Abstract
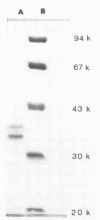
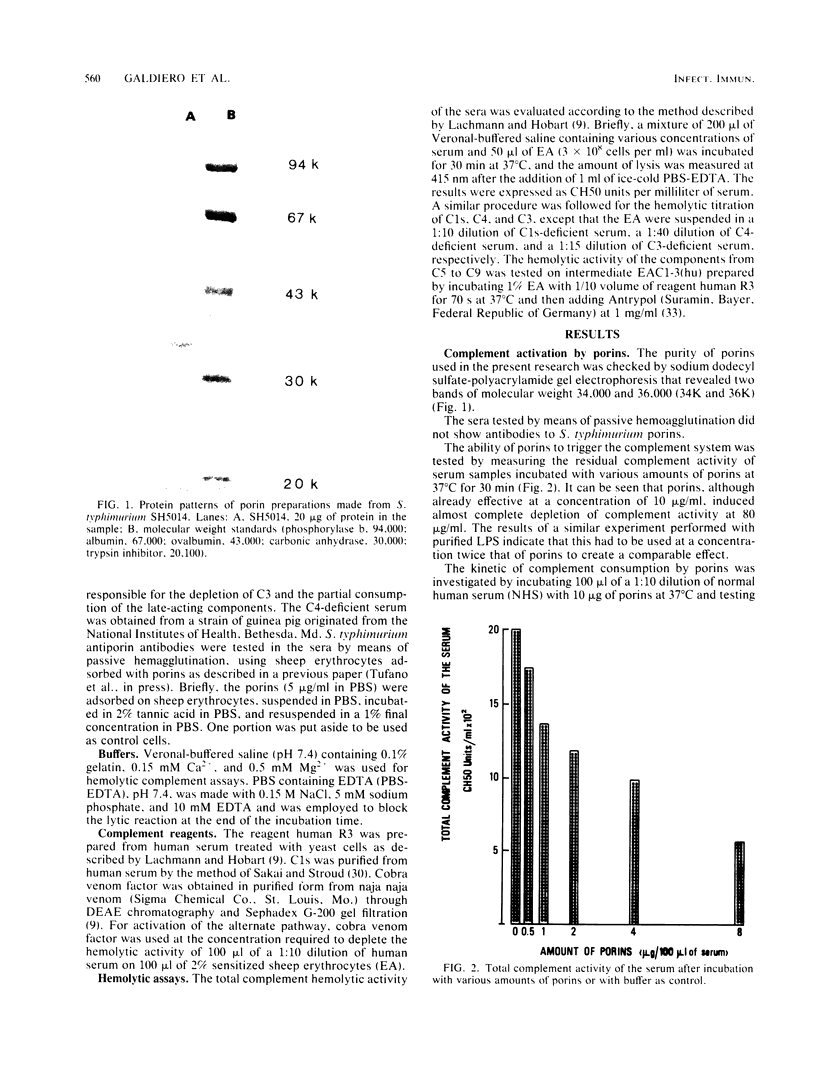
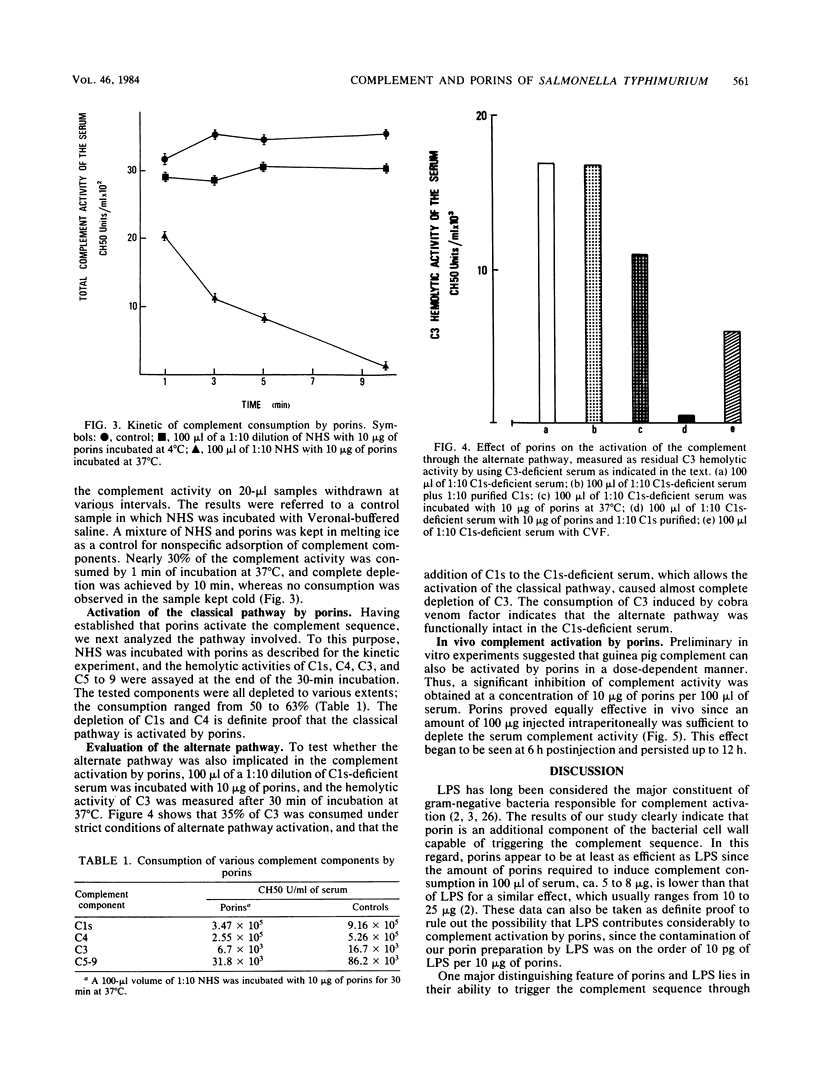
The effect of porins purified from Salmonella typhimurium on the complement system was investigated both in vitro and in vivo. Incubation of porins with either human or guinea pig serum resulted in the consumption of the total complement activity when an amount of porins ranging from 8 to 10 micrograms per 100 microliters of serum was used. The activation of the complement system was temperature dependent, suggesting an active process rather than passive adsorption of the complement components by porins. In addition, the activation had a fast kinetic and proceeded mainly through the classical pathway. This conclusion is supported by the consumption of C1s and C4 in normal human serum treated with porins and also by the depletion of C3 activity in the C1s-deficient serum which was marked only when purified C1s was added to the serum before incubation with porins. Injection of 100 micrograms of porins into guinea pigs induced profound complement consumption at 6 h postinjection that persisted up to 12 h. We conclude from this study that porins can effectively contribute to complement activation and to subsequent biological events induced by gram-negative bacteria.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dierich M. P., Bitter-Suermann D., König W., Hadding U., Galanos C., Rietschel E. T. Analysis of bypass activation of C3 by endotoxic LPS and loss of this potency. Immunology. 1973 Apr;24(4):721–733. [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma J. Y. The protein moiety of the endotoxin of Pseudomonas aeruginosa. Z Allg Mikrobiol. 1968;8(3):227–248. doi: 10.1002/jobm.3630080310. [DOI] [PubMed] [Google Scholar]
- Kiley P., Holt S. C. Characterization of the lipopolysaccharide from Actinobacillus actinomycetemcomitans Y4 and N27. Infect Immun. 1980 Dec;30(3):862–873. doi: 10.1128/iai.30.3.862-873.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Grossman N., Leive L. Salmonellae activate complement differentially via the alternative pathway depending on the structure of their lipopolysaccharide O-antigen. J Immunol. 1983 Apr;130(4):1867–1870. [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Saxén H., Mäkelä P. H., Leive L. Complement activation by polysaccharide of lipopolysaccharide: an important virulence determinant of salmonellae. Infect Immun. 1983 Aug;41(2):563–569. doi: 10.1128/iai.41.2.563-569.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos M., Bitter-Suermann D., Dierich M. Interaction of the first (C1), the second (C2) and the fourth (C4) component of complement with different preparations of bacterial lipopolysaccharides and with lipid A. J Immunol. 1974 Mar;112(3):935–940. [PubMed] [Google Scholar]
- Madonna G. S., Allen R. C. Shigella sonnei phase I and phase II: susceptibility to direct serum lysis and opsonic requirements necessary for stimulation of leukocyte redox metabolism and killing. Infect Immun. 1981 Apr;32(1):153–159. doi: 10.1128/iai.32.1.153-159.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus R. L., Shin H. S., Mayer M. M. An alternate complement pathway: C-3 cleaving activity, not due to C4,2a, on endotoxic lipopolysaccharide after treatment with guinea pig serum; relation to properdin. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1351–1354. doi: 10.1073/pnas.68.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F., Oades Z. G., Henson P. M. Mechanisms of lipopolysaccharide-initiated rabbit platelet responses: alternative complement pathway dependence of the lytic response. Infect Immun. 1978 Jun;20(3):744–751. doi: 10.1128/iai.20.3.744-751.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn C. B., Ishiguro E. E., Kay W. W., Trust T. J. Role of surface components in serum resistance of virulent Aeromonas salmonicida. Infect Immun. 1982 Jun;36(3):1069–1075. doi: 10.1128/iai.36.3.1069-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschel L. H., Jackson J. E. The reactivity of serum against protoplasts and spheroplasts. J Immunol. 1966 Jul;97(1):46–51. [PubMed] [Google Scholar]
- Muschel L. H., Larsen L. J. The sensitivity of smooth and rough gram-negative bacteria to the immune bactericidal reaction. Proc Soc Exp Biol Med. 1970 Jan;133(1):345–348. doi: 10.3181/00379727-133-34472. [DOI] [PubMed] [Google Scholar]
- Nelson B. W., Roantree R. J. Analyses of lipopolysaccharides extracted from penicillin-resistant, serum-sensitive salmonella mutants. J Gen Microbiol. 1967 Aug;48(2):179–188. doi: 10.1099/00221287-48-2-179. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PILLEMER L., SCHOENBERG M. D., BLUM L., WURZ L. Properdin system and immunity. II. Interaction of the properdin system with polysaccharides. Science. 1955 Sep 23;122(3169):545–549. doi: 10.1126/science.122.3169.545. [DOI] [PubMed] [Google Scholar]
- Rowley D. Antibacterial action of antibody and complement. J Infect Dis. 1973 Jul;128(Suppl):170–175. doi: 10.1093/infdis/128.supplement_1.s170. [DOI] [PubMed] [Google Scholar]
- Rowley D. Sensitivity of rough gram-negative bacteria to the bactericidal action of serum. J Bacteriol. 1968 May;95(5):1647–1650. doi: 10.1128/jb.95.5.1647-1650.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D., Turner K. J. Passive sensitization of Salmonella adelaide to the bactericidal action of antibody and complement. Nature. 1968 Feb 17;217(5129):657–658. doi: 10.1038/217657a0. [DOI] [PubMed] [Google Scholar]
- Siraganian R. P. Platelet requirement in the interaction of the complement and clotting systems. Nat New Biol. 1972 Oct 18;239(94):208–210. doi: 10.1038/newbio239208a0. [DOI] [PubMed] [Google Scholar]
- Taylor P. W. Genetical studies of serum resistance in Escherichia coli. J Gen Microbiol. 1975 Jul;89(1):57–66. doi: 10.1099/00221287-89-1-57. [DOI] [PubMed] [Google Scholar]
- Tedesco F., Bardare M., Giovanetti A. M., Sirchia G. A familial dysfunction of the eight component of complement (C8). Clin Immunol Immunopathol. 1980 Jun;16(2):180–191. doi: 10.1016/0090-1229(80)90202-0. [DOI] [PubMed] [Google Scholar]
- Traub W. H., Kleber I. Selective activation of classical and alternative pathways of human complement by "promptly serum-sensitive" and "delayed serum-sensitive" strains of Serratia marcescens. Infect Immun. 1976 May;13(5):1343–1346. doi: 10.1128/iai.13.5.1343-1346.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufano M. A., Berlingieri M. T., Sommese L., Galdiero F. Immune response in mice and effects on cells by outer membrane porins from Salmonella typhimurium. Microbiologica. 1984 Oct;7(4):353–366. [PubMed] [Google Scholar]
- Yin E. T., Galanos C., Kinsky S., Bradshaw R. A., Wessler S., Lüderitz O., Sarmiento M. E. Picogram-sensitive assay for endotoxin: gelation of Limulus polyphemus blood cell lysate induced by purified lipopolysaccharides and lipid A from Gram-negative bacteria. Biochim Biophys Acta. 1972 Jan 28;261(1):284–289. doi: 10.1016/0304-4165(72)90340-6. [DOI] [PubMed] [Google Scholar]