Abstract
Background and objectives: Anemia is a well known complication of chronic kidney disease (CKD); however, the prevalence of anemia within CKD stages in the pediatric population has not been established. Additionally, the associated morbidity of anemia in the pediatric CKD population has not been elucidated.
Design, setting, participants, & measurements: 2779 patients ages 2 yr and older in the North American Pediatric Renal Trials and Collaborative Studies database with CKD stage II to V (excluding dialysis or previous transplant patients) were identified. Descriptive statistics and multivariate modeling using logistic regression was performed to determine the prevalence of anemia and to evaluate the correlation between baseline anemia and hospitalization.
Results: The prevalence of anemia (hematocrit < 33%) increased from 18.5% in CKD stage II to 68% in CKD stage V (predialysis). Anemic children were 55% more likely to be hospitalized when compared with nonanemic children (odds ratio 1.55; 95% confidence interval 1.23 to 1.94). Similar results were obtained using hematocrit cutoffs of 36 and 39%.
Conclusions: In this pediatric predialysis CKD population, anemia increases with increasing CKD stage and is significantly associated with hospitalization risk. Hematocrit levels above 36 and 39% were not associated with increased risk of hospitalization. Further examination into the effect of correcting anemia on hospitalization rates may provide additional useful information.
Anemia in chronic kidney disease (CKD) is largely due to decreased production of erythropoietin (1). Correction of anemia with erythropoietin has been associated with a variety of beneficial effects in children, including improvement in quality of life, appetite, exercise tolerance, and Wechsler intelligence score (2,3).
The risk of anemia in children with CKD increases as the GFR decreases (1,4,5). Whereas studies in children with ESRD have shown that anemia is associated with an increased risk of hospitalization and mortality (6,7), the relationship between anemia and morbidity in children with predialysis CKD has yet to be studied in a large cohort. Moreover, prior studies have analyzed small numbers of children, included children with renal transplants, and used adult definitions of anemia without adjusting for the variation in normative values that occur during childhood.
Recently, several large adult studies of both dialysis and nondialysis CKD patients have demonstrated an increased risk of morbidity and mortality with complete correction of hematocrit (8–10). This possible association has not been examined in the pediatric predialysis CKD population.
Accordingly, this study describes the prevalence of anemia in a large cohort of children with predialysis CKD. Additionally, we describe the relationship between anemia and morbidity (hospitalization) in this cohort. As a secondary aim, two definitions of anemia are compared: an age-independent definition using the National Kidney Foundation–Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines and an age-dependent definition using age- and gender-appropriate norms.
Materials and Methods
Study Population
A retrospective cohort study of pediatric patients with CKD (estimated GFR < 75 ml/min/1.73m2) enrolled in the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Chronic Renal Insufficiency (CRI) registry was performed. Patients are eligible for enrollment through their 20th birthday. Patients on chronic dialysis are not included in the CRI registry. NAPRTCS is a voluntary registry that began collecting data on children with chronic renal insufficiency in 1994. Currently, more than 6400 patients are enrolled in the registry. Information is collected at enrollment and every 6 mo thereafter, until disenrollment due to the initiation of dialysis, kidney transplant, loss to follow-up, or death.
Patients were classified by disease severity into one of five stages using the KDOQI CKD staging system (11). Estimated GFR (eGFR) was determined via the Schwartz equation (12). All patients with CKD stage II to V and at least 12 mo of follow-up data were included. Patients were excluded if they had a history of a prior kidney transplant or dialysis, or if they were <2 yr of age because the KDOQI staging system does not include patients less than 2 yr (11). Patients without either a baseline or a 1-yr follow-up hematocrit, or the data needed to estimate GFR (height and serum creatinine) were also excluded.
Definition of Terms
Primary diagnosis was defined as the primary etiology of CKD. Patients were divided into five categories: obstructive uropathy, focal segmental glomerulosclerosis (FSGS), reflux nephropathy, renal dysplasia, and other. These are the categories of CKD etiology used in the NAPRTCS database.
We used the KDOQI CKD staging system: Stage I GFR ≥ 90 ml/min/1.73m2; Stage II GFR 60 to 89 ml/min/1.73m2; Stage III GFR 30 to 59 ml/min/1.73m2; Stage IV GFR 15 to 29 ml/min/1.73m2; and Stage V GFR <15 ml/min/1.73m2 or dialysis (11).
The serum calcium was adjusted for serum albumin as follows: measured calcium (mg/dl) + 0.8 × [4-serum albumin (g/dl)]. We defined a calcium value of 8.5 to 10.0 mg/dl as normal. Calcium values below 8.5 mg/dl were classified as low and values above 10.0 mg/dl were classified as high.
The definition of hyperphosphatemia was adjusted for age in the following manner: ≥6.5 mg/dl for 2 to 5 yr; ≥5.8 mg/dl for 6 to 12 yr; and ≥4.5 mg/dl for 13 to 20 yr.
Parathyroid hormone (PTH) values were divided into a dichotomous variable on the basis of being above or below twice the upper limit of the KDOQI target based on CKD stage (13). Hence, intact PTH values >140 pg/ml (stage II or stage III), 220 pg/ml (stage IV), or 600 pg/ml (stage V) were defined as >2 times the upper limit of normal.
The year of entry into the registry was also divided into a dichotomous variable. The early cohort included those patients registered from 1994 to 1997 and the late cohort included those registered from 1998 to 2006.
Two definitions of anemia were used in the analysis. The first was a hematocrit < 33%, the lower limit recommended by KDOQI (14). The second was a hematocrit z-score below −2. Data from the Third National Health and Nutrition Examination Survey was used to determine age- and gender-appropriate normal hematocrit values and SD for the general pediatric population in the United States (15). z-scores were generated using these means and SD. Hematocrit values were used because hemoglobin data are not available in the NAPRTCS database.
Study Objectives
The primary outcome measure was hospitalization during the 1-yr postenrollment follow-up period. Hospitalization was only analyzed as having occurred or not occurred. The number of hospitalizations, duration of hospitalization, and elective versus acute were not considered because the data were either incomplete or not collected by the database.
The relationship between baseline anemia (hematocrit < 33%) and hospitalization during the subsequent year was analyzed. To address the possible morbidity associated with a higher hematocrit, separate analyses were also run using cutoffs of 36 and 39%. The following covariates were included in the analysis: age, gender, race, primary diagnosis, registry year, height z-score, weight z-score, body mass index (BMI) z-score, erythropoietin use, human growth hormone use, PTH, iron supplementation, antihypertensive medication use, CKD stage, eGFR, corrected serum calcium, serum albumin, and serum phosphorus. Additionally, we considered the possibility of confounding between hematocrit and CKD stage as well as hematocrit and erythropoietin use. The same covariates were also examined for their association with baseline hematocrit.
As a secondary aim, the above analyses were repeated using the standardized age-dependent z-score definition of anemia to assess which definition was a more appropriate measure of anemia in this patient population.
Statistical Analyses
The prevalence of anemia by CKD stage was determined using descriptive statistics. Univariate logistic regression analyses were used to assess the factors associated with anemia at baseline and hospitalization during the first year after registration. All identified factors were entered into the model and removed in a backwards-stepwise manner using a significance level of 0.05. Multivariate logistic regression modeling was used to estimate the relative risk and 95% confidence intervals of hospitalization of anemic patients compared with nonanemic patients. Receiver operating characteristic (ROC) curves were generated to determine the clinically relevant cutoff for anemia as a predictor of hospitalization during the first year. The two alternate definitions of anemia were compared using two statistical methods: NcNemar's test for marginal homogeneity and the Kappa statistic to measure agreement. The analysis was performed using SAS version 8.2 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Study Population Demographics
A total of 6405 patients were enrolled in the NAPRTCS CRI database. Of those, 2950 did not have complete data available (either eGFR or hematocrit) at either registry initiation or at the 1-yr follow-up. The 719 patients under 2 yr of age and a single patient with missing gender data were excluded. Thus, there were 2779 remaining patients ages 2 and older with complete data (eGFR and hematocrit) available for analysis.
We compared the characteristics of the patients who met the inclusion criteria to those who were excluded. All variables except gender were significantly different (all P values < 0.0003, data not shown). In general, included patients tended to be younger, Caucasian, carry the diagnosis of obstructive uropathy, and have better renal function. Additionally, included patients were less likely to be anemic or to be receiving erythropoietin.
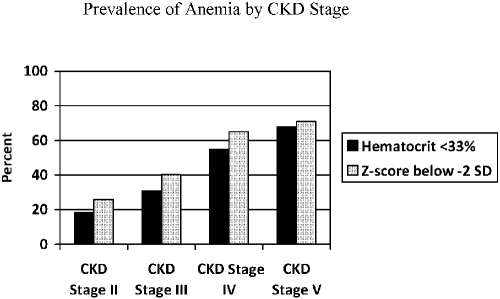
The prevalence of anemia (hematocrit < 33%) by CKD stage is presented in Figure 1. It increased from 18.5% in CKD stage II to 68% in CKD stage V.
Figure 1.
Prevalence of anemia at registry enrollment by the Kidney Disease Outcomes Quality Initiative (KDOQI) anemia definition of hematocrit < 33% and the NHANES anemia definition of hematocrit below −2 SD for age.
There are some notable differences when comparing those patients who were hospitalized to those who were not hospitalized (Table 1). Younger patients (ages 2 to 5 yr) had a higher frequency of hospitalizations compared with older patients (24.9 versus 20.7%). As expected, 28% of CKD stage V patients were hospitalized (28 of 100) whereas only 18.7% of patients with CKD stage II were hospitalized (105 of 561). More anemic (hematocrit < 33%) patients were hospitalized than nonanemic patients (27.5 versus 18.2%). Notably, patients with a higher hematocrit did not have an increased rate of hospitalization (Table 2).
Table 1.
Characteristics of hospitalized and nonhospitalized patientsa
| Characteristics | Hospitalized n (%) | Nonhospitalized n (%) |
|---|---|---|
| Total cases (≥2 yr of age) | 597 (21.5) | 2182 (78.5) |
| Age (yr) | ||
| 2 to 5 | 154 (24.9) | 465 (75.1) |
| 6 to 12 | 237 (20.3) | 929 (79.7) |
| >12 | 206 (20.7) | 788 (79.3) |
| Gender | ||
| male | 353 (20.2) | 1391 (79.8) |
| female | 224 (21.6) | 791 (76.4) |
| Race | ||
| Caucasian | 356 (20.2) | 1409 (79.8) |
| African-American | 123 (25.1) | 367 (74.9) |
| Hispanic | 87 (23.0) | 291 (77.0) |
| other | 31 (22.0) | 110 (78.0) |
| Primary diagnosis | ||
| obstructive uropathy | 148 (23.0) | 496 (77.0) |
| FSGS | 50 (20.6) | 193 (79.4) |
| renal dysplasia | 60 (14.7) | 349 (85.3) |
| reflux nephropathy | 66 (23.5) | 215 (76.5) |
| other | 256 (22.3) | 894 (77.7) |
| Anemia (KDOQI) | ||
| HCT ≥ 33% | 328 (18.2) | 1474 (81.8) |
| HCT < 33% | 269 (27.5) | 708 (72.5) |
| Anemia (NHANES III) | ||
| z-score above −2 | 292 (18.8) | 1262 (81.2) |
| z-score below −2 | 305 (24.9) | 920 (75.1) |
| CKD stage | ||
| II | 105 (18.7) | 456 (81.3) |
| III | 316 (21.3) | 1171 (78.7) |
| IV | 148 (23.5) | 483 (76.5) |
| V | 28 (28.0) | 72 (72.0) |
| CRI registry year | ||
| before 1998 | 406 (22.2) | 1424 (77.8) |
| 1998 and after | 191 (20.1) | 758 (79.9) |
| Erythropoietin use | ||
| yes | 98 (29.8) | 231 (70.2) |
| no | 499 (20.4) | 1951 (79.6) |
| Growth hormone use | ||
| yes | 46 (20.9) | 174 (79.1) |
| no | 551 (21.5) | 2007 (78.5) |
| Calcium | ||
| low | 28 (22.6) | 96 (77.4) |
| normal | 386 (21.5) | 1413 (78.5) |
| high | 106 (23.8) | 340 (76.2) |
| Phosphorus | ||
| normal | 394 (21.0) | 1483 (79.0) |
| elevated | 160 (23.0) | 535 (77.0) |
| Parathyroid hormone | ||
| <2× upper limit | 200 (19.1) | 846 (80.1) |
| >2× upper limit | 130 (24.4) | 403 (75.6) |
| unknown | 267 (22.3) | 933 (77.7) |
| Iron supplementation | ||
| yes | 179 (28.2) | 456 (71.8) |
| no | 417 (19.5) | 1722 (80.5) |
| Antihypertensive medication use | ||
| yes | 258 (22.3) | 901 (77.8) |
| no | 339 (20.9) | 1280 (79.1) |
FSGS, focal segmental glomeruloscerosis; KDOQI, Kidney Disease Outcomes Quality Initiative; HCT, hematocrit; NHANES III, National Health and Nutrition Examination Survey III; CKD, chronic kidney disease; CRI, Chronic Renal Insufficiency registry.
Table 2.
Hospitalization rates by baseline hematocrit
| Baseline Hematocrit, % | Hospitalization, n (%) | Total Number of Patients, n |
|---|---|---|
| <31 | 201 (30.7) | 654 |
| 31 to <33 | 68 (21.1) | 323 |
| 33 to <35 | 87 (20.7) | 421 |
| 35 to <37 | 93 (22.0) | 422 |
| 37 to <39 | 59 (15.7) | 376 |
| 39 to <41 | 51 (18.8) | 271 |
| 41 to <43 | 23 (13.9) | 166 |
| 43 to <45 | 8 (10.8) | 74 |
| ≥45 | 7 (9.7) | 72 |
| Total | 597 (21.5) | 2779 |
Hospitalizations
There were 597 (21.5%) patients hospitalized within the first year of enrollment. The median number of hospitalizations was one (maximum of 12) and the median number of days in the hospital was five (maximum of 125). Reasons for hospitalization available in the database include infection, hypertension, and other cardiovascular problems. Of those hospitalizations that listed an etiology, 39.5% were infection related, whereas 10% were hypertension related.
The multivariate logistic regression analysis of factors associated with hospitalization is summarized in Table 3. All factors that were significant in the univariate analysis were included in the multivariate analysis. Age, gender, and CKD stage were not significant in the univariate analysis. Age and gender were therefore not included in the multivariate analysis. However, CKD stage was included given its relationship to anemia. Factors associated with hospitalization in the multivariate analysis included anemia, albumin, height z-score, primary diagnosis, erythropoietin use, race, and cohort year. After adjusting for the above factors, anemic patients (hematocrit < 33%) were 55% more likely to be hospitalized than were nonanemic patients [odds ratio (OR) 1.55, 95% confidence interval (CI) 1.23 to 1.94]. The OR was unchanged when age and gender were included in the model. Additionally, the OR were similar when hematocrit cutoffs of 36 and 39% were evaluated (OR 1.74, 95% CI 1.40 to 2.17 and OR 1.57, 95% CI 1.19 to 2.07, respectively).
Table 3.
Multivariate logistic regression analysis of factors associated with hospitalization during the first year of registry participationa
| Variable | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| Anemia | ||
| yes (HCT < 33%) | 1.55 | 1.23 to 1.54b |
| no (HCT ≥ 33%) | 1.00 | — |
| Height z-score | 0.89 | 0.83 to 0.95b |
| Primary diagnosis | ||
| dysplasia | 0.50 | 0.34 to 0.72b |
| reflux nephropathy | 1.16 | 0.80 to 1.68 |
| FSGS | 0.63 | 0.40 to 0.98c |
| other | 0.83 | 0.63 to 1.09 |
| obstructive uropathy | 1.00 | — |
| Erythropoietin use | ||
| yes | 1.51 | 1.11 to 2.07c |
| no | 1.00 | — |
| Race | ||
| African-American | 1.45 | 1.11 to 1.90b |
| Hispanic | 1.26 | 0.93 to 1.69 |
| other/unknown | 0.85 | 0.50 to 1.44 |
| Caucasian | 1.00 | — |
| Cohort year | ||
| 1994 to 1997 | 1.31 | 1.04 to 1.65 |
| 1998 to 2004 | 1.00 | — |
| Albumin | 0.72 | 0.61 to 0.86b |
Factors included in the multivariate analysis include: albumin, CKD stage, primary diagnosis, cohort year, height z-score, weight z-score, parathyroid hormone, calcium, phosphorus, anemia, antihypertensive medication use, iron supplementation, growth hormone use, and erythropoietin use.
P value < 0.005;
P value < 0.05.
Table 4 is an analysis of risk factors associated with anemia (hematocrit < 33%) at baseline. The significant risk factors were CKD stage, primary diagnosis, use of iron supplementation, use of erythropoietin, albumin, elevated serum phosphorus level, elevated PTH level, age, weight z-score, elevated serum calcium, antihypertensive medication use, and cohort year.
Table 4.
Multivariate logistic regression analysis of factors associated with anemia (hematocrit < 33%) at the time of enrollmenta
| Variable | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| CKD stage | ||
| V | 8.50 | 4.51 to 16.00b |
| IV | 4.83 | 3.37 to 6.92b |
| III | 1.89 | 1.39 to 2.56b |
| II | 1.00 | — |
| Weight z-score | 0.88 | 0.82 to 0.93c |
| Primary diagnosis | ||
| dysplasia | 0.93 | 0.66 to 1.30 |
| reflux nephropathy | 1.09 | 0.73 to 1.62 |
| FSGS | 1.00 | 0.62 to 1.59 |
| other | 2.05 | 1.56 to 2.70b |
| obstructive uropathy | 1.00 | — |
| Iron supplementation | ||
| yes | 2.39 | 1.85 to 3.08b |
| no | 1.00 | — |
| Erythropoietin use | ||
| yes | 0.70 | 0.50 to 0.97d |
| no | 1.00 | — |
| Serum calcium | ||
| high | 0.50 | 0.38 to 0.66b |
| low | 1.31 | 0.85 to 2.03 |
| normal | 1.00 | — |
| Serum phosphorus | ||
| high | 1.82 | 1.41 to 2.35b |
| normal | 1.00 | — |
| Parathyroid hormone level | ||
| >2× upper limit of normal | 2.14 | 1.61 to 2.83b |
| unknown | 1.29 | 1.02 to 1.63d |
| <2× upper limit of normal | 1.00 | — |
| Age (yr) | ||
| 2 to 5 | 1.84 | 1.34 to 2.54c |
| 6 to 12 | 1.81 | 1.40 to 2.34b |
| >12 | 1.00 | — |
| Antihypertensive medication use | ||
| yes | 1.27 | 1.02 to 1.59d |
| no | 1.00 | — |
| Cohort year | ||
| 1994 to 1997 | 1.43 | 1.14 to 1.79c |
| 1998 to 2004 | 1.00 | — |
| Albumin | 0.48 | 0.40 to 0.57b |
Factors included in the multivariate analysis include: age, race, gender, primary diagnosis, cohort year, height z-score, weight z-score, parathyroid hormone, calcium, phosphorus, CKD stage, albumin, antihypertensive medication use, iron supplementation, growth hormone use, and erythropoietin use.
P value < 0.0001;
P value < 0.005;
P value < 0.05.
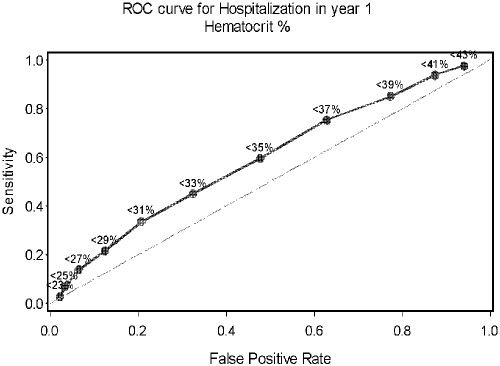
ROC curves demonstrating the thresholds for anemia in relation to hospitalization are depicted in Figures 2 and 3. Figure 2 shows the curve for the KDOQI anemia definition of <33%. After examining multiple cutpoints by hematocrit percent, the optimal cutpoint for predicting hospitalization was found to be 31%.
Figure 2.
Receiver operator characteristic (ROC) curve for hospitalization within 1 yr of registry enrollment using the KDOQI anemia definition of hematocrit < 33%.
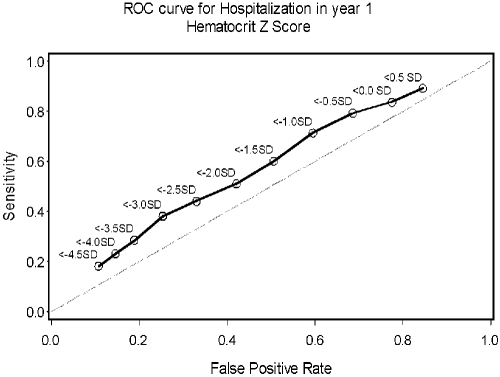
Figure 3.
ROC curve for hospitalization within 1 yr of registry enrollment using the NHANES anemia definition of hematocrit below −2 SD for age.
Table 5 compares the two alternate definitions of anemia. On the basis of z-score, 44% of all patients were anemic. This contrasts with 35% having anemia on the basis of the KDOQI definition. The two methods agreed in 89% of the patients (34% had anemia and 55% did not have anemia). In contrast, 10% of patients fulfilled the z-score definition of anemia, but did not have a hematocrit < 33%. Only 1% of patients had a hematocrit < 33% while not meeting the criteria for anemia on the basis of their z-score. McNemar's test for the marginal differences in distributions of the two definitions was statistically significant (P value < 0.0001). However, the kappa coefficient measuring agreement between the two definitions was 0.767, with 0.81 to 1.00 indicating almost perfect agreement.
Table 5.
Comparison between alternate definitions of anemiaa
| Z-score Definition | KDOQI Definition
|
||
|---|---|---|---|
| ≥33% n (%b) | <33% n (%b) | Total n (%b) | |
| Above −2 SD | 1522 (54.8) | 32 (1.2) | 1554 (55.9) |
| Below −2 SD | 280 (10.1) | 945 (34.0) | 1225 (44.1) |
| Total | 1802 (64.8) | 977 (35.2) | 2779 (100.0) |
McNemar's test P < 0.0001, kappa coefficient 0.77.
Percent of total.
When the multivariate logistic regression analysis of factors associated with hospitalization was analyzed using standardized age- and gender-specific normal values for hematocrit (the anemia z-score), we obtained a similar increase in risk for hospitalization in anemic patients (OR 1.39, 95% CI 1.11 to 1.74) using the same covariates from the previous regression analysis.
The analysis of risk factors associated with anemia was also repeated with the z-score definition. Using the same statistical model, we identified the same risk factors for anemia, except cohort year lost significance; whereas gender, height z-score, and BMI z-score became significant.
Figure 3 shows the ROC curve for the alternate anemia definition based on z-score. After evaluating multiple cutpoints by z-score, less than −3.0 was determined to be the best for predicting hospitalization.
Discussion
Anemia is a well described complication of CKD in children, but there is little information about its prevalence or effect on morbidity in the pediatric predialysis population. We found that the prevalence of anemia increases with increasing CKD stage, approaching 71% of children with CKD stage V. In the multivariate analysis, pediatric CKD patients with anemia have a significant increase risk for hospitalization during the first year of follow-up when compared with nonanemic CKD patients after adjusting for other potentially confounding factors. Furthermore, hematocrit levels above either 36 or 39% were not associated with increased risk of hospitalization.
Although the prevalence of anemia in the adult CKD population has been reported to be as high as 47.7% (16), the prevalence of anemia in the predialysis pediatric CKD population has previously not been well characterized. A recent single-center study from Ontario reported that anemia (hematocrit < 33%) was present in 36.6% of the pediatric CKD patients, and the prevalence of anemia increased from 31% in stage I to 93.3% in patients who were in either stage IV or V (4). However, their cohort included dialysis and transplant patients and was limited to a single center's population, with only 15 children in CKD stages IV and V combined. Moreover, more than 50% of their patients had stage I CKD. Although the prevalence of anemia in our cohort was only slightly less (35% overall using the KDOQI definition and 44% using the z-score definition) than what was seen in the Ontario experience, comparisons are difficult because of significant differences in study design (definitions and patient populations).
The most recent KDOQI guidelines recommend a target hemoglobin of 11 to 12 g/dl, not to exceed 13 g/dl in children and adults (14). Studies evaluating the effect of normalization of hematocrit in the adult population have had mixed results, including two studies noting an increased morbidity associated with higher hemoglobin concentrations (8,9,17,18). Subsequent to the publication of these trials, the U.S. Food and Drug Administration issued a black box warning for all erythropoiesis-stimulating agents noting an increase risk of death or serious cardiovascular events when dosed to achieve a hemoglobin greater than 12 g/dl (19). We did not note any association between higher hematocrit (either >36% or >39%) and risk of hospitalization in the first year in this cohort of patients. However, the correct target hemoglobin for children remains uncertain given the absence of data correlating hemoglobin to outcome in children, in addition to the significant variation in normal hemoglobin values at different ages.
Anemia is associated with increased morbidity and mortality in adults and children with CKD (20,21). In children with CKD, Gerson et al. have shown that anemia (hematocrit < 36%) is associated with a decreased quality of life in adolescents, whereas Warady and Ho found anemia (hematocrit < 33%) to be associated with an increased frequency of hospitalization and an increased mortality risk in children initiating dialysis (7,22). Additionally, when looking at hospitalization rates, peritoneal dialysis patients with anemia had a hospitalization probability rate of 17.2% compared with 12.3% for nonanemic patients (7). We found similar results pertaining to hospitalization risk in nondialysis CKD patients.
A study of adolescent patients receiving hemodialysis demonstrated that anemia (hemoglobin < 11 g/dl) was associated with a significant increase in mortality. Interestingly, there was no statistically significant association between anemia and risk of hospitalization (6). This difference from our results may be due to the smaller number of patients analyzed in the hemodialysis cohort (type II error), but could also be related to different effects of anemia in different patient populations. This emphasizes the importance of studying predialysis CKD patients separately from patients receiving dialysis.
ROC curves for accuracy of predicting hospitalization were developed for both hematocrit percent as well as hematocrit z-score. Reviewing the ROC curve data illustrates that the hematocrit percent definition appears to have a slight advantage as a predictor of hospitalization compared with the z-score definition of anemia; however, neither definition of anemia is particularly predictive of hospitalization risk.
The multivariate analysis did show an association between both iron supplementation and erythropoietin use and risk for hospitalization. Although this association seems counterintuitive, it may be explained by the close correlation between anemia and both iron supplementation and erythropoietin use. These two variables are likely acting as surrogate markers for anemia in the analysis.
Of the prevalent patients with CKD stage IV and V, only 5.7 and 2.0%, respectively, have a primary diagnosis of FSGS. Although this diagnosis may seem underrepresented in the higher CKD stages, it is likely due to the more rapid progression of disease in this subset of patients, leading to smaller numbers in the higher CKD stages, and likely higher numbers in the ESRD categories. This is supported by the high number of patients with FSGS that have been excluded from this analysis.
We looked at the question of the appropriate definition of anemia in the pediatric predialysis CKD population and found no substantial difference in the risk of hospitalization between the two definitions (OR 1.55 for hematocrit < 33% and OR 1.39 for z-score).
Although the two definitions did agree on the anemia status of 89% of the patients, there was a noteworthy difference in the prevalence of anemia between the alternate definitions, with the z-score definition classifying 44% of the cohort as anemic and the KDOQI definition classifying only 35% as anemic. Interestingly, the most recent KDOQI clinical practice recommendations for target hemoglobin do suggest that consideration should be given to the age specific normal values when targeting therapy (14).
There are limitations of our study. The NAPRTCS database, although the largest database of its kind, is a voluntary registry. Thus, there may be a significant number of pediatric CKD patients who are not enrolled. Additionally, there may be hospitalizations that were not entered into the database; indeed, there were many patients excluded from the analysis because of missing data and incomplete follow-up. Moreover, the available data are limited to information that is routinely collected in the database at 6-mo intervals. This has limited the analysis to that presented here, not allowing for a time-variant analysis, which may more accurately portray the association between hospitalization and anemia.
We acknowledge that were many patients excluded and that there were significant differences between the patients included in the study and those excluded. These differences are likely due to the inclusion criteria developed for the study. Children with more severe CKD and those with acquired forms of renal disease such as FSGS may be more likely to progress to ESRD before completing 1 yr of follow-up and thus be excluded from the study. Given the large number of excluded patients, it must be acknowledged that this population represents a specific cohort of patients with slower progression of renal disease (because they remained in the CRI database longer).
It is important to emphasize that this study demonstrated an association between anemia and risk of hospitalization and no evidence of causality can be established in this population. Although we controlled for many potentially confounding variables, it is possible that anemia is acting as a surrogate marker for other conditions that are causally linked to increased hospitalization risk, such as chronic inflammation or more significant underlying disease. Indeed, inflammation has been shown to be associated with anemia and morbidity in CKD patients (23). Unfortunately, we were not able to evaluate markers of inflammations, such as C-reactive protein, because of limitations of the database. However, this association is currently being investigated by the National Institute of Health funded, multicenter, Chronic Kidney Disease in Children study.
Conclusions
We observed an increasing prevalence of anemia associated with increasing severity of CKD. Use of erythropoietin was more common in higher CKD stages, but many anemic patients were not treated with this therapy. Anemia was associated with an increased risk of hospitalization in nondialysis dependent children with CKD. Complete correction of anemia was not associated with any increased risk of hospitalization as has been seen in the adult population. Future studies should address the effect that correction of anemia has on hospitalization rates, as well as quality of life, learning capacity, and other important outcomes in children with CKD.
Disclosures
None.
Acknowledgments
This study was presented in part at the American Society of Nephrology Annual meeting in Philadelphia, Pennsylvania in November 2005. A substantial portion of this work was performed while the first author, Amy Staples, was receiving postdoctoral research support at the University of Washington from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Disease Pediatric Nephrology Research Training Program Grant (# T32 DK007662).
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Chandra M, Clemons GK, McVicar MI: Relation of serum erythropoietin levels to renal excretory function: Evidence for lowered set point for erythropoietin production in chronic renal failure. J Pediatr 113: 1015–1021, 1988 [DOI] [PubMed] [Google Scholar]
- 2.Burke JR: Low-dose subcutaneous recombinant erythropoietin in children with chronic renal failure. Australian and New Zealand Paediatric Nephrology Association. Pediatr Nephrol 9: 558–561, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Montini G, Zacchello G, Baraldi E, Zanconato S, Suppiej A, Fabris F, Andreetta B, Pavanello L, Zacchello F: Benefits and risks of anemia correction with recombinant human erythropoietin in children maintained by hemodialysis. J Pediatr 117: 556–560, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Wong H, Mylrea K, Feber J, Drukker A, Filler G: Prevalence of complications in children with chronic kidney disease according to KDOQI. Kidney Int 70: 585–590, 2006 [DOI] [PubMed] [Google Scholar]
- 5.McGonigle RJ, Boineau FG, Beckman B, Ohene-Frempong K, Lewy JE, Shadduck RK, Fisher JW: Erythropoietin and inhibitors of in vitro erythropoiesis in the development of anemia in children with renal disease. J Lab Clin Med 105: 449–458, 1985 [PubMed] [Google Scholar]
- 6.Amaral S, Hwang W, Fivush B, Neu A, Frankenfield D, Furth S: Association of mortality and hospitalization with achievement of adult hemoglobin targets in adolescents maintained on hemodialysis. J Am Soc Nephrol 17: 2878–2885, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Warady BA, Ho M: Morbidity and mortality in children with anemia at initiation of dialysis. Pediatr Nephrol 18: 1055–1062, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A: Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D: Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol 16: 2180–2189, 2005 [DOI] [PubMed] [Google Scholar]
- 11.K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 12.Schwartz GJ, Haycock GB, Edelmann CM Jr., Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 13.K/DOQI clinical practice guidelines for bone metabolism and disease in children with chronic kidney disease. Am J Kidney Dis 46: S1–S121, 2005 [PubMed] [Google Scholar]
- 14.KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis 50: 471–530, 2007 [DOI] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services. Third National Health and Nutrition Examination Survey, 1988–94, NHANES III. Hyattsville, MD, National Center for Health Statistics.
- 16.McClellan W, Aronoff SL, Bolton WK, Hood S, Lorber DL, Tang KL, Tse TF, Wasserman B, Leiserowitz M: The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin 20: 1501–1510, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Ma JZ, Ebben J, Xia H, Collins AJ: Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 10: 610–619, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Paoletti E, Cannella G: Update on erythropoietin treatment: Should hemoglobin be normalized in patients with chronic kidney disease? J Am Soc Nephrol 17: S74–S77, 2006 [DOI] [PubMed] [Google Scholar]
- 19.FDA Alert: Erythropoiesis Stimulating Agents (ESA). Rockville, MD, U.S. Food and Drug Administration, 2007.
- 20.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: The impact of anemia on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Am J Kidney Dis 28: 53–61, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Robinson BM, Joffe MM, Berns JS, Pisoni RL, Port FK, Feldman HI: Anemia and mortality in hemodialysis patients: Accounting for morbidity and treatment variables updated over time. Kidney Int 68: 2323–2330, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Gerson A, Hwang W, Fiorenza J, Barth K, Kaskel F, Weiss L, Zelikovsky N, Fivush B, Furth S: Anemia and health-related quality of life in adolescents with chronic kidney disease. Am J Kidney Dis 44: 1017–1023, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD: Malnutrition-inflammation complex syndrome in dialysis patients: Causes and consequences. Am J Kidney Dis 42: 864–881, 2003 [DOI] [PubMed] [Google Scholar]