Abstract
Background and objectives: Measurement of GFR is important for the management of chronic kidney disease (CKD). Although bolus administration of radiocontrast agents is commonly used to measure GFR, the optimal duration of sampling to assess their plasma clearance is unknown. The purpose of this study was to evaluate whether the duration of plasma sampling influences precision and estimation of GFR.
Design, setting, participants, & measurements: GFR was measured by sampling plasma 12 times over 5 h in 56 patients with CKD (mean age 64 yr, 98% men, 79% Caucasian, 34% diabetics, estimated GFR 31.8 ± 14.2 ml/min/1.73 m2). In a subset of 12 patients we measured GFR by sampling plasma 17 times over 10 h.
Results: Short sampling intervals considerably overestimated GFR measured using total plasma iothalamate clearance, especially in larger patients. In the higher estimated GFR group (>30 ml/min/1.73m2), the 5-h GFR was 17% higher and 2-h GFR 54% higher compared with the 10-h GFR, which averaged 40.3 ml/min/1.73 m2. In the lower estimated GFR group (<30 ml/min/1.73m2), the 5-h GFR was 36% higher and 2-h GFR 126% higher compared with the 10-h GFR, which averaged 22.2 ml/min/1.73 m2. Short sampling duration also reduced the precision of the estimated GFR from 1.67% for 10-h GFR, to 3.48% for 5-h GFR, and to 7.07% for 2-h GFR.
Conclusions: GFR measured over a longer duration with multiple plasma samples spanning the distribution and elimination phases may improve precision and provide a better measure of renal function.
The clinical manifestations of chronic kidney disease (CKD) are heterogenous, but it is generally accepted that the staging of CKD rests upon an accurate knowledge of GFR (1). Although urinary clearance of radioactive iothalamate has been used as the reference method to measure GFR (2), many clinical and research laboratories now use plasma clearance of nonradioactive radiocontrast dyes instead (3–7). Plasma clearance of iothalamate can be measured either after continuous infusion of iothalamate to achieve steady state and measuring plasma iothalamate (4,5,7), or after an intravenous bolus (3,6,8). The latter technique involves administering a bolus dose of iothalamate or another radiocontrast dye and sampling blood at timed intervals to study its pharmacokinetics. We and others have reported that plasma iothalamate clearance provides improved precision over urinary clearances (8,9). Because of improved precision, the plasma iothalamate clearance technique appears attractive for longitudinal studies in which sample size can be reduced to detect a given change in GFR (9).
The optimal duration of plasma sampling to best ascertain GFR remains undefined—no minimum duration of sampling is recommended. Accordingly, uncertainty exists when planning the optimal duration of GFR studies for the long-term follow-up renal function. Review of published work reveals that the duration of plasma iothalamate clearances measurement has varied anywhere between 2 to 10 h (8,10–12). In a study that measured plasma iothalamate concentration time profile over 10 h (12), plasma clearance was noted to be log-linear in all instances after 120 min, whereas another study reported that a 2-h time frame was perfectly adequate (10). A more recent multicenter study in children in the United States suggested 5 h as an adequate time frame for sampling (6).
We sought to evaluate the optimal duration of measurement of plasma iothalamate clearance in a cohort of patients with CKD. We hypothesized that short studies would overestimate GFR and that longer studies would reduce this error. We reasoned that short studies in patients with lower GFR would be associated with greater discrepancy compared with studies with longer sampling duration and tested the hypothesis that shorter duration studies would sacrifice precision compared with longer studies.
Materials and Methods
Between March 14, 2008 and July 16, 2008 we enrolled 60 veterans attending the Renal Clinic at the Veterans Administration hospital, Indianapolis, Indiana. Our recruitment goal was to enroll 30 patients each in stage 3 and stage 4 CKD. However, we made 3 exceptions to the protocol. To better estimate the GFR, we enrolled one patient with nephrotic proteinuria due to membranous GN on cyclosporine and metformin with an estimated GFR (eGFR) of 68 ml/min/1.73 m2. Two other patients with eGFR of 8 and 11 ml/min/1.73 m2 were completely free of uremic symptoms and were recruited at the request of their treating physicians. Those with allergy to radiocontrast or iodine or those with organ transplants were excluded from the study.
The study protocol was approved by the institutional review boards and the Veterans Administration Research and Development Committee and all patients provided written informed consent.
GFR Measurement Protocol
Patients generally reported to the clinical research laboratory in the morning. The patients voided on arrival to the laboratory. This sample was used to estimate the spot urine protein/creatinine ratio. Anthropometric measurements were made and blood pressure was obtained in the seated position in triplicate in each arm after a 5-min rest.
After placing an intravenous catheter in the arm, blood was drawn for analysis of routine chemistries, including serum creatinine using an isotope dilution mass spectroscopy traceable method that utilizes a modified Jaffe method (Roche Diagnostics, Indianapolis, Indiana). A 5-ml syringe was weighted on an analytic balance with an accuracy of 1 mg. Using this syringe, 5 ml (3000 mg) of iothalamate meglumine (Conray 60, Malinckrodt, St Louis, Missouri) was drawn and reweighed. Iothalamate was injected over 15 s and the catheter flushed with 10 to 15 ml of saline. The iothalamate syringe was weighted again to calculate the difference between the full and empty syringe. The exact volume of iothalamate injected was then calculated from the density of iothalamate for GFR calculations.
The subjects were asked to drink 750 ml of water over 10 to 15 min and blood was obtained 12 times using a stop watch at 5, 10, 20, 30, 45, 60, 90, 120, 150, 180, 240, and 300 min after the end of injection. In a subset of 12 patients we carried out plasma sampling at hourly intervals beyond 300 min until 10 h. All samples were stored on ice and plasma separated in a refrigerated centrifuge. Plasma was aliquoted and stored at −86°C until analyzed using a previously published HPLC method (13). Plasma from each patient obtained before iothalamate injection was analyzed for a detectable iothalamate peak. Despite the intake of many medications in these patients with CKD, in no instance did we observe interfering peaks. A total of 934 plasma iothalamate HPLC analyses were performed.
Modeling Methods
The plasma iothalamate concentration versus time curve was modeled using a two-compartment model. Specifically, the plasma concentration versus time data after bolus injection were analyzed using the equation Ct = Ae−αt + Be−βt, where Ct is the iothalamate concentration at time t, A and B are y-intercepts, and α and β are disposition rate constants. The initial rapid disappearance, measured by α, reflects the distribution of iothalamate in the extravascular compartment followed by a slow disappearance β that represents the renal elimination. The α-phase half-life was measured by ln(2)/α and β-phase half-life by ln(2)/β. The area under the plasma concentration time curve (AUC0to∞) was calculated as A/α + B/β, in which A/α represents the area under the “fast curve” and B/β the area under the “slow curve.” The plasma clearance of iothalamate was calculated as Dose/AUC0to∞. A higher order three-compartment model that has been described in normal, healthy volunteers could not be fitted to the data (14).
An iterative least-square model minimized the square of the residual to fit the best curve using the WinNonLin computer program version 2.0 (PharSight Corporation, Palo Alto, California). We first fitted a model that included all 12 samples over 300 min. We then dropped the last sample and refitted the model to obtain plasma iothalamate clearance over 240 min. We repeated the model fitting until we reached eight samples spanning 120 min. Thus each patient had a 5-, 4-, 3-, 2.5-, and 2-h GFR estimates. In the subset of 12 patients who had measurements over 10 h, we performed a similar analysis such that we had 10-, 9-, 8-, 7-, and 6-h plasma iothalamate clearances in addition to the above GFR estimates.
Statistical Methods
Plasma clearances of iothalamate obtained using the various time intervals were compared using a nominal mixed model (15). On visual examination of the plasma iothalamate clearances, the magnitude of overestimation was noted to diminish exponentially with increasing sampling duration. Thus, clearances were log-transformed before model fitting. The repeated measurement on each subject is accounted for by this statistical model. A second model was created in which we tested the effect of Modification of Diet in Renal Disease study-eGFR on the magnitude of overestimation of iothalamate plasma clearances. For this model we created a nominal variable with eGFR < 30 or 30 ml/min/1.73 m2 or more and interacted this variable with the five durations of plasma iothalamate measurements. Because this model was nested within the first, we tested the significance of the two model fits with a likelihood ratio test.
A linear mixed model was then created using random slopes for each subject. In this model each subject had a random intercept and random slope. We also tested the time-dependency of the overestimation in clearances by introducing a quadratic term for time.
To test the hypothesis that shorter studies can reduce the precision of the measurement, we used a two-stage approach. We first calculated the coefficient of variation as the ratio of standard error of the estimate and plasma iothalamate clearance for each individual expressed as a percent. A nominal mixed model was then used to test the significance of differences between various durations of sampling on the precision of the results after log transforming the coefficient of variation. All statistical analyses were performed with Stata 10.0 (Stata Corp, College Station, Texas).
Results
Of the 60 patients who participated, all but one had adequate iothalamate clearances measured. The latter patient had leakage of iothalamate due to dislodged intravenous catheter. In the first 9 participants, we measured only 2 h plasma clearances. However, when we discovered a large discrepancy between estimated and measured iothalamate clearances, we modified the protocol to measure iothalamate clearances over 5 h in the subsequent participants. We were able to repeat the plasma iothalamate clearances in all but 3 of the initial 9 participants.
Demographic and clinical characteristics of the 56 participants who had at least 5 h iothalamate clearances are shown in Table 1. Participants were typically older men as would be expected of the veteran population. The racial mix was typical of the CKD population and the median eGFR was 31.8 ml/min/1.73m2. In addition to the three protocol exceptions, there were four patients outside the range of our CKD stage because of variation in serum creatinine from screening visit to the measurement visit. Slightly fewer participants (48%) had earlier stages of CKD (stage 2 and 3) than later stages of CKD (stage 4 and 5). Two patients complained of slight burning at the injection site and two vomited within 2 min of the injection. No other adverse events due to iothalamate were noted.
Table 1.
Baseline characteristics of the study populationa
| Characteristic | Result |
|---|---|
| Number of subjects | 56 |
| Age (yr) | 64.3 ± 10.7 |
| Men | 55 (98%) |
| Height (in) | 68.3 ± 3.5 |
| Weight (kg) | 99.3 ± 24.2 |
| Hip/waist ratio | 1.01 ± 0.08 |
| Body mass index (kg/m2) | 32.8 ± 6.9 |
| Body surface area (m2) | 2.12 ± 0.27 |
| Race | |
| White | 44 (79%) |
| Black | 10 (18%) |
| American Indian | 2 (4%) |
| Etiology of CKDb | |
| diabetes | 19 (34%) |
| hypertension | 20 (36%) |
| GN | 9 (16%) |
| other | 8 (14%) |
| Hemoglobin (g/dl) | 12.3 ± 1.6 |
| Albumin (g/dl) | 4.1 ± 0.3 |
| Plasma creatinine (mg/dl) | 2.6 ± 1.2 |
| Blood urea nitrogen (mg/dl) | 44.7 ± 25.5 |
| Estimated GFR (ml/min/1.73 m2) | 31.8 ± 14.2 |
| stage 2 | 3 (5%) |
| stage 3 | 24 (43%) |
| stage 4 | 25 (45%) |
| stage 5 | 4 (7%) |
| Median urine protein/creatinine (g/g) (interquartile range) | 0.40 (0.11–1.36) |
± indicates SD, parentheses indicate percent of patients.
Chronic kidney disease (CKD) stage is based on estimated GFR by Modification of Diet in Renal Disease formula.
Results of 5-h Studies
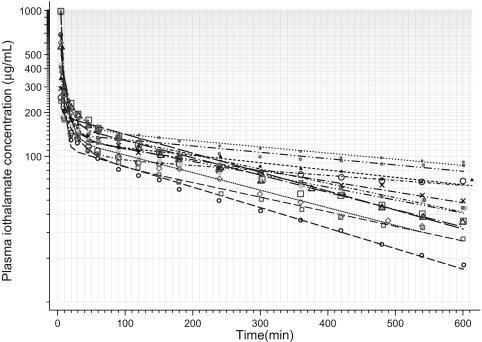
The pharmacokinetics of iothalamate in blood is best described by a double exponential curve (Figure 1). Nonlinear modeling of these plasma concentration time profiles in 56 patients yielded pharmacokinetic parameters that are shown in Table 2. The initial disappearance of iothalamate from plasma was rapid (<5 min median half-life) compared with the slower later removal. The elimination half-life from the central compartment was typically 66 min.
Figure 1.
Pharmacokinetic profile of plasma iothalamate concentration versus time curves in 12 patients with chronic kidney disease (CKD) who had measurements over 10 h. An exponential decline is visible although the data are plotted on a logarithmic ordinate. Thus, a two-compartment pharmacokinetic model was fitted. Symbols are the actual plasma iothalamate concentration in each patient, whereas the lines are the model fitted curves.
Table 2.
Pharmacokinetic characteristics of iothalamatea
| Pharmacokinetic Parameter | Median | Q1 | Q3 |
|---|---|---|---|
| Plasma iothalamate clearance (ml/min) | 47.8 | 33.9 | 65.6 |
| Plasma iothalamate clearance (ml/min/1.73m2) | 37.7 | 30.1 | 50.6 |
| Volume of distribution central (ml) | 4797 | 2275 | 6266 |
| Volume of distribution (ml/kg) | 50.1 | 20.0 | 67.4 |
| Volume of distribution, steady state (ml) | 15908 | 13822 | 18827 |
| Volume of distribution, steady state (ml/kg) | 172.4 | 147.1 | 190.6 |
| Elimination half-life (min) | 66 | 24 | 115 |
| Area under curve (μg × min/ml) | 62856 | 45847 | 89286 |
| Fast component | |||
| intercept A (μg/ml) | 452 | 320 | 1207 |
| half-life ln(2)/α (min) | 4.83 | 2.39 | 7.18 |
| area A/α (μg·min/ml) | 5335 | 2843 | 3624 |
| Slow component | |||
| intercept B (μg/ml) | 161 | 136 | 183 |
| half-life ln(2)/β (min) | 271 | 199 | 336 |
| area B/β (μg·min/ml) | 57615 | 40924 | 81973 |
Q1, first quartile; Q3, third quartile.
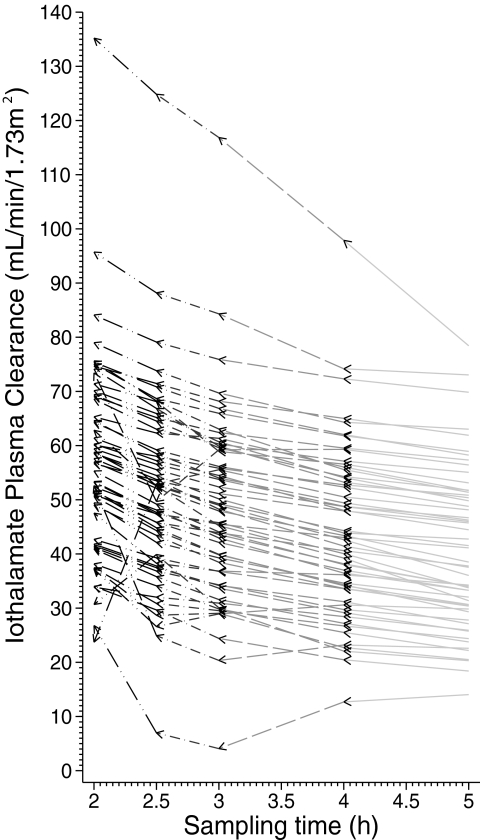
We next calculated the GFR from plasma iothalamate clearances using the two-compartment model. First, all of the 12 plasma iothalamate concentrations collected over 300 min were used for curve fitting and plasma clearance was calculated. Next, the last point was dropped and the curve was refitted to obtain another GFR. This yielded the GFR as if it was measured over 240 min. The process was repeated until we reached 2-h GFR. Table 3 shows the results of the GFR thus calculated and the magnitude of overestimation with shortened studies. For the higher GFR category, (eGFR > 30 ml/min/1.73m2) the magnitude of overestimation increased from 6% for 4-h study to 34% for 2-h study when each was compared with 5-h study as the reference category. For the lower GFR category, this overestimation was even more and increased from 10% for the 4-h study to 53% for the 2-h study. Figure 2 shows the results for each individual patient.
Table 3.
Sampling duration and GFR estimates stratified by estimated GFR (eGFR)a
| Sampling Duration | Mean GFR (ml/min/1.73 m2) | 95% CIb | Ratio Compared to 5-h GFR | 95% CI of Ratio | P |
|---|---|---|---|---|---|
| eGFR stratum >30 ml/min/1.73m2 | |||||
| 5 h | 49.3 | 44.4 to 54.8 | |||
| 4 h | 52.5 | 47.2 to 58.3 | 1.06 | 0.99 to 1.14 | 0.084 |
| 3 h | 57.2 | 51.5 to 63.5 | 1.16 | 1.08 to 1.25 | <0.001 |
| 2.5 h | 60.4 | 54.4 to 67.2 | 1.23 | 1.14 to 1.32 | <0.001 |
| 2 h | 66.1 | 59.5 to 73.4 | 1.34 | 1.25 to 1.44 | <0.001 |
| eGFR stratum <30 ml/min/1.73m2 | |||||
| 5 h | 29.1 | 26.3 to 32.2 | |||
| 4 h | 32.0 | 28.9 to 35.4 | 1.10 | 1.03 to 1.18 | 0.007 |
| 3 h | 34.5 | 31.2 to 38.2 | 1.19 | 1.11 to 1.27 | <0.001 |
| 2.5 h | 38.2 | 34.6 to 42.3 | 1.31 | 1.23 to 1.41 | <0.001 |
| 2 h | 44.4 | 40.1 to 49.1 | 1.53 | 1.42 to 1.63 | <0.001 |
Mean GFR calculated using a nominal mixed model with maximal likelihood estimates.
CI, confidence interval.
Figure 2.
Body surface area-corrected GFR as measured by plasma iothalamate clearance are overestimated if shorter sampling times are used. The individual changes in plasma iothalamate clearance are denoted by arrows. As the sampling time shortens, the plasma iothalamate clearance is overestimated. Some individuals have large changes in measured GFR.
Results of 10-h Studies
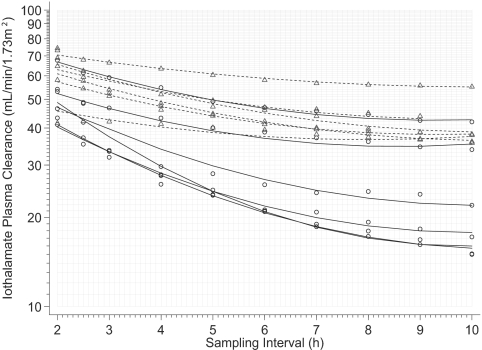
From the above study, it was not clear if 5 h was sufficient to accurately measure GFR. To further characterize the nature of this overestimation, we studied iothalamate pharmacokinetics in a subset of 12 patients over 10 h. Thus, each patient now had 17 plasma samples for curve fitting and the effect of longer duration sampling on GFR estimation could be evaluated. Table 4 shows the magnitude of overestimation with shorter studies. The modeled relationship of GFR thus obtained plotted against the sampling interval demonstrates that plasma iothalamate clearances are dependent on the duration of the study (Figure 3). The regression model that describes the relationship of GFR with sampling interval and the baseline level of GFR is shown in Table 5. The quadratic term has a positive coefficient reflecting the time-dependent reduction in overestimation of GFR.
Table 4.
Sampling duration and GFR estimates stratified by eGFR for 10-h GFRa
| Sampling Duration | Mean GFR (ml/min/1.73 m2) | 95% CI | Ratio Compared to 10-h GFR | 95% CI of Ratio | P |
|---|---|---|---|---|---|
| eGFR stratum >30 ml/min/1.73m2 | |||||
| 10 h | 40.3 | 33.2 to 48.9 | |||
| 9 h | 41.2 | 34.1 to 49.8 | 1.02 | 0.92 to 1.14 | >0.2 |
| 8 h | 42.1 | 34.8 to 50.9 | 1.04 | 0.94 to 1.16 | >0.2 |
| 7 h | 43.7 | 36.1 to 52.8 | 1.08 | 0.97 to 1.21 | 0.15 |
| 6 h | 45.2 | 37.4 to 54.7 | 1.12 | 1.01 to 1.25 | 0.037 |
| 5 h | 47.2 | 39.0 to 57.1 | 1.17 | 1.05 to 1.31 | 0.004 |
| 4 h | 49.3 | 40.7 to 59.6 | 1.22 | 1.10 to 1.36 | <0.001 |
| 3 h | 53.1 | 43.8 to 64.4 | 1.32 | 1.18 to 1.48 | <0.001 |
| 2.5 h | 56.7 | 46.9 to 68.6 | 1.41 | 1.26 to 1.57 | <0.001 |
| 2 h | 62.3 | 51.5 to 75.3 | 1.54 | 1.39 to 1.72 | <0.001 |
| eGFR stratum <30 ml/min/1.73m2 | |||||
| 10 h | 22.2 | 18.4 to 26.9 | |||
| 9 h | 23.6 | 19.5 to 28.6 | 1.06 | 0.96 to 1.18 | >0.2 |
| 8 h | 24.8 | 20.5 to 30.0 | 1.12 | 1.01 to 1.24 | 0.037 |
| 7 h | 25.9 | 21.4 to 31.3 | 1.16 | 1.05 to 1.29 | 0.004 |
| 6 h | 27.5 | 22.7 to 33.3 | 1.24 | 1.12 to 1.37 | <0.001 |
| 5 h | 30.3 | 25.0 to 36.6 | 1.36 | 1.23 to 1.51 | <0.001 |
| 4 h | 33.4 | 27.6 to 40.4 | 1.50 | 1.36 to 1.67 | <0.001 |
| 3 h | 39.4 | 32.5 to 47.8 | 1.77 | 1.59 to 1.98 | <0.001 |
| 2.5 h | 44.6 | 36.9 to 54.0 | 2.01 | 1.81 to 2.23 | <0.001 |
| 2 h | 50.2 | 41.5 to 60.7 | 2.26 | 2.04 to 2.51 | <0.001 |
Mean GFR calculated using a nominal mixed model with maximal likelihood estimates.
Figure 3.
The overestimation in GFR plateaus at about 6 to 7 h in people with estimated GFR (eGFR) of 30 ml/min/1.73 m2. However, this plateau may not occur until a later time point in those with lower kidney function. The solid lines are modeled relationships between iothalamate clearances and sampling times in those with lower eGFR (<30 ml/min/1.73 m2) whereas the dashed lines are relationships in those with higher eGFR (>30 ml/min/1.73 m2). Open triangles are measured plasma iothalamate clearances in those with higher eGFR and circles in those with lower eGFR.
Table 5.
Regression model for GFR estimation: 10-h studiesa
| Parameter | Coefficient | 95% CI | P |
|---|---|---|---|
| Fixed effects | |||
| constant (ml/min/1.73 m2) | 58.6 | 46.4 to 70.8 | <0.001 |
| sampling time (h) | −11.7 | −14.2 to −9.2 | <0.001 |
| sampling time2 (h2) | 0.676 | 0.48 to 0.87 | <0.001 |
| eGFR (ml/min/1.73 m2) | 0.369 | −0.01 to 0.75 | 0.057 |
| eGFR × sampling time | 0.119 | 0.04 to 0.20 | 0.003 |
| eGFR × sampling time2 | −0.007 | −0.013 to −0.001 | 0.021 |
| Random effects | |||
| intercept SD | 7.66 | 5.01 to 11.72 | |
| sampling time SD | 0.624 | 0.380 to 1.027 | |
| correlation (time, intercept) | −0.85 | −0.96 to −0.53 | |
| residual SD | 2.56 | 2.22 to 2.96 |
Random coefficient model with maximal likelihood estimates.
We fitted another model to describe the relationship of body surface area with overestimation of iothalamate clearance with short sampling intervals and found that the overestimation was greater in those with greater body surface area and the quadratic component was also more positive (data not shown).
Mechanism of Underestimation
The provenance of overestimation of GFR with shorter duration studies was analyzed by calculating the AUC estimates with shorter duration studies. Given that plasma iothalamate clearance is calculated by dividing the dose administered by the AUC, it follows that AUC may be underestimated. If so, the resulting plasma clearance will be overestimated. Shorter duration GFR indeed underestimate the AUC and this underestimation relates to the slow phase of the curve (Table 6). For those with higher GFR, the 2-h clearance underestimated AUC by 45% compared with the 10-h clearance study. For those with lower GFR, the 2-h clearance underestimated the AUC by 56% compared with the full study.
Table 6.
Sampling duration and area under the curve (AUC) estimates stratified by eGFRa
| Sampling Duration | Mean AUC (μg · min/ml) | 95% CI | Ratio compared to 10-h AUC | 95% CI of Ratio | P |
|---|---|---|---|---|---|
| eGFR stratum >30 ml/min/1.73m2 | |||||
| 10 h | 65,427 | 51,731 to 82,749 | |||
| 9 h | 63,968 | 50,707 to 80,697 | 0.98 | 0.88 to 1.09 | >0.2 |
| 8 h | 62,603 | 49,625 to 78,975 | 0.96 | 0.86 to 1.07 | >0.2 |
| 7 h | 60,365 | 47,851 to 76,151 | 0.92 | 0.83 to 1.03 | 0.15 |
| 6 h | 58,271 | 46,191 to 73,510 | 0.89 | 0.80 to 0.99 | 0.037 |
| 5 h | 55,796 | 44,229 to 70,388 | 0.85 | 0.76 to 0.95 | 0.004 |
| 4 h | 53,466 | 42,382 to 67,449 | 0.82 | 073 to 0.91 | <0.001 |
| 3 h | 49,628 | 39,240 to 62,768 | 0.76 | 0.68 to 0.85 | <0.001 |
| 2.5 h | 46,464 | 36,831 to 58,615 | 0.71 | 0.64 to 0.79 | <0.001 |
| 2 h | 42,330 | 33,555 to 53,400 | 0.65 | 0.58 to 0.72 | <0.001 |
| eGFR stratum <30 ml/min/1.73m2 | |||||
| 10 h | 105,432 | 83,576 to 133,005 | |||
| 9 h | 99,198 | 78,633 to 125,140 | 0.94 | 0.85 to 1.04 | >0.2 |
| 8 h | 94,490 | 74,901 to 119,201 | 0.90 | 0.81 to 0.99 | 0.037 |
| 7 h | 90,584 | 71,805 to 114,273 | 0.86 | 0.77 to 0.95 | 0.004 |
| 6 h | 85,141 | 67,491 to 107,407 | 0.81 | 0.73 to 0.90 | <0.001 |
| 5 h | 77,359 | 61,322 to 97,590 | 0.73 | 0.66 to 0.81 | <0.001 |
| 4 h | 70,104 | 55,571 to 88,438 | 0.66 | 0.60 to 0.74 | <0.001 |
| 3 h | 59,402 | 46,968 to 75,126 | 0.56 | 0.51 to 0.63 | <0.001 |
| 2.5 h | 52,466 | 41,589 to 66,187 | 0.50 | 0.45 to 0.55 | <0.001 |
| 2 h | 46,656 | 36,984 to 58,857 | 0.44 | 0.40 to 0.49 | <0.001 |
AUC calculated using a nominal mixed model with maximal likelihood estimates.
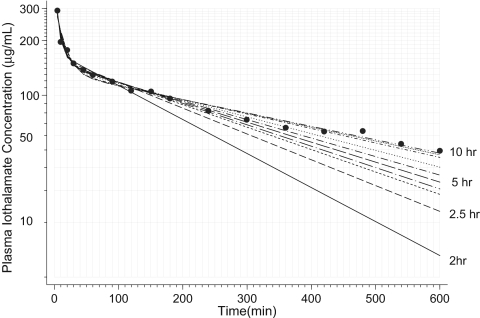
Figure 4 illustrates the kinetics of iothalamate in the plasma compartment and curve fits when GFR was analyzed over 10 h (full data) and by progressively fitting curves by censoring the last data point until only 2-h data remained in one representative patient. The slow-phase distribution constant (β) was overestimated (steeper slopes), with short studies resulting in lower AUC.
Figure 4.
Measured plasma iothalamate concentrations versus time are shown on a logarithmic ordinate in one patient. The lines represent modeled curves when various durations of sampling are considered. When GFR was analyzed over 10 h (full data) the topmost curve was obtained, which yielded a GFR of 37 ml/min. The bottom solid line shows a modeled curve when only 2 h of data were considered. GFR using the 2-h data were 72 ml/min. Overestimation of the terminal elimination constant by short studies is why GFR appear to be overestimated with short durations of measurement.
Effect of Short Studies on Precision of GFR Measurement
Each plasma iothalamate clearance is measured with some error because of technical issues or biologic variation. The standard error in measurement divided by the mean GFR when expressed as a percent yields the coefficient of variation. The mean coefficient of variation decreased with increasing sampling duration from 7.07% for 2-h studies to 1.67% for 10-h studies (Table 7). Thus, the precision increased more than fourfold by increasing the duration of the studies.
Table 7.
Precision of GFR as a function of sampling durationa
| Sampling Duration | Mean GFR CV (%) | 95% CI | Difference from 10-h GFR | 95% CI of Difference | P |
|---|---|---|---|---|---|
| 10 h | 1.67 | 1.38 to 2.01 | |||
| 9 h | 1.81 | 1.50 to 2.18 | 1.09 | 0.93 to 1.27 | >0.2 |
| 8 h | 2.06 | 1.71 to 2.48 | 1.24 | 1.06 to 1.44 | 0.007 |
| 7 h | 2.38 | 1.98 to 2.87 | 1.43 | 1.23 to 1.67 | <0.001 |
| 6 h | 2.85 | 2.37 to 3.43 | 1.71 | 1.47 to 1.99 | <0.001 |
| 5 h | 3.48 | 2.89 to 4.19 | 2.09 | 1.79 to 2.44 | <0.001 |
| 4 h | 4.31 | 3.58 to 5.19 | 2.59 | 2.22 to 3.02 | <0.001 |
| 3 h | 4.86 | 4.01 to 5.89 | 2.91 | 2.48 to 3.42 | <0.001 |
| 2.5 h | 6.11 | 5.08 to 7.36 | 3.67 | 3.15 to 4.28 | <0.001 |
| 2 h | 7.07 | 5.87 to 8.51 | 4.24 | 3.63 to 4.95 | <0.001 |
Mean GFR coefficient of variation (CV) calculated using a nominal mixed model with log-transformed CV using maximal likelihood estimates.
Discussion
The standard method to measure GFR as developed by Homer Smith involved maintenance of an intravenous infusion of inulin at a constant rate, the collection of frequent blood samples, and bladder catheterization to accurately measure urinary flow rate (16). Emphasizing the importance of this technique, Dr. Smith in a subsequent paper commented, “the use of constant, slow infusions gives reliable blood curves of inulin and phenol red, and catheterization of the bladder followed by a careful washing with saline eliminates what is perhaps the largest source of error. This last precaution is obviously necessary if single clearance determinations are to be given any physiologic significance.” (17) Recognizing this limitation of GFR measurement, many investigators, almost since Smith published his clearance technique, have sought to develop a measurement method that is devoid of urine collections (8,11,18–21). It is generally reported that compared with the standard clearance method, the bolus plasma clearance technique is more precise but slightly overestimates the GFR (8,11,21). However, the effect of duration of the study to measure the precision of plasma clearance or compare the GFR estimates resulting from different durations have not been rigorously analyzed although several investigators have pointed to the need for longer sampling intervals when measuring GFR (22–24). For example, Groth et al. suggested frequent and prolonged sampling for reliable estimates of GFR from curve-fitting techniques after a single intravenous bolus of radiolabeled iothalamate (24). Frennby et al. suggested a 24-h sample of iohexol in patients with low GFR (22). Sterner et al. recommended that the sampling duration should be inversely proportional to the GFR (23). In children, for GFR below 20 ml/min/1.73m2, GFR was overestimated by 50% from plasma iohexol sampled 3.5 h after injection when compared with plasma sampled at 24 h (25).
Our data demonstrate that short studies spanning just a few hours overestimate plasma iothalamate clearance by a clinically and statistically significant amount. This overestimation is greater when the GFR is more severely impaired and more likely to occur in patients with larger body surface areas. Not only does the short sampling duration overestimate GFR, it also reduces the precision of the estimate. Thus, GFR measured over a longer duration improves precision and provides a better measure of renal function.
The optimal timing to measure GFR using a two-component pharmacokinetic model requires knowledge of α and β distribution constants. An ideal study informed by sampling theory would be one in which measurements of plasma iothalamate would be made at times 1/α and 1/β (26). Because β is dependent on GFR, it follows that the duration of sampling should follow the degree of impairment of renal function—those with more impaired GFR would require longer sampling periods. On the basis of the β observed in our study, 5-h GFR would be adequate for those with eGFR of 50 ml/min/1.73 m2 or more. But those with eGFR of 10 ml/min/1.73 m2 would require 15-h sampling, for 20 ml/min/1.73 m2 12-h sampling, for 30 ml/min/1.73 m2 9-h sampling, and for 40 ml/min/1.73 m2 6-h sampling, a strategy similar to that suggested by Sterner et al. (23). However, if such a strategy is followed, in many patients with stage 4 CKD the sampling duration can extend well beyond 9 h, which would make the technique of plasma iothalamate clearance to measure GFR cumbersome. For such patients a continuous infusion technique may be useful but then this would require two visits to the hospital (7). Thus, frequent sampling over 5 to 7 h appears to be an adequate compromise between scientific theory and feasibility of long-term GFR measurements.
The mechanism of overestimation of GFR with short-term studies can be understood by analyzing Figure 4, which shows the curve fits when various sampling intervals are used to model the pharmacokinetic profile. In this patient, using the two-compartment model, the 2-h GFR was calculated as 72 ml/min, 2.5-h as 59 ml/min, 5-h as 49 ml/min, and 10-h as 37 ml/min, illustrating the progressive overestimation of GFR with shorter duration studies. It is clear that the AUC with 2-h study is less than that obtained with 10-h study. Because of the difficulty in describing the terminal kinetics with short studies, it is evident that it is the slow component of the AUC that is underestimated with shorter duration studies that leads to the overestimation of GFR.
Several studies have suggested limited plasma sampling after bolus clearance techniques to make the procedure less cumbersome (27–31). The scientific rationale offered by these studies is that limited sampling technique and full study are highly correlated, therefore limited sampling technique provides good information. Whereas the mean level of GFR may well be stable with limited sampling, our data suggest that shorter duration studies reduce precision of the measurements. Figure 2 shows that in some individuals large errors in GFR may occur with limited sampling. Thus, whenever possible, a longer duration and a richer sampling method for GFR measurement are recommended. We suggest an eight-sample technique to adequately capture the entire plasma pharmacokinetic profile. Sampling at 5, 15, 30, 45, 60, 120, 240, and 360 (or longer) min after bolus iothalamate should adequately capture the distribution and elimination phase of this drug. Others have suggested a similar approach (8,30).
A limitation of the study is that it included mostly older men. Whether the results would be applicable in women is a not clear although prior studies have not revealed gender difference in iothalamate pharmacokinetics (10).
Our data have clinical and research implications. Because the overestimation in GFR is greater in those with lower GFR, it follows that patients who progress to a lower GFR are more likely to be missed with shorter studies. Such patients would most benefit with longer studies. In clinical trials designed to assess progression of CKD, power estimates are dependent on the precision of the measurement. Short-duration studies reduce the precision of GFR measurements as much as fourfold. This may increase sample size requirements in contrast to more precise measurements with longer duration studies. Thus longer studies are likely to increase both precision and accuracy when measuring GFR via plasma iothalamate clearances.
Disclosures
None.
Acknowledgments
Supported by a grant from the National Institutes of Health 5R44DK071370-03
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G: National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Greene T, Schluchter MD, Cleary PA, Teschan PE, Lorenz RA, Molitch ME, Mitch WE, Siebert C, Hall PM: Glomerular filtration rate measurements in clinical trials. Modification of Diet in Renal Disease Study Group and the Diabetes Control and Complications Trial Research Group. J Am Soc Nephrol 4: 1159–1171, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erley CM, Bader BD, Berger ED, Vochazer A, Jorzik JJ, Dietz K, Risler T: Plasma clearance of iodine contrast media as a measure of glomerular filtration rate in critically ill patients. Crit Care Med 29: 1544–1550, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Holliday MA, Heilbron D, Al-Uzri A, Hidayat J, Uauy R, Conley S, Reisch J, Hogg RJ: Serial measurements of GFR in infants using the continuous iothalamate infusion technique. University of California San Francisco (UCSF) and Southwest Pediatric Nephrology Study Group (SPNSG). Kidney Int 43: 893–898, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Al-Uzri A, Holliday MA, Gambertoglio JG, Schambelan M, Kogan BA, Don BR: An accurate practical method for estimating GFR in clinical studies using a constant subcutaneous infusion. Kidney Int 41: 1701–1706, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GJ, Furth S, Cole SR, Warady B, Munoz A: Glomerular filtration rate via plasma iohexol disappearance: Pilot study for chronic kidney disease in children. Kidney Int 69: 2070–2077, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Agarwal R: Ambulatory GFR measurement with cold iothalamate in adults with chronic kidney disease. Am J Kidney Dis 41: 752–759, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Sambataro M, Thomaseth K, Pacini G, Robaudo C, Carraro A, Bruseghin M, Brocco E, Abaterusso C, DeFerrari G, Fioretto P, Maioli M, Tonolo GC, Crepaldi G, Nosadini R: Plasma clearance rate of 51Cr-EDTA provides a precise and convenient technique for measurement of glomerular filtration rate in diabetic humans. J Am Soc Nephrol 7: 118–127, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Agarwal R: Reproducibility of renal function measurements in adult men with diabetic nephropathy: Research and clinical implications. Am J Nephrol 27: 92–100, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Isaka Y, Fujiwara Y, Yamamoto S, Ochi S, Shin S, Inoue T, Tagawa K, Kamada T, Ueda N: Modified plasma clearance technique using nonradioactive iothalamate for measuring GFR. Kidney Int 42: 1006–1011, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Dowling TC, Frye RF, Fraley DS, Matzke GR: Comparison of iothalamate clearance methods for measuring GFR. Pharmacotherapy 19: 943–950, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Gaspari F, Mosconi L, Vigano G, Perico N, Torre L, Virotta G, Bertocchi C, Remuzzi G, Ruggenenti P: Measurement of GFR with a single intravenous injection of nonradioactive iothalamate. Kidney Int 41: 1081–1084, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Agarwal R, Vasavada N, Chase SD: Liquid chromatography for iothalamate in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci 785: 345–352, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Edelson J, Shaw D, Palace G: Pharmacokinetics of iohexol, a new nonionic radiocontrast agent, in humans. J Pharm Sci 73: 993–995, 1984 [DOI] [PubMed] [Google Scholar]
- 15.Holden JE, Kelley K, Agarwal R: Analyzing change: A primer on multilevel models with applications to nephrology. Am J Nephrol 28: 792–801, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shannon JA, Smith HW: The excretion of inulin, xylose and urea by normal and phlorizinized man. J Clin Invest 14: 393–401, 1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith HW, Goldring W, Chasis H: The measurement of the tubular excretory mass, effective blood flow and filtration rate in the normal human kidney. J Clin Invest 17: 263–278, 1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tacket HS, Houck CR: Measurement of renal hemodynamics in man by the “slope method” without urinalysis. Proc Soc Exp Biol Med 74: 317–321, 1950 [DOI] [PubMed] [Google Scholar]
- 19.Vogeli B, Riedwyl H, Donath A, Oetliker O: Comparison of glomerular filtration rate and effective renal plasma flow determinations obtained by a single injection technique and by means of a standard clearance technique in children. Acta Paediatr Scand 60: 528–532, 1971 [DOI] [PubMed] [Google Scholar]
- 20.Landowne M, Alving AS: A method of determining the specific renal functions of glomerular filtration, maximal tubular excretion (or reabsorption), and “effective blood flow” using a single injection of a single substance. J Lab Clin Med 32: 931–942, 1947 [PubMed] [Google Scholar]
- 21.Florijn KW, Barendregt JN, Lentjes EG, van Dam W, Prodjosudjadi W, van Saase JL, van Es LA, Chang PC: Glomerular filtration rate measurement by “single-shot” injection of inulin. Kidney Int 46: 252–259, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Frennby B, Sterner G, Almen T, Hagstam KE, Hultberg B, Jacobsson L: The use of iohexol clearance to determine GFR in patients with severe chronic renal failure—A comparison between different clearance techniques. Clin Nephrol 43: 35–46, 1995 [PubMed] [Google Scholar]
- 23.Sterner G, Frennby B, Hultberg B, Almen T: Iohexol clearance for GFR-determination in renal failure—single or multiple plasma sampling? Nephrol Dial Transplant 11: 521–525, 1996 [PubMed] [Google Scholar]
- 24.Groth T, Tengstrom B: The usefulness of 125I-sodium lothalamate as a GFR-indicator in single intravenous injection tests. Ups J Med Sci 83: 53–63, 1978 [DOI] [PubMed] [Google Scholar]
- 25.Stake G, Monn E, Rootwelt K, Monclair T: The clearance of iohexol as a measure of the glomerular filtration rate in children with chronic renal failure. Scand J Clin Lab Invest 51: 729–734, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Gabrielsson J, Weiner D: Pharmacokinetic and Pharmacodynamic Data Analysis: Concepts and Applications, 4th Ed., Stockholm, Sweden, Swedish Pharmaceutical Press, 2006
- 27.Fisher M, Veall N: Glomerular filtration rate estimation based on a single blood sample. Br Med J 2: 542, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan WD, Birks JL, Sivyer A, Ghose RR: An efficient technique for the simultaneous estimation of GFR and ERPF, involving a single injection and two blood samples. Int J Nucl Med Biol 4: 79–83, 1977 [DOI] [PubMed] [Google Scholar]
- 29.Smart R, Trew P, Burke J, Lyons N: Simplified estimation of glomerular filtration rate and effective renal plasma flow. Eur J Nucl Med 6: 249–253, 1981 [DOI] [PubMed] [Google Scholar]
- 30.Pucci L, Bandinelli S, Pilo M, Nannipieri M, Navalesi R, Penno G: Iohexol as a marker of glomerular filtration rate in patients with diabetes: Comparison of multiple and simplified sampling protocols. Diabet Med 18: 116–120, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Chowdhury TA, Dyer PH, Bartlett WA, Legge ES, Durbin SM, Barnett AH, Bain SC: Glomerular filtration rate determination in diabetic patients using iohexol clearance—Comparison of single and multiple plasma sampling methods. Clin Chim Acta 277: 153–158, 1998 [DOI] [PubMed] [Google Scholar]