Abstract
C4d deposition in peritubular capillaries is a specific marker for the presence of antidonor antibodies in renal transplant recipients and is usually associated with antibody-mediated rejection (AMR) in conventional allografts. In ABO-incompatible grafts, however, peritubular capillary C4d is often present on protocol biopsies lacking histologic features of AMR; the significance of C4d in this setting remains unclear. For addressing this, data from 33 patients who received ABO-incompatible renal allografts (after desensitization) were retrospectively reviewed. Protocol biopsies were performed at 1 and/or 3 and 6 mo after transplantation in each recipient and at 12 mo in 28 recipients. Twenty-one patients (group A) had strong, diffuse peritubular capillary C4d staining without histologic evidence of AMR or cellular rejection on their initial protocol biopsies. The remaining 12 patients (group B) had negative or weak, focal peritubular capillary C4d staining. Three grafts (two in group B) were lost but not as a result of AMR. Excluding these three patients, serum creatinine levels were similar in the two groups at 6 and 12 mo after transplantation and at last follow-up; however, recipients in group A developed significantly fewer overall chronic changes, as scored by the sum of Banff chronic indices, than group B during the first year after transplantation. These results suggest that diffuse peritubular capillary C4d deposition without rejection is associated with a lower risk for scarring in ABO-incompatible renal allografts; the generalizability of these results to conventional allografts remains unknown.
During the past 10 to 15 yr, an increasing number of transplant centers worldwide have successfully expanded the potential pool of living kidney donors by performing transplants of cross-match–positive or ABO-incompatible kidneys into recipients who are preconditioned to remove antibodies specific for donor HLA or blood group antigens.1–7 A potential risk of such procedures, however, is the continued presence or reappearance of such antibodies with resulting antibody-mediated rejection (AMR) of the graft. In conventional and HLA-incompatible renal allografts, the presence of peritubular capillary (PTC) C4d staining on an allograft biopsy, even a protocol biopsy of a stably functioning graft, is usually associated with histologic features of AMR, namely neutrophil and monocyte margination in PTC and/or thrombotic microangiopathy (TMA).8–12 Furthermore, PTC C4d deposition plus neutrophil margination and/or TMA on protocol biopsies of HLA-incompatible grafts was found to be associated with development of chronic changes, including chronic transplant glomerulopathy (TG), providing strong evidence that the former changes indeed represent subclinical AMR in these grafts.11
By contrast, we and others have noted that the majority of protocol biopsies of ABO-incompatible grafts show PTC C4d deposition that is often diffuse but only infrequently associated with histologic changes of AMR.12–14 The significance of C4d staining in the absence of these histologic changes remains unclear.15
In this study, we retrospectively examined renal allograft biopsies and clinical data from 33 patients who received an ABO-incompatible renal allograft after desensitization to remove blood group antibodies (BGA). Each patient had protocol biopsies 1 and/or 3 and 6 mo after transplantation, and most also had 12-mo protocol biopsies. The specific questions addressed in the study were as follows: (1) How often, if at all, do patients with early (1- and/or 3-mo) protocol biopsies showing diffuse, strong C4d staining without histologic changes of rejection subsequently develop AMR, and (2) how do graft function and graft scarring as assessed on 6- and 12-mo protocol biopsies compare in these patients versus those whose early protocol biopsies show absent or weak and focal C4d staining?
RESULTS
Of the 33 study patients, 21 had an initial protocol biopsy showing strong (>1+, 0 to 4+ scale), diffuse PTC C4d staining without histologic evidence of AMR or acute cellular rejection (ACR; Banff ’97 grade 1A or greater); these patients are classified as group A. Four of the 21 group A patients had borderline inflammation on their initial protocol biopsy, and six had mild (g1) glomerulitis. Of the remaining 12 patients, comprising group B, six had negative PTC C4d staining on their initial protocol biopsy, and six had weak (≤1+) C4d staining involving an estimated 10 to 25% of PTC present on their initial protocol biopsy. As with the group A patients, none of the 12 group B patients showed significant (PTC score ≥1) margination of neutrophils or mononuclear leukocytes in cortical PTC, more than mild glomerulitis (all had g0), or other histologic features of AMR on their initial protocol biopsy. In addition, none of these 12 biopsies showed ACR or even borderline inflammation. Four additional recipients of ABO-incompatible renal allografts during the study period had ≥1+, diffuse PTC C4d staining on their initial protocol biopsy, but with margination of leukocytes (mononuclear cells and neutrophils) in PTC, consistent with subclinical AMR.11 These four patients were not included in this study. Notably, none of the 12 patients (group B) whose initial protocol biopsy showed absent or weak and focal PTC C4d had a subsequent protocol biopsy that demonstrated strong, diffuse C4d without histologic evidence of AMR. By contrast, 14 of the 21 group A patients whose initial protocol biopsy showed strong, diffuse C4d without histologic evidence of AMR or ACR continued to show diffuse C4d staining (>1+ in all but two) without margination of neutrophils or mononuclear leukocytes in PTC on each subsequent protocol biopsy during the first year after transplantation.
Staining for C3d was done on the initial protocol biopsies from 16 of the group A patients and all 12 group B patients. Only two biopsies showed PTC C3d, both from group A patients. In one biopsy, the C3d staining was diffuse, and in the other it was focal. Interestingly, the one group A patient whose initial protocol biopsy showed diffuse C3d later developed AMR. Our previous results12 suggested that PTC C3d in addition to C4d may be better correlated with AMR than C4d alone in ABO-incompatible renal allografts; however, the number of patients with C3d-positive biopsies in this study is far too few to draw any clear conclusions.
Table 1 compares demographic and pretransplantation serologic findings in group A and B patients. There were no significant differences between the groups of patients with respect to age, gender, racial composition, mean and median maximum BGA titer before desensitization, or numbers of pre- or postoperative plasmapheresis (PP)/intravenous Ig treatments. Group A had a higher fraction of allografts from donors of blood group A1, and group B had a higher fraction of patients receiving kidneys from blood type B donors, although variations in the distribution of donor blood types among the two groups of recipients did not reach statistical significance (Table 1). In addition, similar fractions of patients in each group underwent splenectomy or received anti-CD20 antibody as part of their desensitization protocol.
Table 1.
Demographic and serologic data for patient cohortsa
| Parameter | Group A | Group B | P |
|---|---|---|---|
| No. of patients | 21 | 12 | |
| Age (yr)b | 0.25d | ||
| mean ± SD | 49 ± 12 | 54 ± 13 | |
| rangeb | 21 to 64 | 33 to 73 | |
| Men/women | 12/9 | 8/4 | 0.72e |
| Race (white/black/Hispanic) | 18/3/0 | 9/2/1 | 0.58e |
| Donor blood group type | 0.09e | ||
| A1 | 14 | 4 | |
| A2 | 2 | 1 | |
| A1B | 1 | 1 | |
| B | 3 | 6 | |
| Initial BGA titerc | |||
| mean ± SD | 182 ± 217 | 133 ± 134 | 0.51c |
| range | 16 to 1024 | 32 to 512 | |
| median | 128 | 96 | 0.44f |
| IQR | 64 to 256 | 64 to 128 | |
| No. of PP/IVIG treatments | |||
| pretransplantation | |||
| mean ± SD | 6.1 ± 2.3 | 5.8 ± 2.4 | 0.48f |
| range | 3 to 12 | 3 to 12 | |
| posttransplantation | |||
| mean ± SD | 4.7 ± 1.5 | 4.7 ± 2.1 | 0.74f |
| range | 2 to 8 | 2 to 10 | |
| Other pretransplantation treatments | 0.99e | ||
| splenectomy | 4 | 3 | |
| anti-CD20 | 4 | 2 | |
| splenectomy and anti-CD20 | 1 | 0 | |
| none | 12 | 7 |
IQR, interquartile range; IVIG, intravenous Ig.
At the time of transplantation.
Pretransplantation titers of anti-A1, anti-A2, or anti-B before initiation of PP/IVIG treatments.
By t test.
By Fisher exact test.
By Wilcoxon rank sum test.
As shown in Table 2, patients in groups A and B had similar mean serum creatinine (SCr) levels at the times of their initial, 6-mo, and 12-mo protocol biopsies, as well as at last follow-up, the last excluding one patient in group A and two in group B who ultimately lost their grafts. The group A patient was lost to follow-up after postoperative day (POD) 257, at which time his SCr was 1.6 mg/dl. He later presented on POD 576 with a SCr of 7.0 mg/dl after stopping all immunosuppressive medications for >3 wk. A biopsy at that time showed severe ACR (Banff ’97 type 1B, C4d-negative) that did not respond to treatment, and the patient became dialysis dependent. The group B patients’ graft losses occurred 27 and 38 mo after transplantation, respectively. Both of these patients had one or more episodes of ACR (Banff ’97 grades 2A and 1B, respectively). Both patients also had FSGS, clearly representing recurrent disease in one case, although neither patient had a biopsy with diagnostic changes of AMR.
Table 2.
Clinical findings in patient cohorts
| Parameter | Group A (n = 21) | Group B (n = 12) | P |
|---|---|---|---|
| Posttransplantation day of initial protocol biopsy (d) | 0.90c | ||
| mean ± SD | 49 ± 29 | 44 ± 18 | |
| range | 22 to 115 | 24 to 81 | |
| Posttransplantation day of most recent follow-up (d) | 0.53c | ||
| mean ± SDa | 852 ± 515 | 887 ± 218 | |
| range | 324 to 1956 | 504 to 1267 | |
| SCr (mg/dl) | |||
| at diagnostic/initial biopsy | |||
| mean ± SD | 1.2 ± 0.3 | 1.2 ± 0.4 | 0.84d |
| range | 0.7 to 1.8 | 0.5 to 2.2 | |
| at 6-mo protocol biopsy | |||
| mean ± SD | 1.3 ± 0.2 | 1.4 ± 0.5 | 0.50d |
| range | 0.8 to 1.7 | 1.0 to 2.7 | |
| at 12-mo protocol biopsy | |||
| mean ± SDb | 1.3 ± 0.3 | 1.4 ± 0.7 | 0.65d |
| range | 0.9 to 2.3 | 1.0 to 3.1 | |
| at most recent follow-up | |||
| mean ± SDa | 1.3 ± 0.3 | 1.3 ± 0.2 | 0.59d |
| range | 0.9 to 2.3 | 1.1 to 1.7 | |
| Graft losses (n [%]) | 1 (5) | 2 (17) | 0.54e |
Does not include the three patients whose grafts were lost.
n = 19 for group A, n = 9 for group B.
By Wilcoxon rank sum test.
By t test.
By Fisher exact test.
Table 3 compares renal biopsy findings in the groups of patients. All 33 patients had protocol biopsies done at 6 mo after transplantation, although one such biopsy in a group B patient was inadequate, containing only medullary tissue. Nineteen group A patients and nine group B patients also had a 12-mo protocol biopsy. One patient in group A and three in group B developed AMR associated with a rise in SCr (clinical AMR; Table 3) between 3 and 12 mo after transplantation. In one of the group B patients, the biopsy showing clinical AMR was done 28 d after a 3-mo protocol biopsy showing subclinical AMR. Similar fractions of patients in groups A and B had one or more biopsies showing ACR (Banff ’97 grade 1A or greater) during the first year after transplantation (Table 3). Two group A patients each had three separate biopsies showing ACR, and one patient each in groups A and B had two biopsies with ACR. Incidence rates of BK virus nephropathy and recurrent glomerular disease (FSGS in each case) were similar in the two groups (Table 3).
Table 3.
Renal biopsy findings
| Parameter | Group A (n = 21) | Group B (n = 12) | P |
|---|---|---|---|
| Patients with AMR (n [%])a | 1 (5) | 3 (25) | 0.12c |
| Patients with clinical AMR (n [%])b | 1 (5) | 3 (25) | 0.12c |
| Patients with ACR (including clinical and subclinical; n [%])a | 7 (33) | 4 (33) | 0.99c |
| ACR type (Banff ’97)a | 0.82c | ||
| none or borderline | 14 | 8 | |
| 1A | 0 | 1 | |
| 1B | 2 | 1 | |
| 2A | 5 | 2 | |
| Patients with BKV (n [%])a | 5 (24) | 2 (17) | 0.99c |
| Patients with recurrent FSGS (n [%])a | 1 (5) | 1 (8) | 0.99c |
| (cg + ci + ct + cv) | |||
| at initial protocol biopsy | |||
| mean ± SD | 1.52 ± 1.29 | 1.33 ± 1.37 | 0.72d |
| range | 0 to 4 | 0 to 5 | |
| at 6-mo protocol biopsy | |||
| mean ± SD | 1.86 ± 1.82 | 3.00 ± 2.57 | 0.22d |
| range (n) | 0 to 7 (21) | 0 to 9 (11) | |
| difference | |||
| mean ± SD | 0.33 ± 1.71 | 1.64 ± 2.38 | 0.044d |
| range | −1 to 6 | −2 to 7 | |
| at 12 mo protocol biopsy | |||
| mean ± SD | 2.53 ± 1.22 | 3.89 ± 2.42 | 0.032d |
| range (n) | 1 to 6 (19) | 0 to 9 (9) | |
| difference | |||
| mean ± SD | 1.21 ± 1.27 | 2.44 ± 1.74 | 0.030d |
| range | 0 to 5 | −1 to 5 |
On any biopsy of the graft in question during the first year after transplantation. When more than one biopsy showed ACR, the highest Banff ’97 type (grade) of ACR is listed.
On any nonprotocol biopsy of the graft in question during the first year after transplantation, done because of a rise in SCr.
By Fisher exact test.
By Wilcoxon rank sum test.
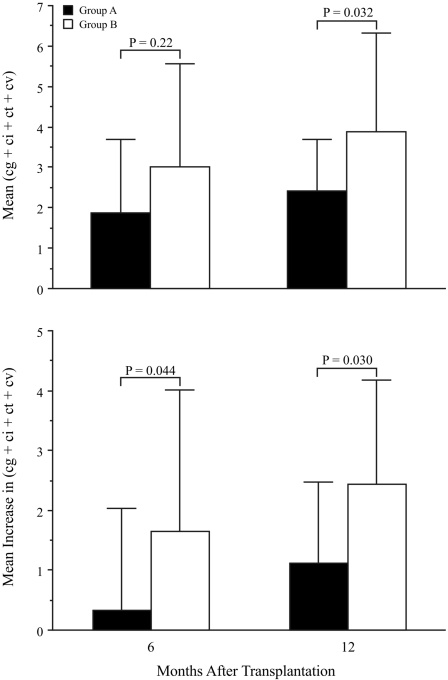
Mean chronic sum scores (comprising the sum of Banff indices for TG [cg], interstitial fibrosis [ci], tubular atrophy [ct], and arterial intimal fibrosis [cv]) on 6- and 12-mo protocol biopsies were lower in group A than in group B, and this reached statistical significance at 12 mo (Table 3, Figure 1, top). Furthermore, increases in mean chronic sum scores from initial to 6-mo protocol biopsies and from initial to 12-mo protocol biopsies both were significantly lower in group A patients compared with group B patients (Table 3; Figure 1, bottom). In group A (but not group B), the mean chronic sum score at 6 mo was not significantly different from that on the initial protocol biopsies (P = 0.80 in group A and P = 0.043 in group B by Wilcoxon rank sum test), although the mean score at 12 mo was different from that on the initial protocol biopsies for both groups (P = 0.012 for group A and P = 0.007 for group B).
Figure 1.
Comparison of mean chronic sum scores (cg + ci + ct + cv) on 6- and 12-mo protocol biopsies (top) and mean increases in this score from the initial biopsy to the 6- and 12-mo biopsies (bottom) in group A and group B patients. Error bars indicate 1 SD. P values shown all are by the Wilcoxon rank sum test. Mean and SD values are also listed in Table 3.
There were no significant differences between the two patient groups with respect to individual Banff chronic indices (cg, ci, ct, and cv) at 6 mo after transplantation; however, at 12 mo, mean ci and ct scores were significantly lower in group A than in group B (both 0.74 ± 0.56 versus 1.33 ± 0.87; P = 0.043 by Wilcoxon). Mean cg (0.05 ± 0.23 in group A, 0.22 ± 0.44 in group B; P = 0.18) and cv (1.00 in both groups) scores on the 12-mo protocol biopsies were not different in the two groups.
DISCUSSION
The findings of this study document that ABO-incompatible renal allografts showing diffuse PTC C4d staining in the presence of circulating BGA but the absence of histologic features of AMR or ACR (Banff ’97 grade 1A or greater) on an early protocol biopsy of a stably functioning graft (group A) develop significantly less overall chronic change, as assessed by the mean chronic sum score (cg + ci + ct + cv), than grafts not showing these findings during the first year after transplantation (group B). Mean ci, ct, and chronic sum scores on 12-mo protocol biopsies, as well as mean increases in chronic sum score between initial and both 6- and 12-mo protocol biopsies, were significantly lower in group A than in group B. Furthermore, the degree of chronic change seen in ABO-incompatible grafts showing diffuse C4d staining without evidence of rejection in this study is essentially identical to that reported for conventional (ABO-compatible with no preoperative anti-HLA donor-specific antibodies) live donor renal allografts at 6 to 12 mo after transplantation,16–18 taking into account somewhat higher mean baseline levels of arterial intimal fibrosis (cv) and, to a lesser extent, tubular atrophy (ct) and interstitial fibrosis (ci) in our donor population that includes a relatively high fraction of older (≥50 yr old) donors.19
Our donor population not only had higher mean baseline ci, ct, and cv values but also greater variability in these parameters than in conventional live-donor transplantation, for which baseline scores are rarely >0 for ci and ct or >1 for cv.18 It is because of this variability at baseline that we feel justified in comparing increases in chronic sum scores between the two patient groups as well as absolute sum scores at 6 and 12 mo. The relatively short-term nature of our findings and the limited number of patients also led us to focus more on chronic sum scores than on individual indices of chronicity (e.g., cg, ct) to demonstrate significant differences between patient groups, acknowledging that a possible drawback of the sum score is that it treats increases in each of the different indices as being equivalent, when in actuality increases in different parameters may have different predictive values regarding graft survival. Still, we did find significant differences in mean ci and ct (but not cg and cv) values at 12 mo between groups A and B. Histologic changes of TG, which is the chronic lesion most closely associated with antibodies against the graft,20 are quite uncommon 1 yr after transplantation in both conventional and ABO-incompatible renal allografts,16,20 although early changes of TG may be detectable within 2 mo by electron microscopy.21
Only four of our study patients (one in group A, three in Group B; P = 0.12) developed diagnostic changes of AMR. This suggests that factors other than prevention of morphologically identifiable AMR contribute to the beneficial effect of PTC C4d deposition without histologic evidence of rejection in reducing graft scarring during the first year after transplantation. Perhaps future use of microarray22 and/or other molecular methods may prove useful in identifying possible antibody-mediated graft injury in the absence of diagnostic morphologic changes of AMR.
It will also be important to investigate whether the apparent beneficial effect associated with diffuse C4d staining without evidence of rejection on relatively short-term graft scarring persists in the long term and translates into significantly improved long-term survival of ABO-incompatible grafts. Especially as BGA persist in all of our recipients of ABO-incompatible renal allografts, such longer term studies are needed to determine whether diffuse C4d staining without evidence of rejection truly reflects a state of graft accommodation, in which the organ continues to function well despite the presence of antibodies directed against it.23 The finding of diffuse PTC C4d staining without histologic evidence of rejection on one or more early protocol biopsies did not preclude later development of AMR, although the latter was rare in this study. Only one of 21 group A patients compared with three of 12 group B patients developed AMR, although this difference did not reach statistical significance and no grafts in either group were lost to AMR.
Whereas in the majority of biopsies of ABO-incompatible grafts the presence of C4d is not associated with histologic changes of AMR, this is not the case in biopsies of ABO-compatible renal allografts, even protocol biopsies.9–11,24–27 Furthermore, in the latter grafts, even PTC C4d deposition without histologic evidence of AMR or ACR may signify low-level, antibody-mediated graft injury. In a study by Dickenmann et al.,28 of 22 patients with ABO-compatible grafts and a biopsy showing such findings, death-censored renal allograft survival 3 yr after biopsy was 69% in patients who were not given increased immunosuppression, compared with 100% in those who were. It must therefore be emphasized that the findings of our study, namely that PTC C4d deposition without histologic evidence of AMR or ACR is associated with less graft scarring during the first year after transplantation, seem to be applicable only to ABO-incompatible renal transplantation and do not represent a general paradigm.
Antibodies directed against the carbohydrate blood group antigens may well be less likely to promote AMR, including subclinical AMR, than are antibodies against HLA protein antigens. In the absence of early T cell help (possibly prevented by multidrug immunosuppressive therapy), antibodies produced against a carbohydrate antigen structurally similar to human blood group antigens in a mouse heart xenograft model result in graft accommodation, with these antibodies binding to the carbohydrate antigen on the graft but not producing AMR.29,30 Still, in this model, AMR did occur when antibodies to the same carbohydrate antigen were produced in the setting of early T cell help,29 and AMR (both clinical and subclinical) does occur in some ABO-incompatible renal allografts,12–14,16,31,32 including four (12%) of 33 in this study. Conversely, a small number of ABO-compatible, positive cross-match (HLA-incompatible) grafts showed C4d staining on protocol biopsies without histologic changes of AMR or subsequent development of graft dysfunction or scarring, including TG.11,12 This suggests that factors in addition to the nature of the antigen determine whether a graft exposed to antidonor antibody develops AMR or simply shows C4d deposition without rejection. Inhibition of the complement cascade distal to C4 cleavage by factors such as decay-accelerating factor, CD59, and heparan sulfate may play a vital role in determining whether grafts exposed to such antibody develop AMR or show accommodation.23,33,34 Indeed, recent studies in a heart xenograft model similar to that noted already demonstrated elevated expression of decay-accelerating factor, CD59, and Crry (a rodent complement regulatory protein) on capillary endothelial cells of accommodating grafts.34 The regulation of expression of these factors in human allografts and how this is affected by binding of antibodies against blood group and HLA antigens expressed on the graft remain important areas for future study.
There are several limitations of this study. First, it is a retrospective study, and patients were not treated according to standardized protocols. Still, group A and group B patients were quite similar with respect to demographics; serology; desensitization procedures (mean numbers of PP/intravenous Ig treatments plus fraction of patients undergoing splenectomy and receiving anti-CD20); baseline immunosuppression; and incidence rates of ACR, BK virus nephropathy, and recurrent glomerular disease. Second, as noted already, follow-up intervals were relatively short, and it will need to be determined whether the association of diffuse PTC C4d deposition without histologic evidence of AMR with reduced graft scarring during the first year after transplantation persists in the long term. Although there is considerable evidence that development of chronic histopathologic changes during the first year after transplantation is associated with reduced graft survival,18,35,36 these studies are in conventional allografts that are less likely to be at risk for antibody-mediated graft injury than are ABO-incompatible grafts.16 Finally, because the number of patients studied was relatively small, there is potential for the results to be disproportionately influenced by just a few patients with poor outcomes. Our “control” group consisted of only 12 patients with negative or weak and focal C4d staining on their initial protocol biopsy, two of whom lost their grafts to causes other than AMR. Thus, as ABO-incompatible renal transplantation becomes more widespread, it will be important to examine correlations between PTC C4d deposition without histologic evidence of AMR and subsequent graft function and histology in larger patient populations from different transplant centers.
CONCISE METHODS
Patients
All patients receiving a live-donor, ABO-incompatible renal transplant at our center from January 2000 through July 2007 were considered for inclusion in this study, with the exception of patients receiving both HLA- and ABO-incompatible grafts. Of a total of 45 such recipients, 37 patients met the following inclusion criteria: (1) One or more protocol biopsies were performed during the first approximately 3 mo after transplantation and met adequacy criteria defined in the Banff ’97 working classification for renal allograft pathology,37 (2) one or more subsequent protocol biopsies were performed at 6 and/or 12 mo after transplantation, and (3) C4d staining was done on all protocol biopsies. Four of the 37 patients had ≥1+, diffuse PTC C4d staining on their initial protocol biopsy with margination of leukocytes (mononuclear cells and neutrophils) in PTC, consistent with subclinical AMR,11 and were not included in this study.
All patients receiving ABO-incompatible renal transplants were treated before transplantation with every-other-day PP and 100 mg/kg cytomegalovirus hyperimmune globulin (CMVIg; Cytogam; Medimmune, Gaithersburg, MD) to remove BGA. PP/CMVIg treatments were continued until a BGA titer of ≤16 was achieved. Detectable levels of circulating BGA, however, were present throughout the postoperative course of each patient. Patients in the early part of the study period underwent splenectomy, those in the middle part received anti-CD20 (Rituxan; IDEC-Genentech, San Francisco, CA), usually without splenectomy, and those in the last part underwent neither splenectomy nor anti-CD20 therapy. Patients also received two to 10 posttransplantation PP/CMVIg treatments per protocol as described previously.5 In addition, all patients received a sequential quadruple-drug immunosuppressive regimen consisting of tacrolimus (0.1 mg/kg per d, adjusted to an initial target level of 8 to 12 ng/ml), mycophenolate mofetil (2 g/d), daclizumab (2 mg/kg before reperfusion, then 1 mg/kg every other week for a total of five doses), and corticosteroids (methylprednisolone 500 mg intraoperatively and 125 mg every 6 h, followed by prednisone 30 mg/d, then 20 mg/d after target level of tacrolimus was reached). All study procedures were approved by the institutional review board of Johns Hopkins Medical Institutions.
C4d and C3d Staining
For each biopsy, a 3- to 4-mm portion of renal cortical and/or medullary tissue was taken for C4d staining. For the majority of biopsies, staining for C3d was also performed. This staining was done by indirect immunofluorescence on cryostat sections using mouse mAb to human C4d and C3d (Quidel, San Diego, CA) as described previously.11,12 Staining for C4d and C3d in PTC was graded for intensity (0 to 4+ scale, increments of 0.5+) and was also graded as diffuse (estimated ≥50% of specimen), focal (≥10% but <50%), or negative (absent or involving <10% of specimen).
Grading of Histologic Parameters and Diagnosis of AMR
Histologic slides from all biopsies performed on the 33 patients meeting study criteria were stained with hematoxylin and eosin, periodic acid-Schiff, silver methenamine, and Masson's trichrome stains and were reviewed by a renal pathologist (M.H.) who was blinded to the clinical, immunofluorescence, and serologic data. For each biopsy, margination of leukocytes (neutrophils and mononuclear cells) in cortical PTC was scored according to the following schema15: 0, minimal margination (fewer than three cells in the most involved PTC and/or margination in [estimated] ≤10% of PTC in nonsclerotic cortex present); 1+, margination in >10% of PTC, with three to four cells in the most involved PTC cross-sections; 2+, margination in >10% of PTC, with five to 10 cells in the most involved PTC cross-sections; and 3+, margination in >10% of PTC, with >10 cells in the most involved PTC cross-sections. It was also noted for each biopsy whether there were histologic findings of TMA, defined by one or more glomerular and/or arteriolar fibrin thrombi, arteriolar necrosis, and/or fragmented red blood cells in one or more vessel walls. In addition, the Banff ’97 grade (type) of acute rejection, all Banff acute and chronic indices,37 and a chronic sum score composed of the sum of the Banff chronic indices (cg + ci + ct + cv) were recorded for all biopsies. A diagnosis of AMR was made when each of the following three criteria was met: (1) Diffuse PTC C4d staining with intensity ≥1+, (2) PTC margination score ≥1+ with primarily neutrophils or a mixture of neutrophils and mononuclear cells and/or presence of TMA, and (3) presence of BGA in serum with specificity for donor blood group antigen(s). C4d staining without evidence of rejection was determined to be present when each of the following five criteria were met: (1) Diffuse PTC C4d staining with intensity >1+; (2) PTC margination score of 0 and no evidence of TMA; (3) no concurrent ACR (Banff ’97 grade 1A or greater); (4) Banff g and cg indices of ≤1 and 0, respectively; and (5) presence of BGA in serum with specificity for donor blood group antigen(s).
SCr levels at the time of each biopsy and at most recent follow-up were recorded on spreadsheets separate from those containing the biopsy results noted and results of serologic tests; the spreadsheets identified each patient only by a study number. Titers of IgG antibodies against blood group antigens were determined by indirect isoagglutination assay using anti-human globulin as described previously.5 None of the patients in this study was found to have donor-specific anti-HLA antibodies at any point during desensitization or after transplantation.
Statistical Analysis
The significance of differences between sample means was determined by t test for normally distributed data and by Wilcoxon rank sum test for data that were not normally distributed. Categorical data were compared using Fisher exact test. All tests were two-sided with statistical significance set at P < 0.05. Statistical analyses were performed using Stata 10 statistical software for Linux (Stata Corp., College Station, TX).
DISCLOSURES
None.
Acknowledgments
This work was supported in part by a grant from the Kidney & Urology Foundation of America, Inc.
A preliminary report of some of these findings was presented at the 9th Banff Conference on Allograft Pathology; June 23 through 29, 2007; La Coruna, Spain.
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Ross CN, Gaskin G, Gregor-MacGregor S, Patel AA, Davey NJ, Lechler RI, Williams G, Rees AJ, Pusey CD: Renal transplantation following immunoadsorption in highly sensitized recipients. Transplantation 55: 785–789, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Alkhunaizi AM, de Mattos AM, Barry JM, Bennett WM, Norman DJ: Renal transplantation across the ABO barrier using A2 kidneys. Transplantation 67: 1319–1324, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Glotz D, Haymann J-P, Sansonetti N, Francois A, Menoyo-Calonge V, Bariety J, Druet P: Suppression of HLA-specific alloantibodies by high-dose intravenous immunoglobulins (IVIg). Transplantation 56: 335–337, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Tyden G, Kumlien G, Fehrman I: Successful ABO-incompatible kidney transplantations without splenectomy using antigen-specific immunoadsorption and rituximab. Transplantation 76: 730–731, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Montgomery RA, Zachary AA, Racusen LC, Leffell MS, King KE, Burdick J, Maley WR, Ratner LE: Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation 70: 887–895, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Tanabe K, Takahashi K, Sonda K, Tokumoto T, Ishikawa N, Kawai T, Fuchinoue S, Oshima T, Yagisawa T, Nakazawa H, Goya N, Koga S, Kawaguchi H, Ito K, Agishi T, Ota K: Long-term results of ABO-incompatible living kidney transplantation: A single-center experience. Transplantation 65: 224–228, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Gloor JM, Lager DJ, Moore SB, Pineda AA, Fidler ME, Larson TS, Grande JP, Schwab TR, Griffin MD, Prieto M, Nyberg SL, Velosa JA, Textor SC, Platt JL, Stegall MD: ABO-incompatible kidney transplantation using both A2 and non-A2 living donors. Transplantation 75: 971–977, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Mengel M, Bogers J, Bosmans J-L, Seron D, Moreso F, Carrera M, Gwinner W, Schwarz A, De Broe M, Kreipe H, Haller H: Incidence of C4d stain in protocol biopsies from renal allografts: Results from a multicenter trial. Am J Transplant 5: 1050–1056, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Collins AB, Schneeberger EE, Pascual M, Saidman SL, Williams WW, Tolkoff-Rubin N, Cosimi AB, Colvin RB: Complement activation in acute humoral renal allograft rejection: Diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol 10: 2208–2214, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Mauiyyedi S, Crespo M, Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Tolkoff-Rubin NE, Williams WW, Delmonico FL, Cosimi AB, Colvin RB: Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol 13: 779–787, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Haas M, Montgomery RA, Segev DL, Rahman MH, Racusen LC, Bagnasco SM, Simpkins CE, Warren DS, Lepley D, Zachary AA, Kraus ES: Subclinical acute antibody-mediated rejection in positive crossmatch renal allografts. Am J Transplant 7: 576–585, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Haas M, Rahman MH, Racusen LC, Kraus ES, Bagnasco SM, Segev DL, Simpkins CE, Warren DS, King KE, Zachary AA, Montgomery RA: C4d and C3d staining in biopsies of ABO- and HLA-incompatible renal allografts: Correlation with histologic findings. Am J Transplant 6: 1829–1840, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Fidler ME, Gloor JM, Lager DJ, Larson TS, Griffin MD, Textor SC, Schwab TR, Prieto M, Nyberg SL, Ishitani MB, Grande JP, Kay PA, Stegall MD: Histologic findings of antibody-mediated rejection in ABO blood-group-incompatible living-donor kidney transplantation. Am J Transplant 4: 101–107, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Setoguchi K, Ishida H, Shimura H, Shimizu T, Shirakawa H, Omoto K, Toki D, Iida S, Setoguchi S, Tokumoto T, Horita S, Nakayama H, Yamaguchi Y, Tanabe K: Analysis of renal transplant protocol biopsies in ABO-incompatible kidney transplantation. Am J Transplant 8: 86–94, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DSR, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Gloor JM, Cosio FG, Rea DJ, Wadel HM, Winters JL, Moore SB, DeGoey SR, Lager DJ, Grande JP, Stegall MD: Histologic findings one year after positive crossmatch or ABO blood group incompatible living donor kidney transplantation. Am J Transplant 6: 1841–1847, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR: The natural history of chronic allograft nephropathy. N Engl J Med 349: 2326–2333, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Cosio FG, Grande JP, Larson TS, Gloor JM, Velosa JA, Textor SC, Griffin MD, Stegall MD: Kidney allograft fibrosis and atrophy early after living donor renal transplantation. Am J Transplant 5: 1130–1136, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Haas M, Segev DL, Racusen LC, Bagnasco SM, Melancon JK, Tan M, Kraus ES, Rabb H, Ugarte RM, Burdick JF, Montgomery RA: Arteriosclerosis in kidneys from healthy live donors: Comparison of wedge and needle core perioperative biopsies. Arch Pathol Lab Med 132: 37–42, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Gloor JM, Sethi S, Stegall MD, Park WD, Moore SB, DeGoey S, Griffin MD, Larson TS, Cosio FG: Transplant glomerulopathy: Subclinical incidence and association with alloantibody. Am J Transplant 7: 2124–2132, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Wavamunno MD, O'Connell PJ, Vitalone M, Fung CL, Allen RD, Chapman JR, Nankivell BJ: Transplant glomerulopathy: Ultrastructural abnormalities occur early in longitudinal analysis of protocol biopsies. Am J Transplant 7: 1–12, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Mueller TF, Einecke G, Reeve J, Sis B, Mengel M, Jhangri GS, Bunnag S, Cruz J, Weishart D, Meng C, Broderick G, Kaplan B, Halloran PF: Microarray analysis of rejection in human kidney transplants using pathogenesis-based transcript sets. Am J Transplant 7: 2712–2722, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Williams JM, Holzknecht ZE, Plummer TB, Lin SS, Brunn GJ, Platt JL: Acute vascular rejection and accommodation: Divergent outcomes of the humoral response to organ transplantation. Transplantation 78: 1471–1478, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Herzenberg AM, Gill JS, Djurdjev O, Magil AB: C4d deposition in acute rejection: An independent long-term prognostic factor. J Am Soc Nephrol 13: 234–241, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Bohmig GA, Exner M, Habicht A, Schillinger M, Lang U, Kletzmayr J, Saemann MD, Horl WH, Watschinger B, Regele H: Capillary C4d deposition in kidney allografts: A specific marker of alloantibody-dependent graft injury. J Am Soc Nephrol 13: 1091–1099, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Mauiyyedi S, Della Pelle P, Saidman S, Collins AB, Pascual M, Tolkoff-Rubin NE, Williams WW, Cosimi AA, Schneeberger EE, Colvin RB: Chronic humoral rejection: Identification of antibody-mediated chronic allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol 12: 574–582, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Regele H, Bohmig GA, Habicht A, Gollowitzer D, Schillinger M, Rockenschaub S, Watschinger B, Kerjaschki D, Exner M: Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: A contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol 13: 2371–2380, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Dickenmann M, Steiger J, Descoeudres B, Mihatsch M, Nickeleit V: The fate of C4d positive kidney allografts lacking histological signs of acute rejection. Clin Nephrol 65: 173–179, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Mohiuddin MM, Ogawa H, Yin DP, Galili U: Antibody-mediated accommodation of heart grafts expressing an incompatible carbohydrate antigen. Transplantation 75: 258–262, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Galili U: Immune response, accommodation, and tolerance to transplantation carbohydrate antigens. Transplantation 78: 1093–1098, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Onitsuka S, Yamaguchi Y, Tanabe K, Takahashi K, Toma H: Peritubular capillary deposition of C4d complement fragment in ABO-incompatible renal transplantation with humoral rejection. Clin Transplant 13[Suppl 1]: 33–37, 1999 [PubMed] [Google Scholar]
- 32.Kato M, Morozumi K, Takeuchi O, Oikawa T, Koyama K, Usami T, Shimano Y, Ito A, Horike K, Otsuka Y, Toda S, Takeda A, Uchida K, Haba T, Kimura G: Complement fragment C4d deposition in peritubular capillaries in acute humoral rejection after ABO blood group-incompatible human kidney transplantation. Transplantation 75: 663–665, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Park WD, Grande JP, Ninova D, Nath KA, Platt JL, Gloor JM, Stegall MD: Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am J Transplant 3: 952–960, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Ding JW, Zhou T, Ma L, Yin D, Shen J, Ding CP, Tang IY, Byrne GW, Chong AS: Expression of complement regulatory proteins in accommodated xenografts induced by anti-α-gal IgG1 in a rat-to-mouse model. Am J Transplant 8: 32–40, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Seron D, Moreso F, Boyer J, Condom E, Gil-Vernet S, Canas C, Fulladosa X, Torras J, Carrera M, Grinyo JM, Alsina J: Early protocol renal biopsies and graft outcome. Kidney Int 51: 310–316, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Nankivell BJ, Fenton-Lee CA, Kuypers DR, Cheung E, Allen RD, O'Connell PJ, Chapman JR: Effect of histological damage on long-term kidney transplant outcome. Transplantation 71: 515–523, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo C, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Marcussen N, Mihatsch MJ, Nadasdy T, Nickerson P, Olsen TS, Papadimitriou JC, Randhawa PS, Rayner DC, Roberts I, Rose S, Rush D, Salinas-Madrigal L, Salomon DR, Sund S, Taskinen E, Trpkov K, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]