Abstract
Background and objectives: Vascular access failure (VAF) is associated with increased morbidity and mortality in hemodialysis patients. The most common cause of VAF is stenosis at the arteriovenous anastomosis because of abnormal neointimal proliferation and extracellular matrix deposition. These two changes are also observed in the classic atheroma, which means atherosclerotic lesions and venous stenosis in VAF may share some similar pathogenic mechanisms. The ankle–brachial index (ABI) is a reliable marker for atherosclerosis. The aim of this study was to evaluate the relationship between ABI <0.9 and VAF.
Design, setting, participants, & measurements: All routine hemodialysis patients in one regional hospital were included except for six patients refusing ABI examinations and four patients with atrial fibrillation. Finally, 225 patients formed our study group. The study subjects were observed from arteriovenous access creation until the first episode of VAF. The mean observation period was 42.2 ± 42.8 mo. The relative VAF risk was analyzed by Cox-regression methods with adjustments for demographic and comorbid conditions.
Results: VAF episodes were recorded in 111 patients. In multivariate analysis, ABI <0.9 (hazard ratio, 1.893; P = 0.039), vascular access type of arteriovenous graft (P = 0.004), and serum triglyceride level (P = 0.043) were positively associated with VAF, and serum parathyroid hormone level (P = 0.043) was negatively associated with VAF.
Conclusions: Our findings show that ABI <0.9 is significantly correlated with increased VAF. Screening hemodialysis patients by means of ABI may help to identify a high-risk group for VAF.
Vascular access failure (VAF) is a frequent problem in hemodialysis patients for its association with increased morbidity and hospitalization (1,2). The most common cause of VAF is stenosis at the arteriovenous anastomosis because of abnormal neointimal proliferation and extracellular matrix deposition (3). These two changes are also observed in the classic atheroma, which means atherosclerotic lesions and venous stenosis in VAF may share some similar pathogenic mechanisms (4,5). Identification of patients at high risk for VAF and requiring aggressive preventive and interventional strategies is an initial and essential step in managing patients with end-stage renal disease (ESRD) undergoing hemodialysis.
The ankle-brachial index (ABI) was reported to be a good marker for atherosclerosis and useful in the diagnosis of peripheral artery occlusive disease (PAOD), and an ABI <0.9 has been used to identify this condition in clinical practice and epidemiologic studies (6–8). Previous studies also demonstrated that a low ABI was a good predictor of all-cause and cardiovascular mortality in hemodialysis patients (9,10). However, there is no study to investigate the relationship between ABI and vascular access patency in hemodialysis patients. Thus, this study hypothesizes that an ABI <0.9, an indicator for severe atherosclerosis, may be highly correlated with VAF in patients with hemodialysis. The aim of this study is to evaluate the potential links between the ABI level and vascular access survival.
Materials and Methods
Study Patients and Design
The study was conducted at one dialysis clinic in one regional hospital in southern Taiwan. All routine hemodialysis patients in this hospital were included except for six patients refusing ABI examinations and four patients with atrial fibrillation. Finally, 225 patients (98 males and 127 females) formed our study group. The protocol was approved by our Institutional Review Board and all enrolled patients gave written, informed consent.
The VAF was defined as thrombosis caused by stenosis having received thrombectomy or greater than 50% stenosis shown on angiography requiring either surgical revision or percutaneous transluminal angioplasty. The occurrence of VAF was confirmed from medical records. The study subjects were observed from arteriovenous access creation until the first episode of VAF occurred. The mean observation period was 42.2 ± 42.8 mo (range 1 to 272).
Hemodialysis
All patients underwent their routine hemodialysis three times a week using a Toray 321 machine (Toray Medical Company, Tokyo, Japan). Each hemodialysis session was performed for 3 to 4 h using a dialyzer with a blood flow rate of 250 to 300 ml/min and dialysate flow of 500 ml/min.
ABI Measurement
The values of the ABI were measured 10 to 30 min before hemodialysis. The ABIs were measured by using an ABI-form device (VP1000, Colin, Komaki, Japan), which automatically and simultaneously measures blood pressure (BP) in both arms and ankles using an oscillometric method (11–13). The ABI was calculated by the ratio of the ankle systolic BP divided by the arm systolic BP. The systolic BP of the arm without dialysis access and the lower value of the ankle systolic BP were used for the calculation. The ABI measurement was done once in each patient.
Collection of Demographic, Medical, and Laboratory Data
Demographic and medical data including age, gender, smoking history (ever versus never), and comorbid conditions were obtained from medical records and interviews with patients. The body mass index (BMI) was calculated as the ratio of weight in kilograms divided by square of height in meters. Laboratory data were measured from fasting blood samples using an autoanalyzer (Roche Diagnostics GmbH, D-68298 Mannheim, Germany; COBAS Integra 400). High-sensitivity C-reactive protein (CRP) (Dade Behring Marburg GmbH, Marburg, Germany) was measured by commercially available kits. Serum intact parathyroid hormone (PTH) concentration was evaluated using a commercially available two-sided immunoradiometric assay (CIS bio international, Gif-sur-Yvette, France). Blood samples were obtained within 1 mo of enrollment. Kt/V was evaluated monthly as a marker of dialysis efficiency, and was determined according to the procedure of Gotch (14).
Statistical Analyses
Statistical analyses were performed using SPSS 12.0 for windows (SPSS Inc., Chicago, Illinois). Data are expressed as numbers and percentages, or mean ± SD. The differences between groups were checked by chi-square test for categorical variables, or by independent t test for continuous variables. Time to VAF and covariates of risk factors were modeled using a Cox proportional hazards model. Significant variables in the univariate analysis were further analyzed by multivariate analysis. Survival curves were also derived using Cox regression analysis. A significant difference was considered when the P value was less than 0.05.
Results
The clinical characteristics of study patients are shown in Table 1. The mean age of study patients was 58.7 ± 13.0 yr old. The percentage of hypertriglyceridemia (≧150 mg/dl) and hypercholesterolemia (≧240 mg/dl) (15) were 40% and 6.2%, respectively. An ABI <0.9 was found to be significantly associated with increased age, diabetes mellitus (DM), a history of coronary artery disease and cerebrovascular disease, increased pulse pressure, decreased serum albumin, decreased HDL cholesterol, decreased creatinine, and increased hematocrit levels. Compared with patients with ABI ≧0.9, those with ABI <0.9 had a higher percentage of usage of arteriovenous graft (AVG) than arteriovenous fistula (AVF). Additionally, the percentage of patients with an ABI <0.9 was higher in patients with AVG (32.6%) than those with AVF (11.2%).
Table 1.
The characteristics of the study patients according to the ankle–brachial index
| Characteristics | ABIa < 0.9 (n = 35) | ABI ≧ 0.9 (n = 190) | All patients (n = 225) |
|---|---|---|---|
| Age (yr) | 66.9 ± 9.7b | 57.2 ± 12.9 | 58.7 ± 13.0 |
| Male gender (%) | 31.4 | 45.8 | 43.6 |
| Duration of dialysis (mo) | 62.2 ± 51.7 | 55.3 ± 46.4 | 56.4 ± 47.2 |
| Smoking history (%) | 22.9 | 27.9 | 27.1 |
| Diabetes mellitus (%) | 68.6b | 35.8 | 40.9 |
| Hypertension (%) | 77.1 | 68.9 | 70.2 |
| Coronary artery disease (%) | 48.6c | 26.8 | 30.7 |
| Cerebrovascular disease (%) | 22.8c | 7.4 | 9.8 |
| Vascular access type | |||
| Arteriovenous graft (%) | 40%b | 16.3% | 20% |
| Arteriovenous fistula (%) | 60%b | 83.7% | 80% |
| Systolic BP (mmHg) | 151.8 ± 34.4 | 143.0 ± 24.0 | 144.3 ± 25.9 |
| Diastolic BP (mmHg) | 73.7 ± 18.3 | 79.3 ± 15.3 | 78.5 ± 15.8 |
| Pulse pressure (mmHg) | 78.1 ± 19.2b | 63.7 ± 16.3 | 65.8 ± 17.4 |
| Body mass index (kg/m2) | 24.1 ± 4.3 | 23.8 ± 3.4 | 23.8 ± 3.5 |
| Laboratory parameters | |||
| Albumin (g/dl) | 3.7 ± 0.3b | 3.8 ± 0.3 | 3.8 ± 0.3 |
| Triglyceride (mg/dl) | 161.7 ± 91.6 | 168.6 ± 130.0 | 167.5 ± 124.6 |
| Cholesterol (mg/dl) | 177.3 ± 44.4 | 183.5 ± 42.0 | 182.5 ± 42.3 |
| HDL cholesterol (mg/dl) | 39.9 ± 9.3b | 47.1 ± 15.0 | 46.1 ± 14.5 |
| LDL cholesterol (mg/dl) | 89.5 ± 23.8 | 87.5 ± 27.4 | 87.8 ± 26.8 |
| Creatinine (mg/dl) | 9.0 ± 2.0b | 10.4 ± 2.4 | 10.2 ± 2.4 |
| Hematocrit (%) | 32.0 ± 3.8c | 30.5 ± 3.2 | 30.7 ± 3.3 |
| Calcium (mg/dl) | 9.8 ± 0.8 | 9.8 ± 0.8 | 9.8 ± 0.8 |
| Phosphate (mg/dl) | 4.8 ± 1.2 | 4.8 ± 1.2 | 4.8 ± 1.2 |
| Uric acid (mg/dl) | 7.7 ± 1.8 | 7.6 ± 1.5 | 7.6 ± 1.5 |
| PTH (pg/ml) | 450.2 ± 321.8 | 528.6 ± 475.8 | 516.4 ± 455.5 |
| hsCRP (mg/L) | 0.9 ± 1.0 | 0.7 ± 1.3 | 0.7 ± 1.2 |
| Kt/V | 1.3 ± 0.3 | 1.3 ± 0.2 | 1.3 ± 0.2 |
ABI, ankle-brachial index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PTH, parathyroid hormone; hsCRP, high-sensitivity C-reactive protein.
P < 0.001 compared with ABI ≧ 0.9.
P < 0.05.
VAF episodes were recorded in 111 patients (49.2%), including 67 with vascular stenosis of arteriovenous anastomosis (within 3 cm) and 44 with vascular stenosis distant from anastomosis (more than 3 cm). Table 2 shows a Cox proportional hazards regression analysis for VAF. The univariate regression analysis shows the hazard ratio (HR) of ABI <0.9 was 2.682 (95% confidence interval [CI], 1.746 to 4.118). In addition, other variables including older age, vascular access type of AVG, the presence of DM, higher systolic BP, increased pulse pressure, higher BMI, increased serum triglyceride, decreased creatinine, and decreased PTH level were also associated with a significant increase in VAF. In the multivariate analysis, ABI <0.9 (HR, 1.893; 95% CI, 1.034 to 3.466), AVG usage, and serum triglyceride level were positively associated with VAF and serum PTH level was negatively associated with VAF.
Table 2.
Cox proportional hazards regression analysis for vascular access failure
| Parameter | Univariate
|
Multivariate
|
||
|---|---|---|---|---|
| Hazard Ratios (95% CI) | P Value | Hazard Ratios (95% CI) | P Value | |
| ABI (< 0.9 versus ≧ 0.9) | 2.682 (1.746-4.118) | <0.001 | 1.893 (1.034-3.466) | 0.039 |
| Age (per 1 yr) | 1.018 (1.003-1.033) | 0.019 | 1.003 (0.982-1.023) | 0.453 |
| Male versus female | 0.852 (0.589-1.250) | 0.413 | 1.027 (0.640-1.649) | 0.912 |
| Smoking (ever versus never) | 1.128 (0.740-1.718) | 0.576 | — | — |
| Diabetes mellitus | 2.797 (1.892-4.135) | <0.001 | 1.543 (0.888-2.679) | 0.124 |
| Hypertension | 1.458 (0.958-2.218) | 0.078 | — | — |
| Coronary artery disease | 1.329 (0.916-1.929) | 0.134 | — | — |
| Cerebrovascular disease | 0.988 (0.515-1.894) | 0.988 | — | — |
| Vascular access type | ||||
| (graft versus fistula) | 2.878 (1.883-4.399) | <0.001 | 2.100 (1.268-3.478) | 0.004 |
| Systolic BP (per 1 mmHg) | 1.008 (1.001-1.015) | 0.034 | 1.015 (0.999-1.031) | 0.063 |
| Diastolic BP (per 1 mmHg) | 1.006 (0.995-1.017) | 0.302 | — | — |
| Pulse pressure (per 1 mmHg) | 1.010 (1.001-1.019) | 0.030 | 0.980 (0.954-1.008) | 0.156 |
| Heart rate (per 1 beat/min) | 1.003 (0.990-1.017) | 0.627 | — | — |
| Body mass index (per 1 kg/m2) | 1.058 (1.000-1.119) | 0.049 | 0.998 (0.936-1.064) | 0.951 |
| Laboratory parameters | ||||
| Albumin (per 1 g/dl) | 0.596 (0.315-1.126) | 0.111 | — | — |
| Triglyceride (per 1 mg/dl) | 1.002 (1.000-1.003) | 0.020 | 1.002 (1.000-1.004) | 0.043 |
| Cholesterol (per 1 mg/dl) | 1.004 (0.999-1.009) | 0.094 | — | — |
| HDL-cholesterol (per 1 mg/dl) | 0.991 (0.978-1.005) | 0.219 | — | — |
| LDL-cholesterol (per 1 mg/dl) | 1.006 (0.999-1.013) | 0.105 | — | — |
| Creatinine (per 1 mg/dl) | 0.897 (0.825-0.974) | 0.010 | 0.939 (0.826-1.067) | 0.335 |
| Hematocrit (per 1%) | 0.995 (0.942-1.052) | 0.872 | — | — |
| Calcium (per 1 mg/dl) | 0.974 (0.776-1.221) | 0.817 | — | — |
| Phosphate (per 1 mg/dl) | 0.953 (0.813-1.116) | 0.550 | — | — |
| Uric acid (per 1 mg/dl) | 1.009 (0.890-1.144) | 0.886 | — | — |
| PTH (per 1 pg/ml) | 0.999 (0.999-1.000) | 0.002 | 0.999 (0.999-1.000) | 0.043 |
| hsCRP (per 1 mg/L) | 1.085 (0.935-1.259) | 0.281 | — | — |
| Kt/V (per 1.0) | 0.715 (0.331-1.546) | 0.394 | — | — |
Values expressed as hazard ratios and 95% confidence interval (CI). Abbreviations are the same as Table 1.
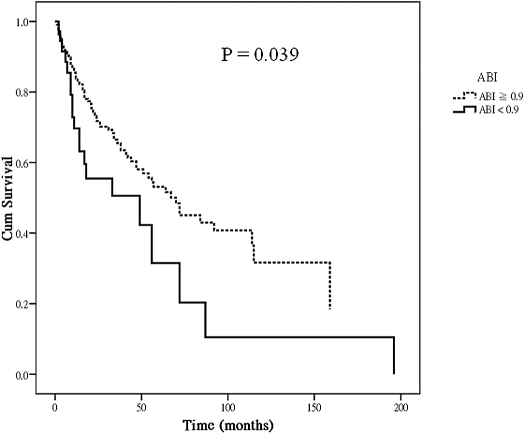
Figure 1 shows the Cox regression survival curves for vascular access patency in hemodialysis patients with ABI < 0.9 versus ≧ 0.9. The curves were adjusted for age, gender, vascular access type, DM, systolic BP, pulse pressure, BMI, serum triglycerides, creatinine, and PTH levels. Patients with ABI < 0.9 had a worse vascular access survival compared with those with ABI ≧ 0.9 (P = 0.039).
Figure 1.
Adjusted vascular access survival (Cox)* by ankle-brachial index (ABI) in hemodialysis patients. *Covariates in the model included age, gender, vascular access type, the presence of diabetes mellitus, systolic BP, pulse pressure, body mass index, serum triglyceride, creatinine, and parathyroid hormone level.
We also analyzed the correlation between an ABI <0.9 and vascular stenosis of different sites. After the multivariate analysis, we found that AVG usage (HR, 3.214; P = 0.001) was positively associated, and serum PTH level (HR, 0.999; P = 0.018) was negatively associated, with VAF in the subgroup analysis involving the 67 patients with stenosis at the site of arteriovenous anastomosis. However, an ABI <0.9 (HR, 1.888; P = 0.134) was not significantly associated with VAF in this subgroup analysis. In contrast, an ABI <0.9 (HR, 2.890; P = 0.024) was significantly associated with VAF in the subgroup analysis involving the 44 patients with stenosis distant from arteriovenous anastomosis.
Because high values (ABI >1.40) could be related to poor arterial compressibility resulting from stiffness and calcification, we performed another subgroup analysis after excluding two patients with DM having abnormally high ABI values (>1.4) and found an ABI <0.9 (HR, 1.903; 95% CI, 1.036 to 3.495, P = 0.038), and AVG usage (HR, 2.146; 95% CI, 1.290 to 3.569, P = 0.003) were positively associated with VAF and serum PTH level (HR, 0.999; 95% CI, 0.999 to 1.000, P = 0.045) was negatively associated with VAF.
We also analyzed the subgroup patients with AVF (n = 180) and found that an ABI <0.9 (HR, 2.652; P = 0.003), and DM (HR, 2.268; P = 0.004) were significantly correlated with VAF in the multivariate analysis.
Discussion
In the present study we evaluated the relationship between ABI level and VAF in 225 hemodialysis patients. We found that ABI <0.9 was significantly associated with a history of atherosclerotic vascular disease and was a marker for poor vascular access outcomes.
ABI is not only a simple, noninvasive, and reliable method to assess PAOD, but also a strong predictor for mortality in hemodialysis patients (10,16). PAOD is prevalent in hemodialysis patients and influences their mortality (17,18). In our study, the prevalence of PAOD, defined as ABI <0.9, was 15.6%, which was close to a large scale of epidemiologic surveys by Ono K et al. (16.5%) (10). Old age, DM, a history of cardiovascular disease and cerebrovascular disease, low albumin and creatinine levels, and high pulse pressure were reported to be associated factors of PAOD in hemodialysis patients (10,19,20), which was consistent with our finding. In addition, we found that the prevalence of PAOD was higher in patients with an AVG (32.6%) than those with an AVF (11.2%). National Kidney Foundation–Kidney Disease Outcomes Quality Initiative guidelines recommended that an AVF with superior access survival and function was a better type of vascular access than AVG (21–23). However, suitable vessels for AVF may be absent in patients with DM or severe atherosclerosis. Therefore, placement of an AVG will be considered in such patients, which may explain the correlation between ABI <0.9 and AVG usage in this study.
Previous studies demonstrated that ABI was not only a marker for diagnosis of PAOD but also for generalized atherosclerotic disease and other cardiovascular events (10,24,25). Patients with low ABI were reported to have an increased risk of coronary artery disease, cardiovascular death, and cerebrovascular disease (26). However, to date, there is no study to evaluate the relationship between ABI and VAF. In the present study, we found that ABI <0.9 is independently associated with increased VAF in hemodialysis patients. This finding implies that patients with severe atherosclerosis, indicated by ABI <0.9, may easily develop VAF.
The vascular access type of AVG has been reported to be a marker for VAF (27,28). It may be because of an accelerated rate of stenosis caused by intimal hyperplasia, flow turbulence, and surgical trauma at the graft-to-vein anastomosis and by the release of growth factors from fibrin and platelets that accumulate on the luminal surface (29–31). Similarly, our study also showed that patients with AVG had a higher incidence of VAF than those with AVG.
Dyslipidemia was well-established atherogenic factor among hemodialysis patients (32,33). In our study, an increased serum triglyceride level was independently associated with VAF as expected, but serum cholesterol level was not. The prevalence of patients with hypercholesterolemia was only 6.2% in this study, and this low prevalence rate may explain no correlation between hypercholesterolemia and VAF.
The present study revealed that there was a correlation between decreased PTH level and VAF. Hyperparathyroidism was reported to be significantly associated with increased morbidity and mortality in hemodialysis patients (34). Grandaliano et al. (35) evaluated the relationship between VAF and serum PTH level in 87 hemodialysis patients, and found hyperparathyroidism was an independent risk factor for VAF. They explained their finding by vascular calcification caused by hyperparathyroidism. In their study, serum PTH levels were evaluated every 3 mo during the observational period of 5 yr, and those measurements were averaged for analysis. In our study, only single measurement of PTH level was made, which might explain the inconsistent result with Grandaliano. However, there were several studies reporting a negative association between PTH levels and cardiac calcification, calcific aortic stenosis, and peripheral vascular disease among dialysis patients, which was supporting our findings (20,36,37). The reason for the negative association might be related to deposition of calcium at extraskeletal sites, preferentially in low-bone turnover states. Another reasonable explanation is that a low PTH level might be a consequence of high vitamin D use. Vitamin D is involved in vascular calcification and has been suggested as a potential atherogenic factor (38). Furthermore, increased PTH levels should play no or a negative role in VAF in our study. An ABI <0.9, AVG usage, and increased serum triglyceride levels may be the major factors resulting in VAF in the present study.
In ESRD patients, chronic inflammation, as expressed by increased CRP levels, has been identified as accelerating atherosclerosis (39). Data from several studies have demonstrated that elevated levels of the acute-phase reactant CRP were associated with increased cardiovascular events and atherosclerosis (decreased ABI) (40,41). In the present study, there was no correlation between high-sensitivity CRP and ABI <0.9 or vascular access failure. Thus, the role of inflammation was not a major determinant of VAF in our study patients.
Previous studies reported the relative frequency of stenosis at different vascular locations. Stenosis was observed most commonly at the arteriovenous anastomosis (42). The differences in the incidence and distribution of vascular stenosis are affected by many factors, such as age, the diagnosis of DM, vascular access type, and surgeon’s experience (28,43,44). In our study, we divided the patients with VAF into two subgroups, including stenosis at the site of arteriovenous anastomosis (within 3 cm of the anastomosis) and site distant from anastomosis to evaluate the correlation between ABI <0.9 and VAF in different stenosis sites. It revealed that an ABI <0.9 was still significantly associated with VAF in the subgroup analysis involving the 44 patients with stenosis distant from arteriovenous anastomosis, but not in the subgroup analysis involving the 67 patients with stenosis at the site of arteriovenous anastomosis. Previous study demonstrated that ABI was not only a marker for diagnosis of PAOD but also for generalized atherosclerotic disease. Thus, in the subgroup analysis involving the patients with stenosis distant from arteriovenous anastomosis, ABI could be related to VAF because generalized atherosclerotic disease might be existed in the patients with ABI <0.9. However, in the subgroup analysis involving the patients with stenosis at the site of arteriovenous anastomosis, VAF might be affected by surgeon’s experience in addition to the value of ABI. This might partially explain there was no significant correlation between ABI <0.9 and VAF in this subgroup analysis.
Although the presence of DM was strongly associated with VAF in the univariate analysis, this association disappeared in the multivariate analysis. Puskar et al. (45) studied the survival of AVF in 463 hemodialysis patients and found fistula survival was shorter in ESRD patients with DM than those without DM. Compared with our study, their study only included the subjects with AVF. In our subgroup analysis involving the patients with AVF, DM was also significantly associated with VAF in the multivariate analysis. This result was consistent with Puskar’s finding. Moreover, ABI <0.9 was still independently associated with VAF in this subgroup analysis.
An ABI <0.9 has been established as a good marker for PAOD with high sensitivity and specificity (46). However, falsely elevated pressures or incompressible arteries at ankle level are common among patients with extensive vascular calcification of the lower extremities, which may occur in those with diabetes or in treatment with hemodialysis (10,47). The prevalence of PAOD may be underestimated when the criterion of an ABI <0.9 was used because many of our patients had DM (40.9%). The abnormally high ABI value or incompressible arteries had been interpreted as the presence of medial arterial calcification, which renders the diagnosis of PAOD by ABI measurement unreliable. In patients with hemodialysis, an ABI <0.9 is not accurate value in terms of sensitivity to detect PAOD (48). In addition, patients having abnormally high ABIs had poor prognoses for all-cause and cardiovascular mortality in hemodialysis patients (10). Therefore, we performed another subgroup analysis after excluding two cases with ABIs >1.4. The results showed an ABI <0.9 was still significantly associated with increased VAF.
There were several limitations in our study. First, the study subjects were included only in one regional hospital and thus the selection of patients was limited. However, vascular access creation and care were similar in these patients and therefore the impact of these two factors on the VAF could be minimized. Second, since the design of the study is observational, it is susceptible to selection bias. We minimized this bias by enrolling all hemodialysis patients in our dialysis clinics and statistically adjusting for several variables that may influence VAF. In addition, there are limitations when using the oscillometric methods to measure BP instead of using traditional mercury methods (49). However, the ABI form solved this problem by introducing a double-cuff configuration and the agreement was excellent between ankle BP determined by the conventional Doppler method and the oscillometric method (50). Thus, the ABI-form could be a reliable method to measure ankle BP. Finally, this was a cross-sectional study and thus we could not evaluate the predictors of VAF. A prospective trial of screening patients by means of ABI at initial vascular access creation will be needed to validate that an ABI <0.9 is a useful predictor of VAF.
In conclusion, our findings show that an ABI <0.9 is significantly correlated with increased VAF. Screening hemodialysis patients by means of ABI may help to identify a high-risk group for VAF.
Disclosures
None.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Feldman HI, Kobrin S, Wasserstein A: Hemodialysis vascular access morbidity. J Am Soc Nephrol 7: 523–535, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Woods JD, Port FK: The impact of vascular access for haemodialysis on patient morbidity and mortality. Nephrol Dial Transplant 12: 657–659, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Weiss MF, Scivittaro V, Anderson JM: Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. Am J Kidney Dis 37: 970–980, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Hofstra L, Tordoir JH, Kitslaar PJ, Hoeks AP, Daemen MJ: Enhanced cellular proliferation in intact stenotic lesions derived from human arteriovenous fistulas and peripheral bypass grafts. Does it correlate with flow parameters? Circulation 94: 1283–1290, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Ross R: The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 362: 801–809, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Fishbane S, Youn S, Kowalski EJ, Frei GL: Ankle-arm blood pressure index as a marker for atherosclerotic vascular diseases in hemodialysis patients. Am J Kidney Dis 25: 34–39, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ: Edinburgh Artery Study: Prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 20: 384–392, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Newman AB, Tyrrell KS, Kuller LH: Mortality over four years in SHEP participants with a low ankle-arm index. J Am Geriatr Soc 45: 1472–1478, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Kitahara T, Ono K, Tsuchida A, Kawai H, Shinohara M, Ishii Y, Koyanagi H, Noguchi T, Matsumoto T, Sekihara T, Watanabe Y, Kanai H, Ishida H, Nojima Y: Impact of brachial-ankle pulse wave velocity and ankle-brachial blood pressure index on mortality in hemodialysis patients. Am J Kidney Dis 46: 688–696, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Ono K, Tsuchida A, Kawai H, Matsuo H, Wakamatsu R, Maezawa A, Yano S, Kawada T, Nojima Y: Ankle-brachial blood pressure index predicts all-cause and cardiovascular mortality in hemodialysis patients. J Am Soc Nephrol 14: 1591–1598, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S: Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement—A survey of 12,517 subjects. Atherosclerosis 166: 303–309, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y: Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 25: 359–364, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Yokoyama H, Shoji T, Kimoto E, Shinohara K, Tanaka S, Koyama H, Emoto M, Nishizawa Y: Pulse wave velocity in lower-limb arteries among diabetic patients with peripheral arterial disease. J Atheroscler Thromb 10: 253–258, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Gotch FA: Evolution of the single-pool urea kinetic model. Semin Dial 14: 252–256, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285: 2486–2497, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Fowkes FG: The measurement of atherosclerotic peripheral arterial disease in epidemiological surveys. Int J Epidemiol 17: 248–254, 1988 [DOI] [PubMed] [Google Scholar]
- 17.Fishbane S, Youn S, Flaster E, Adam G, Maesaka JK: Ankle-arm blood pressure index as a predictor of mortality in hemodialysis patients. Am J Kidney Dis 27: 668–672, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Testa A, Ottavioli JN: Ankle-arm blood pressure index (AABPI) in hemodialysis patients. Arch Mal Coeur Vaiss 91: 963–965, 1998 [PubMed] [Google Scholar]
- 19.Cheung AK, Sarnak MJ, Yan G, Dwyer JT, Heyka RJ, Rocco MV, Teehan BP, Levey AS: Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int 58: 353–362, 2000 [DOI] [PubMed] [Google Scholar]
- 20.O’Hare AM, Hsu CY, Bacchetti P, Johansen KL: Peripheral vascular disease risk factors among patients undergoing hemodialysis. J Am Soc Nephrol 13: 497–503, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Ascher E, Hingorani A: The Dialysis Outcome and Quality Initiative (DOQI) recommendations. Semin Vasc Surg 17: 3–9, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK: Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int 60: 1443–1451, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Maya ID, Weatherspoon J, Young CJ, Barker J, Allon M: Increased risk of infection associated with polyurethane dialysis grafts. Semin Dial 20: 616–620, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelmark M, Polak JF, Powe NR, Siscovick D: Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol 19: 538–545, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Mehler PS, Coll JR, Estacio R, Esler A, Schrier RW, Hiatt WR: Intensive blood pressure control reduces the risk of cardiovascular events in patients with peripheral arterial disease and type 2 diabetes. Circulation 107: 753–756, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Belch JJ, Topol EJ, Agnelli G, Bertrand M, Califf RM, Clement DL, Creager MA, Easton JD, Gavin JR, 3rd, Greenland P, Hankey G, Hanrath P, Hirsch AT, Meyer J, Smith SC, Sullivan F, Weber MA: Critical issues in peripheral arterial disease detection and management: A call to action. Arch Intern Med 163: 884–892, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Churchill DN, Taylor DW, Cook RJ, LaPlante P, Barre P, Cartier P, Fay WP, Goldstein MB, Jindal K, Mandin H: Canadian Hemodialysis Morbidity Study. Am J Kidney Dis 19: 214–234, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Woods JD, Turenne MN, Strawderman RL, Young EW, Hirth RA, Port FK, Held PJ: Vascular access survival among incident hemodialysis patients in the United States. Am J Kidney Dis 30: 50–57, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Lumsden AB, Ofenloch JC, Noe B, Campbell EJ, Stratford PW, Yianni YP, Taylor AS, Hanson SR: Phosphorylcholine coating of ePTFE grafts reduces neointimal hyperplasia in canine model. Ann Vasc Surg 11: 74–79, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Hofstra L, Bergmans DC, Leunissen KM, Hoeks AP, Kitslaar PJ, Tordoir JH: Prosthetic arteriovenous fistulas and venous anastomotic stenosis: influence of a high flow velocity on the development of intimal hyperplasia. Blood Purif 14: 345–349, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Schwab SJ, Harrington JT, Singh A, Roher R, Shohaib SA, Perrone RD, Meyer K, Beasley D: Vascular access for hemodialysis. Kidney Int 55: 2078–2090, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Deighan CJ, Caslake MJ, McConnell M, Boulton-Jones JM, Packard CJ: Atherogenic lipoprotein phenotype in end-stage renal failure: Origin and extent of small dense low-density lipoprotein formation. Am J Kidney Dis 35: 852–862, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Nishizawa Y, Shoji T, Emoto M, Koyama H, Tahara H, Fukumoto S, Inaba M, Ishimura E, Miki T: Roles of metabolic and endocrinological alterations in atherosclerosis and cardiovascular disease in renal failure: Another form of metabolic syndrome. Semin Nephrol 24: 423–425, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Grandaliano G, Teutonico A, Allegretti A, Losappio R, Mancini A, Gesualdo L, Schena FP, Pertosa G: The role of hyperparathyroidism, erythropoietin therapy, and CMV infection in the failure of arteriovenous fistula in hemodialysis. Kidney Int 64: 715–719, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Eguchi M, Tsuchihashi K, Takizawa H, Nakahara N, Hagiwara M, Ohnishi H, Torii T, Hashimoto A, Marusaki S, Nakata T, Ura N, Shimamoto K: Detection of cardiac calcinosis in hemodialysis patients by whole-body scintigraphy with 99m-technetium methylene diphosphonate. Am J Nephrol 20: 278–282, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Lazarus JM, Lowrie EG, Hampers CL, Merrill JP: Cardiovascular disease in uremic patients on hemodialysis. Kidney Int Suppl 167–175, 1975 [PubMed]
- 38.Rostand SG, Drueke TB: Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int 56: 383–392, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Ross R: Atherosclerosis—An inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Kim BS, Jeon DS, Shin MJ, Kim YO, Song HC, Lee SH, Kim SY, Choi EJ, Chang YS, Bang BK: Persistent elevation of C-reactive protein may predict cardiac hypertrophy and dysfunction in patients maintained on hemodialysis. Am J Nephrol 25: 189–195, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Matsumae T, Abe Y, Murakami G, Ishihara M, Ueda K, Saito T: Determinants of arterial wall stiffness and peripheral artery occlusive disease in nondiabetic hemodialysis patients. Hypertens Res 30: 377–385, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Bozof R, Kats M, Barker J, Allon M: Time to symptomatic vascular stenosis at different locations in patients with arteriovenous grafts. Semin Dial 21: 285–288, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Fassiadis N, Morsy M, Siva M, Marsh JE, Makanjuola AD, Chemla ES: Does the surgeon’s experience impact on radiocephalic fistula patency rates? Semin Dial 20: 455–457, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Hayakawa K, Miyakawa S, Hoshinaga K, Hata K, Marumo K, Hata M: The effect of patient age and other factors on the maintenance of permanent hemodialysis vascular access. Ther Apher Dial 11: 36–41, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Puskar D, Pasini J, Savic I, Bedalov G, Sonicki Z: Survival of primary arteriovenous fistula in 463 patients on chronic hemodialysis. Croat Med J 43: 306–311, 2002 [PubMed] [Google Scholar]
- 46.Feigelson HS, Criqui MH, Fronek A, Langer RD, Molgaard CA: Screening for peripheral arterial disease: the sensitivity, specificity, and predictive value of noninvasive tests in a defined population. Am J Epidemiol 140: 526–534, 1994 [DOI] [PubMed] [Google Scholar]
- 47.Orchard TJ, Strandness DE, Jr.: Assessment of peripheral vascular disease in diabetes. Report and recommendations of an international workshop sponsored by the American Diabetes Association and the American Heart Association September 18–20, 1992, New Orleans, Louisiana. Circulation 88: 819–828, 1993 [DOI] [PubMed] [Google Scholar]
- 48.Okamoto K, Oka M, Maesato K, Ikee R, Mano T, Moriya H, Ohtake T, Kobayashi S: Peripheral arterial occlusive disease is more prevalent in patients with hemodialysis: Comparison with the findings of multidetector-row computed tomography. Am J Kidney Dis 48: 269–276, 2006 [DOI] [PubMed] [Google Scholar]
- 49.O’Brien E, Waeber B, Parati G, Staessen J, Myers MG: Blood pressure measuring devices: Recommendations of the European Society of Hypertension. BMJ 322: 531–536, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cortez-Cooper MY, Supak JA, Tanaka H: A new device for automatic measurements of arterial stiffness and ankle-brachial index. Am J Cardiol 91: 1519–1522, 2003 [DOI] [PubMed] [Google Scholar]