomegalovirus (CMV), was interpreted cautiously in the context of chronic plasma exchange therapy. The donor was CMV-negative, the patient was blood type O-positive, and panel reactive antibody was 0%. Although there were zero matches at the HLA A, B, and DR loci, final lymphocyte and flow cytometry crossmatches were negative.
Organs became available one day after a routine plasma exchange and another treatment was provided just before the operation. The specific treatment parameters for this last exchange were: weight 18.5 kg, hematocrit 35%, 1250 ml plasma removed, and 1204 ml FFP replaced. During the surgical procedure he received 250 ml packed red blood cells and 200 ml FFP after hepatectomy and before donor liver implantation. The donor liver was split in situ and the graft, the left lateral segment, weighed 266 g. The cold/warm ischemia times were 7.5 h for the liver and 12.5 h for the kidney. Initial immunosuppression included a single dose of daclizumab (1 mg/kg) on day 0, corticosteroids, tacrolimus (started on day 1), and mycophenolate mofetil. Both grafts had excellent immediate function. No plasma infusions or plasma exchanges were given postoperatively.
As with our first patient, low-dose aspirin (40 mg daily) and enoxaparin sodium (0.5 mg/kg/dose every 12 h) were administered beginning in the immediate postoperative period. In this patient, enoxaparin was temporarily discontinued from postoperative days 1 to 10 because the prothrombin time exceeded 20 s (international ratio approximately 1.5) and three doses of vitamin K were administered. Enoxaparin was subsequently utilized through 6 mo posttransplant and aspirin for 4 mo.
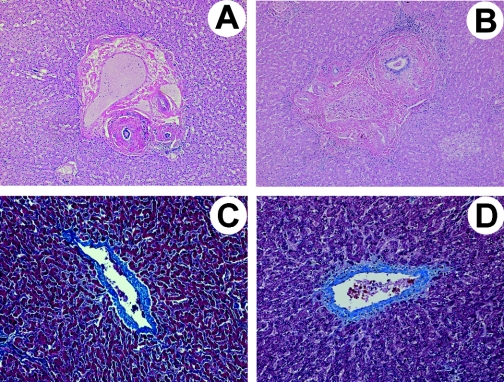
The native liver weighed 627.5 g and showed no gross abnormalities. Microscopic evaluation revealed moderate dense periductal fibrosis of hilar, septal, and large interlobular bile ducts (Figure 1A). The smaller interlobular bile ducts were unremarkable. The hilar blood vessels were unremarkable and no thrombi were identified within the liver. Trichrome stain highlighted the periductal fibrosis and demonstrated focal mild fibrosis of the terminal hepatic venules (Figure 1C).
Figure 1.
Native liver histologic findings in the two examples of children with recurrent hemolytic uremic syndrome (HUS) due to complement factor H gene (CFH) mutation. (A and C) Patient presented here; (B and D) patient 1. (A and B) Periductal fibrosis involving medium-sized bile ducts (hematoxylin and eosin stain, 50×). (C and D) Mild fibrosis of terminal hepatic venules (trichrome stain, 100×).
The initial postoperative period was significant for severe hypertension requiring several hours of nicardipine infusion, followed closely by mild hypotension treated by fluid resuscitation and several hours of dopamine infusion. The endotracheal tube was removed on the fifth postoperative day and he was discharged from the hospital 2 wk later. Over the longer postoperative period, he had persistent ascites requiring a drainage catheter and later surgical closure of the drainage site. Portal vein thrombosis was ruled out by magnetic resonance angiography, although splenomegaly was again noted. Additional evaluation demonstrated moderate pleural and pericardial effusions. Without a significant clinical problem besides abdominal distension, he was treated conservatively with spironolactone and clinical improvement ensued over several weeks. At 14 mo posttransplant he experienced a moderate acute cellular liver rejection (Banff total 6) that was successfully treated with pulse methylprednisolone followed by a slowly tapering dose of corticosteroids.
Now, nearly 2 yr posttransplant, the child's health has been generally good. Both height and weight are approximately 50th percentile for age. His development at home and in kindergarten has been age-appropriate. Because he continues to require four antihypertensive medications, native nephrectomies are being considered. His immunosuppression remains tacrolimus (with levels maintained near 8 ng/ml), prednisone (2.5 mg/d), and mycophenolate mofetil (approximately 350 mg/m2/ twice daily). The ALT and AST were 23 and 27 U/L respectively, albumin 3.8 g/dl, GGT 29 U/L, bilirubin 0.7 mg/dl, and serum creatinine concentration was 0.6 mg/dl.
Discussion
Recurrent HUS is a devastating condition particularly when presenting during infancy. It has been well established that mutations of CFH predispose to aHUS (4,5,19–21). The recurrence risk of HUS after isolated kidney transplant for carriers of mutations in CFH is over 80% (2,4,5).
In addition to the risk implied by the literature, HUS recurrence after isolated kidney transplant in a family member with the same mutation provided a particularly compelling argument against using that approach for this child. The outcome of this patient is a success considering that approximately 80% of recurrences of aHUS occur within 1 mo of isolated kidney transplantation (22).
In addition to its regulatory activity in plasma, CFH plays a key role in downregulating complement activation on glomerular basement membranes and endothelial cell surfaces, greatly contributing to their protection from triggers of complement activation (23). CFH mutations associated with aHUS cluster in the carboxy-terminal region, which is important for binding both to C3b and to cell surfaces. As a consequence of such mutations, the regulatory function of CFH in slowing complement activity particularly at the endothelial cell membrane is impaired, in turn raising the likelihood that once triggered, complement activation will intensify and culminate in injury to the endothelial cell. The specific heterozygous CFH mutation in this child and the functionality of its protein product have been described, suggesting it binds the endothelial surface but fails to bind C3b (24). One could theorize that this mutant CFH might not only fail to regulate complement activity but additionally block cell surface binding of wild-type CFH and therefore manifest a “dominant negative” effect at the protein level.
The first experiences with LKT for treatment of aHUS were problematic (14,15). Retrospectively, early acute liver failure may have resulted from intense intrahepatic complement activation with vascular injury. Complement activation occurs in the graft organ during liver transplantation and triggers systemic complement activation (25,26). Our modified approach introduced a thorough plasma exchange immediately before transplantation and additional plasma infusion during the procedure. This not only provides sufficient CFH to the CFH-deficient recipient, but also removes mutant CFH, reducing its potential interference with wild-type CFH. The half-life of CFH is approximately 6 d (27), which should allow sufficient time for the donor liver to recover its synthetic activity. Still, it is likely that CFH is consumed more rapidly in the context of the complement-activating stimulus of transplantation itself, so the “dose” of plasma exchange and infusion may vary from patient to patient depending on the mutation and how the clinical scenario unfolds. For instance, patients with impaired CFH secretion because of mutation might require no exchange and less plasma than those with circulating dysfunctional CFH. Clinically, an indication of an effective dose might be inferred from the dose of plasma that induced remission of HUS during the pretransplant period.
Another technical aspect of this patient, our prior patient, and those reported by Jalanko et al. (17) should be highlighted. In each instance, the patient was treated with low-molecular-weight heparin. At our center we utilized subcutaneous exoxaparin and aspirin, treatments, which are provided routinely for recipients of this size for concern about hepatic artery thrombosis. Additionally, we worried about thrombus development assuming that some degree of complement activation with endothelial damage was inevitable or that pre-existing endothelial lesions from prior HUS episodes could form a thrombotic nidus, particularly while using large amounts of plasma perioperatively. In our patient 1, we discontinued anticoagulation quickly because of hemorrhagic cystitis (16). In the patient presented here, aside from the transient period when the prothrombin time was prolonged, we continued this treatment for 6 mo. Although we are not sure if anticoagulation is an important part of this transplant protocol, the noted concerns as well as the known interactions between heparin-related compounds and the complement system (28) do not allow us to discard that possibility.
The gravity of liver transplantation for patients with ESRD requires consideration of alternative treatments. Chronic plasma exchange may protect against recurrence after an isolated kidney transplant. The main drawbacks to that approach are the effect on lifestyle, the risk of an indwelling catheter, and the risks of chronic plasma therapy. In the patient presented here, the child routinely suffered treatment reactions including one life-threatening event. There was also a realization that whether because of initial renal injury, incomplete protection from HUS, or other unknown reasons, chronic plasma exchange had not been able to protect the native kidneys from long-term decline. In the literature, variability in response to plasma therapy has been reported (27,29,30). Intravenous Ig (IVIG) might ameliorate TMA (31–33), possibly related to its ability to decrease complement activation (34,35). On the other hand, others have found that IVIG activates complement in vivo (36,37). In one example of recurrent HUS related to CFH mutation in which chronic plasma therapy was failing, IVIG was initially effective in reducing biochemical evidence of HUS but did not prevent ESRD (personal communication: Lawrence S. Milner, Tufts Floating Hospital for Children, Boston, Massachusetts, March 21, 2008). In the future, CFH concentrate or pharmaceutical inhibition of complement activation (38) may provide reliable long-term prophylaxis against HUS and other diseases associated with excessive complement activity.
The abnormal histologic findings in the native liver were unexpected and prompted us to closely re-examine the native liver of our patient 1 (16). Indeed, patient 1 had been evaluated well before LKT because of intermittent transaminase elevations and was found to have mild hepatomegaly with diffusely increased liver echogenicity by sonography. In neither patient were there biochemical signs of native liver disease at the time of transplant. Nonetheless, like the patient presented here, the native liver histology of patient 1 revealed periductal fibrosis of scattered medium and large bile ducts and focal mild fibrosis of the central hepatic venules (Figure 1, B and D) in addition to previously reported focal fatty change, glycogenic foci, and a single granuloma (16). The extent of periductal fibrosis was more extensive in the patient presented here than in patient 1.
Periductal fibrosis falls into the generic classification of sclerosing cholangitis. This finding is typical of primary sclerosing cholangitis, but is also a consequence of such “secondary” causes as biliary surgery, cholelithiasis, biliary neoplasia, toxins, and ischemia. Although episodic hemolysis presents a possible link to biliary tract disease, a more likely cause of periductal fibrosis in our patients is microangiopathic disease within the hepatic circulation. The site of endothelial injury and vascular compromise would appear to be the peribiliary vascular plexus. This plexus is derived from branches of the hepatic arteries and feeds the epithelium and wall of medium and large bile ducts. Microthrombi in the plexus would result in ischemia and reactive fibrosis in these larger caliber bile ducts. Fibrosis of the terminal hepatic venules is nonspecific and although direct endothelial injury to the venous microcirculation from aHUS is one potential cause, this injury could also be explained by other insults including episodes of shock both patients experienced during the course of their illness.
Liver involvement in HUS is not uncommon, and although catastrophic disease has been reported (39), usually the effect is subclinical and limited to alterations of liver enzymes or occasionally manifesting with significant signs of cholestasis (40–42). Our experience allowed direct examination of the native liver and we believe this is the first report to describe periductal fibrosis of the medium-sized and large intrahepatic bile ducts in HUS. Although morphologically noteworthy, this periductal fibrosis was not clinically significant at the time of transplant. Moreover, with only two patients, it is not possible to say that our conclusions are definitive. Nonetheless, this is a relevant finding because it suggests that chronic or recurrent HUS has adverse and likely cumulative effects on the liver. It leaves open the distinct possibility that similar subclinical disease may be widespread in such patients, particularly in those organs occasionally found to manifest severe disease such as the brain, pancreas, and intestines.
We continue to support the idea that patients with aHUS should undergo genotyping of CFH, CFI, and MCP before consideration of kidney transplant. LKT should be considered for patients with CFH or CFI mutations, and isolated liver transplant is an option if native renal function is preserved. Preoperative plasma exchange with FFP is crucial to success, and use of low-molecular-weight heparin may also play a role. We believe that the successful outcomes of this LKT technique support its continued use in individual patients and we favor the development of an international consensus on the approach to isolated liver or LKT under these circumstances.
Disclosures
None.
Acknowledgments
Chiara Mossali (Mario Negri Institute) did the genetic analysis of CFH in this family.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Loirat C, Niaudet P: The risk of recurrence of hemolytic uremic syndrome after renal transplantation in children. Pediatr Nephrol 18: 1095–1101, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Bresin E, Daina E, Noris M, Castelletti F, Stefanov R, Goodship TH, Remuzzi G: Outcome of patients with non-shiga Toxin-associated HUS: Prognostic significance of genetic background. CJASN 1: 88–99, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Miller RB, Burke BA, Schmidt WJ, Gillingham KJ, Matas AJ, Mauer M, Kashtan CE: Recurrence of haemolytic-uraemic syndrome in renal transplants: A single-centre report. Nephrol Dial Transplant 12: 1425–1430, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Neumann HP, Salzmann M, Bohnert-Iwan B, Mannuelian T, Skerka C, Lenk D, Bender BU, Cybulla M, Riegler P, Konigsrainer A, Neyer U, Bock A, Widmer U, Male DA, Franke G, Zipfel PF: Haemolytic uraemic syndrome and mutations of the factor H gene: A registry-based study of German speaking countries. J Med Genet 40: 676–681, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caprioli J, Castelletti F, Bucchioni S, Bettinaglio P, Bresin E, Pianetti G, Gamba S, Brioschi S, Daina E, Remuzzi G, Noris M: Complement factor H mutations and gene polymorphisms in haemolytic uraemic syndrome: The C-257T, the A2089G and the G2881T polymorphisms are strongly associated with the disease. Hum Mol Genet 12: 3385–3395, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Kavanagh D, Richards A, Noris M, Hauhart R, Liszewski MK, Karpman D, Goodship JA, Fremeaux-Bacchi V, Remuzzi G, Goodship TH, Atkinson JP: Characterization of mutations in complement factor I (CFI) associated with hemolytic uremic syndrome. Mol Immunol 45: 95–105, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Nilsson SC, Karpman D, Vaziri-Sani F, Kristoffersson AC, Salomon R, Provot F, Fremeaux-Bacchi V, Trouw LA, Blom AM: A mutation in factor I that is associated with atypical hemolytic uremic syndrome does not affect the function of factor I in complement regulation. Mol Immunol 44: 1835–1844, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Geelen J, van den Dries K, Roos A, van de Kar N, de Kat Angelino C, Klasen I, Monnens L, van den Heuvel L: A missense mutation in factor I (IF) predisposes to atypical haemolytic uraemic syndrome. Pediatr Nephrol 22: 371–375, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Fremeaux-Bacchi V, Dragon-Durey MA, Blouin J, Vigneau C, Kuypers D, Boudailliez B, Loirat C, Rondeau E, Fridman WH: Complement factor I: A susceptibility gene for atypical haemolytic uraemic syndrome. J Med Genet 41: e84, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richards A, Liszewski MK, Kavanagh D, Fang CJ, Moulton E, Fremeaux-Bacchi V, Remuzzi G, Noris M, Goodship TH, Atkinson JP: Implications of the initial mutations in membrane cofactor protein (MCP; CD46) leading to atypical hemolytic uremic syndrome. Mol Immunol 44: 111–122, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Kavanagh D, Goodship TH: Membrane cofactor protein and factor I: Mutations and transplantation. Semin Thromb Haemost 32: 155–159, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Endoh M, Yamashina M, Ohi H, Funahashi K, Ikuno T, Yasugi T, Atkinson JP, Okada H: Immunohistochemical demonstration of membrane cofactor protein (MCP) of complement in normal and diseased kidney tissues. Clin Exp Immunol 94: 182–188, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caprioli J, Noris M, Brioschi S, Pianetti G, Castelletti F, Bettinaglio P, Mele C, Bresin E, Cassis L, Gamba S, Porrati F, Bucchioni S, Monteferrante G, Fang CJ, Liszewski MK, Kavanagh D, Atkinson JP, Remuzzi G: Genetics of HUS: The impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood 108: 1267–1279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Remuzzi G, Ruggenenti P, Codazzi D, Noris M, Caprioli J, Locatelli G, Gridelli B: Combined kidney and liver transplantation for familial haemolytic uraemic syndrome. Lancet 359: 1671–1672, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Remuzzi G, Ruggenenti P, Colledan M, Gridelli B, Bertani A, Bettinaglio P, Bucchioni S, Sonzogni A, Bonanomi E, Sonzogni V, Platt JL, Perico N, Noris M: Hemolytic uremic syndrome: A fatal outcome after kidney and liver transplantation performed to correct factor h gene mutation. Am J Transplant 5: 1146–1150, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Saland JM, Emre SH, Shneider BL, Benchimol C, Ames S, Bromberg JS, Remuzzi G, Strain L, Goodship TH: Favorable long-term outcome after liver-kidney transplant for recurrent hemolytic uremic syndrome associated with a factor H mutation. Am J Transplant 6: 1948–1952, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Jalanko H, Peltonen S, Koskinen A, Puntila J, Isoniemi H, Holmberg C, Pinomaki A, Armstrong E, Koivusalo A, Tukiainen E, Makisalo H, Saland J, Remuzzi G, de Cordoba S, Lassila R, Meri S, Jokiranta TS: Successful liver-kidney transplantation in two children with aHUS caused by a mutation in complement factor H. Am J Transplant 8: 216–221, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 19.Noris M, Ruggenenti P, Perna A, Orisio S, Caprioli J, Skerka C, Vasile B, Zipfel PF, Remuzzi G: Hypocomplementemia discloses genetic predisposition to hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: Role of factor H abnormalities. Italian Registry of Familial and Recurrent Hemolytic Uremic Syndrome/Thrombotic Thrombocytopenic Purpura. J Am Soc Nephrol 10: 281–293, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Warwicker P, Donne RL, Goodship JA, Goodship TH, Howie AJ, Kumararatne DS, Thompson RA, Taylor CM: Familial relapsing haemolytic uraemic syndrome and complement factor H deficiency. Nephrol Dial Transplant 14: 1229–1233, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Rougier N, Kazatchkine MD, Rougier JP, Fremeaux-Bacchi V, Blouin J, Deschenes G, Soto B, Baudouin V, Pautard B, Proesmans W, Weiss E, Weiss L: Human complement factor H deficiency associated with hemolytic uremic syndrome. J Am Soc Nephrol 9: 2318–2326, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Ducloux D, Rebibou JM, Semhoun-Ducloux S, Jamali M, Fournier V, Bresson-Vautrin C, Chalopin JM: Recurrence of hemolytic-uremic syndrome in renal transplant recipients: A meta-analysis. Transplantation 65: 1405–1407, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Noris M, Remuzzi G: Hemolytic uremic syndrome. J Am Soc Nephrol 16: 1035–1050, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Heinen S, Sanchez-Corral P, Jackson MS, Strain L, Goodship JA, Kemp EJ, Skerka C, Jokiranta TS, Meyers K, Wagner E, Robitaille P, Esparza-Gordillo J, Rodriguez de Cordoba S, Zipfel PF, Goodship TH: De novo gene conversion in the RCA gene cluster (1q32) causes mutations in complement factor H associated with atypical hemolytic uremic syndrome. Hum Mutat 27: 292–293, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Bellamy MC, Gedney JA, Buglass H, Gooi JH: Complement membrane attack complex and hemodynamic changes during human orthotopic liver transplantation. Liver Transpl 10: 273–278, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Scoazec JY, Borghi-Scoazec G, Durand F, Bernuau J, Pham BN, Belghiti J, Feldmann G, Degott C: Complement activation after ischemia-reperfusion in human liver allografts: Incidence and pathophysiological relevance. Gastroenterology 112: 908–918, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Licht C, Weyersberg A, Heinen S, Stapenhorst L, Devenge J, Beck B, Waldherr R, Kirschfink M, Zipfel PF, Hoppe B: Successful plasma therapy for atypical hemolytic uremic syndrome caused by factor H deficiency owing to a novel mutation in the complement cofactor protein domain 15. Am J Kidney Dis 45: 415–421, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Ninomiya H, Kawashima Y, Nagasawa T: Inhibition of complement-mediated haemolysis in paroxysmal nocturnal haemoglobinuria by heparin or low-molecular weight heparin. Br J Haematol 109: 875–881, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Gerber A, Kirchhoff-Moradpour AH, Obieglo S, Brandis M, Kirschfink M, Zipfel PF, Goodship JA, Zimmerhackl LB: Successful (?) therapy of hemolytic-uremic syndrome with factor H abnormality. Pediatr Nephrol 18: 952–955, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Nathanson S, Ulinski T, Fremeaux-Bacchi V, Deschenes G: Secondary failure of plasma therapy in factor H deficiency. Pediatr Nephrol 21: 1769–1771, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Heyman MR, Sweet T: Thrombotic thrombocytopenic purpura treated with high-dose intravenous gamma globulin. South Med J 83: 1471–1474, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Centurioni R, Bobbio-Pallavicini E, Porta C, Rodeghiero F, Gugliotta L, Billio A, Tacconi F, Ascari E: Treatment of thrombotic thrombocytopenic purpura with high-dose immunoglobulins. Results in 17 patients. Italian Cooperative Group for TTP. Haematologica 80: 325–331, 1995 [PubMed] [Google Scholar]
- 33.Ito S, Okuyama K, Nakamura T, Tetanishi J, Saito K, Matsumoto M, Fujimura Y, Aihara Y, Yokota S: Intravenous gamma globulin for thrombotic microangiopathy of unknown etiology. Pediatr Nephrol 22: 301–305, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Watanabe T: Thrombotic microangiopathy and intravenous immunoglobulin therapy. Pediatr Nephrol 22: 907–908; author reply 909, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Basta M, Fries LF, Frank MM: High doses of intravenous Ig inhibit in vitro uptake of C4 fragments onto sensitized erythrocytes. Blood 77: 376–380, 1991 [PubMed] [Google Scholar]
- 36.Aukrust P, Gullestad L, Lappegard KT, Ueland T, Aass H, Wikeby L, Simonsen S, Froland SS, Mollnes TE: Complement activation in patients with congestive heart failure: Effect of high-dose intravenous immunoglobulin treatment. Circulation 104: 1494–1500, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Mollnes TE, Hogasen K, De Carolis C, Vaquero E, Nielsen EW, Fontana L, Perricone R: High-dose intravenous immunoglobulin treatment activates complement in vivo. Scand J Immunol 48: 312–317, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Smith RJ, Alexander J, Barlow PN, Botto M, Cassavant TL, Cook HT, de Cordoba SR, Hageman GS, Jokiranta TS, Kimberling WJ, Lambris JD, Lanning LD, Levidiotis V, Licht C, Lutz HU, Meri S, Pickering MC, Quigg RJ, Rops AL, Salant DJ, Sethi S, Thurman JM, Tully HF, Tully SP, van der Vlag J, Walker PD, Wurzner R, Zipfel PF: New approaches to the treatment of dense deposit disease. J Am Soc Nephrol 18: 2447–2456, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarthy DW, Mutabagani K, Mahan JD, Caniano DA, Cooney DR: Infarction of the choledochus, liver, gallbladder, and pancreas: A unique complication of the hemolytic uremic syndrome. J Pediatr Surg 35: 502–504, 2000 [DOI] [PubMed] [Google Scholar]
- 40.de Buys Roessingh AS, de Lagausie P, Baudoin V, Loirat C, Aigrain Y: Gastrointestinal complications of post-diarrheal hemolytic uremic syndrome. Eur J Pediatr Surg 17: 328–334, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Siegler RL: Spectrum of extrarenal involvement in postdiarrheal hemolytic-uremic syndrome. J Pediatr 125: 511–518, 1994 [DOI] [PubMed] [Google Scholar]
- 42.Jeffrey G, Kibbler CC, Baillod R, Farrington K, Morgan MY: Cholestatic jaundice in the haemolytic-uraemic syndrome: A case report. Gut 26: 315–319, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]