Abstract
The 5-yr survival rate of renal allografts is significantly lower for grafts from older deceased donors than from younger deceased donors. For evaluation of the potential contribution of renal senescence in this shortened graft survival, glomerular function and structure were analyzed in allografts from deceased donors older than 55 yr (“aging”) or younger than 40 yr (“youthful”). Aging donors had a significantly higher prevalence of sclerotic glomeruli (P < 0.002), and their nonsclerotic glomeruli tended to be larger, had a larger filtration surface area (P = 0.02), and had a higher single-nephron ultrafiltration coefficient (Kf; P = 0.07), suggesting a compensatory response to functional loss of glomeruli. After serum creatinine reached a stable nadir in the transplant recipients, GFR and its hemodynamic determinants were evaluated and the whole allograft Kf was computed. Compared with the allografts from youthful donors, allografts from aging donors exhibited a 32% lower GFR, which was exclusively attributable to a 45% reduction in allograft Kf (both P < 0.001). In addition, the number of functioning glomeruli per allograft was profoundly lower in grafts from aging donors than from youthful donors (3.6 ± 2.1 × 105 versus 8.5 ± 3.4 × 105; P < 0.01), and this could not be explained by the relatively modest 17% prevalence of global glomerulosclerosis in the aging group. The marked reduction in overall glomerular number in many aging donors may lead to a “remnant kidney” phenomenon, potentially explaining the shorter mean survival of these allografts.
According to the United Network for Organ Sharing (UNOS), the recipient waiting list for kidney transplantation (Tx) is currently in excess of 76,000, and median waiting time is more than 3 yr.1 One response to the donor deficit by transplant centers has been to increase the number of grafts from deceased donors who are aging. According to the UNOS database, the number of transplants from deceased donors older than 50 yr increased by 50% and those older than 65 yr by 55% during between 1996 and 2006.1 This increase is being fueled by the creation of a parallel waiting list for so-called “expanded donor criteria” (ECD) kidneys, which come predominantly from aging donors; however, allografts from older deceased donors exhibit a striking reduction in the 5-yr graft survival rate. This study was designed to evaluate a possible contribution to poor graft survival of aging-related changes in the kidney donor, known collectively as renal senescence.
By renal senescence, we mean a series of physiologic and structural alterations that lower renal function in aging individuals. The best documented physiologic change is depression of the GFR, which has been shown to decline linearly as a function of age beyond the end of the fifth decade.2–4 A substantial body of evidence from the autopsy literature points to structural abnormalities, with an ensuing decline in glomerular ultrafiltration capacity, as a likely basis for the declining GFR. Such abnormalities include declining renocortical volume5 and an increasing prevalence of global glomerulosclerosis, interstitial fibrosis, and atherosclerosis.6–8
In addition to a prevalence of global sclerosis between 10 and 40% beyond age 60 yr,6,7 renocortical avascularity is thought to be associated with resorption of obsolescent, sclerosed glomeruli.9,10 The latter process is in keeping with the demonstration by Nyengaard and Bendtsen11 that the number of patent (nonsclerosed) glomeruli at autopsy was significantly lower in individuals who were older than versus younger than 55 yr. Thus, glomerulopenia, whether a result of global sclerosis or resorption of glomeruli, could severely limit glomerular filtration capacity of an allograft when a single kidney is transplanted from an aging donor. To address this possibility, we used a novel approach to compare glomerular number (NFG) and ultrafiltration capacity in recently transplanted allografts from deceased donors who were aging (>55 yr) with those from youthful donors (<40 yr). Our findings form the basis of this report.
RESULTS
Donor Characteristics and Morphology
The median (range) age in the aging donor group was 59 yr (55 to 72 yr) compared with 24 yr (18 to 32 yr) in the youthful donor group. Serum creatinine concentrations of the donor upon admission to hospital for the terminal illnesses were 1.0 ± 02 and 0.9 ± 0.1 mg/dl; corresponding nadir values after resuscitation were 0.8 ± 0.3 in the aging and 0.7 ± 0.2 mg/dl in the youthful donors, indicating a satisfactory level of kidney function. A documented history of hypertension was present in 64% of the aging donors versus only 10% of the youthful donors. None of the deceased donors in this study had a history of diabetes.
We analyzed the effects of aging on glomerular structure by performing a morphometric analysis of glomeruli obtained during Tx by wedge biopsy in the first 25 individuals to be recruited into our study. Of these, 13 were in the aging and 12 in the youthful group (Table 1). The percentage of glomeruli exhibiting global sclerosis was significantly higher in the aging than the youthful group (17 ± 12 versus 2 ± 3%, respectively; P = 0.003; Table 1). A parallel relationship between fractional interstitial area and age revealed a significant expansion of the interstitial compartment in aging compared with youthful donors (Table 1).
Table 1.
Glomerular morphometry
| Parameter | Youthful (n = 12) | Aging (n = 13) |
|---|---|---|
| No. of glomeruli sampled (median [10th to 90th percentiles]) | 23 (20 to 88) | 53 (27 to 96) |
| Global sclerosis (%) | 1.6 ± 3.0a | 16.8 ± 12.2 |
| Fractional interstitial area (%) | 12.0 ± 2.8a | 17.7 ± 7.3 |
| Glomerular volume (μ3 × 106) | 2.7 ± 0.8b | 3.4 ± 0.9 |
| Filtration surface area (μ2 × 105) | 3.1 ± 0.9a | 4.1 ± 0.9 |
| Hydraulic permeability (m/s per Pa × 10−9) | 2.45 ± 0.33 | 2.42 ± 0.40 |
| SNKf (nl/[min · mmHg]) | 8.6 ± 2.6b | 11.1 ± 3.5 |
P < 0.05.
P = 0.07.
That the glomerulosclerosis could be associated with glomerulopenia of greater magnitude than attributable to the percentage of global sclerosis alone is suggested by a strong trend to increased volume of nonsclerosed glomeruli in the aging versus youthful group (3.4 ± 0.9 versus 2.7 ± 0.8 μ3× 106; P = 0.07; Table 1). This 24% increase in patent glomerular volume in aging versus youthful donors resulted in a corresponding and significant parallel increase in the computed filtration surface area (S) by 32% (P = 0.02; Table 1). Minor insignificant differences in basement membrane thickness and filtration slit frequency resulted in a similar computed value for hydraulic permeability (k) between aging and youthful individuals (Table 1). Nevertheless, because single-nephron ultrafiltration coefficient (SNKf) = k × S, the larger value of S resulted in a strong trend to an excess of the computed SNKf in aging versus youthful donors, respectively (11.1 ± 3.5 versus 8.6 ± 2.6 nl/(min · mmHg); P = 0.07; Table 1). The findings of a sclerosing glomerulopathy accompanied by strong trends to compensatory hypertrophy and adaptive hyperfiltration (increased SNKf) in aging donors before Tx is consistent with a degree of functional glomerulopenia greater than can be accounted for by an observed prevalence of global glomerulosclerosis of only 17 ± 12%.12
Recipient Characteristics
Of the 43 recipients recruited into the study, 38 completed the physiologic study on average 106 ± 67 and 87 ± 121 d after Tx in the recipients of aging versus youthful donors, respectively. Recipients of aging kidneys were older than recipients of youthful kidneys (64 ± 6 versus 46 ± 15 yr; P < 0.05; Table 2). There were no clinical episodes of rejection in either group. The degree of HLA mismatches and delayed graft function were similar in the two groups. Immunosuppression in both groups included calcineurin inhibitors, mycophenolate mofetil, and steroids and did not differ between groups. Nadir serum creatinine concentration on the day of physiologic study was significantly higher at 1.2 ± 0.5 in recipients of aging versus 0.9 ± 0.4 mg/dl in recipients of youthful donors (P < 0.05; Table 2).
Table 2.
Filtration dynamics
| Parameter | Youthful (n = 20) | Aging (n = 17) |
|---|---|---|
| Recipient age (yr) | 46 ± 15a | 64 ± 6 |
| Serum creatinine (mg/dl) | 0.9 ± 0.4a | 1.2 ± 0.5 |
| GFR (ml/min per 1.73 m2) | 69 ± 17a | 48 ± 21 |
| RPF (ml/min per 1.73 m2) | 304 ± 71a | 248 ± 76 |
| Filtration fraction | 0.24 ± 0.06a | 0.19 ± 0.06 |
| Mean arterial pressure (mmHg) | 90 ± 10 | 93 ± 10 |
| πA (mmHg) | 25.4 ± 2.3 | 24.2 ± 1.9 |
| πGC (mmHg) | 29.0 ± 3.3a | 26.6 ± 2.5 |
| Whole-kidney Kf (ml/[min · mmHg]) | 6.6 ± 3.4a,b | 3.7 ± 2.2 b or 2.5 ± 1.9c |
P < 0.05.
Assume ΔP = 40 or
ΔP = 45.
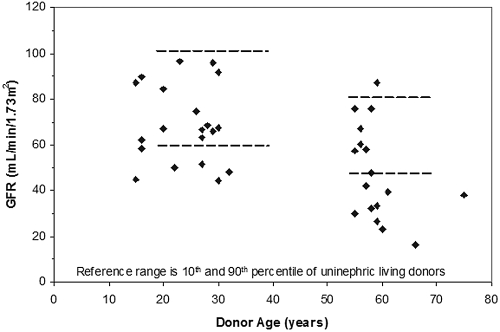
GFR levels are illustrated in Figure 1. A normal range (10th to 90th percentiles depicted by horizontal parallel lines) for a healthy uninephric individual is provided by our control population of 51 post-Tx living donors. Note that allograft GFR from 10 of 17 aging and six of 21 youthful donors fell below the 10th percentile for corresponding native kidney GFR in the uninephric living donors. An inverse relationship between GFR and (donor) age is evident (R2 = 0.3, P < 0.01). Also noteworthy is that, in a substantial subset of those with allografts from aging donors, the GFR is <40 ml/min, a phenomenon not seen in the youthful group (Figure 1).
Figure 1.
Allograft “optimal” GFR versus donor age, approximately 3 mo after Tx. The parallel dashed lines represent the normal range for GFR (10th to 90th percentiles) in healthy uninephric living donors.
GFR in the aging group was significantly lower by 30% than in the youthful donor group, 48 ± 21 versus 69 ± 17 ml/min, respectively (Table 2). Aging renal plasma flow (RPF) was also depressed below youthful levels but proportionately less than GFR, with the result that the filtration fraction was significantly lower in the aging than the youthful donor group (Table 2). From the filtration fraction and afferent arteriolar oncotic pressure (πA), which tended to be lower, we computed13 that mean oncotic pressure of plasma in glomerular capillaries (πGC) is significantly depressed in aging versus youthful groups, 26.6 ± 2.5 versus 29.0 ± 3.3 mmHg, respectively (Table 2). This difference reflects a lower serum albumin after Tx surgery in the older recipients who received the aging donor kidneys, 3.59 ± 0.39 versus 3.75 ± 0.39 g/dl in the younger recipients who received youthful donor kidneys. Because oncotic pressure is the pressure opposing filtration, the difference should favor a higher GFR in the aging donor than youthful donor group and not a lower GFR as was observed.
By exclusion, a loss of intrinsic filtration capacity likely explains the lower GFR and filtration fraction in the aging group. Using our “best guess” value for transcapillary hydraulic pressure difference (ΔP; 40 mmHg) in each group, we computed this whole kidney Kf to be substantially lower in the aging than youthful group (3.7 ± 2.2 versus 6.6 ± 3.4 ml/(min/mmHg); P < 0.001; Table 2). In the event that ΔP is not 40 mmHg in the aging group, but elevated instead to 45 mmHg, the whole-kidney Kf in the latter would be depressed even more to only 2.5 ± 1.9 ml/(min/mmHg). Given that SNKf is higher in the aging than youthful donor groups, we speculated that a reduction in glomerular number (NFG) likely accounts for the GFR and whole-allograft Kf difference detected between the groups. Clearly, the observed 17% prevalence of global sclerosis (Table 1) is insufficient to explain the 44 to 62% mean depression in computed Kf in the aging group (Table 2).
Glomerular Number
Nineteen of the 25 recipients subjected to morphometric analysis of a wedge biopsy completed their post-Tx physiologic study: Nine from the aging and 10 from the youthful donor groups, respectively. From the whole-kidney KF/SNKf quotient (equation 5), we approximated the number of functioning (nonsclerosed) glomeruli (NFG). As shown in Table 3, values for whole-kidney Kf and of SNKf in the aging and youthful donor subgroups were similar to the respective values for the full corresponding groups (Tables 1 and 2). As shown in Figure 2, an inverse relationship was observed between NFG and age (R2 = 0.46, P < 0.01). On average, NFG in the youthful donor group was 8.5 ± 3.4 × 105, a number similar to that reported from autopsy studies.11 In contrast, mean (±1 SD) NFG was depressed below youthful values to only 3.6 ± 2.1 × 105 or 2.5 ± 1.9 × 105 in the aging donor group depending on whether the prevailing ΔP was 40 or 45 mmHg, respectively (P < 0.01; Table 3). This level of functional glomerulopenia is sufficient to explain the observed depression of GFR and whole-kidney Kf in the recipients of aging allografts.
Table 3.
Number of functioning glomeruli
| Parameter | Youthful (n = 10)
|
Aging (n = 9)
|
|
|---|---|---|---|
| ΔP = 40 mmHg | ΔP = 40 mmHg | ΔP = 45 mmHg | |
| Whole-kidney Kf (ml/[min · mmHg]) | 7.4 ± 3.4 | 3.6 ± 1.9a | 2.5 ± 1.2a |
| SNKf (nl/[min · mmHg]) | 9.0 ± 2.5 | 11.2 ± 3.9 | 11.2 ± 3.9 |
| NFG × 105 | 8.5 ± 3.4 | 3.5 ± 2.1a | 2.5 ± 1.4a |
P < 0.01 versus youthful.
Figure 2.
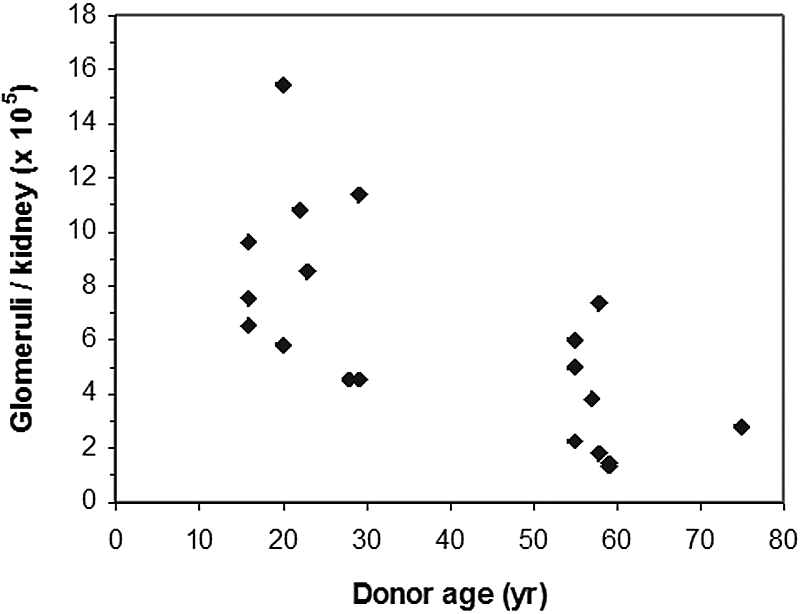
NFG per allograft as a function of donor age assuming ΔP = 40 mmHg in each group. By linear regression, the inverse relationship is significant (R2 = 0.46, P < 0.01).
DISCUSSION
We have shown that optimal allograft GFR in transplant recipients varies inversely with the age of a deceased donor (Figure 1). The inverse relationship is similar to that observed in healthy, binephric humans2–4 and to that reported by us recently in uninephric living donors in the wake of their transplant nephrectomy.14 In an effort to understand the basis of GFR depression in allografts from aging donors, we estimated simultaneous glomerular pressures and flows and used these to compute an allograft Kf that was lowered >40% in recipients of aging (>55 yr) compared with youthful (<40 yr) kidney donors. The extent of Kf depression accounted entirely for the aging-related GFR depression (Table 2).
In an attempt to gain insights into the structural basis for the diminished filtration capacity of aging donors, we undertook a morphometric analysis of glomeruli obtained in abundant numbers from a wedge biopsy performed immediately before Tx. We found a high prevalence of global glomerulosclerosis in the aging donors (Table 1) but not high enough to account for the extent of Kf depression. Rather, a finding of hypertrophy of remaining patent glomeruli with an ensuing enhancement of filtration S and SNKf suggested an adaptive response in these aging donors to a profound reduction in the prevailing number of nonsclerotic glomeruli. Indeed, an inverse relationship between glomerular volume and NFG has been previously documented at autopsy with the use of accurate stereologic techniques in aging but not youthful individuals.15 This has been interpreted to indicate that glomerular hypertrophy is a response to and serves as a surrogate marker for declining NFG in aging populations.12,15,16
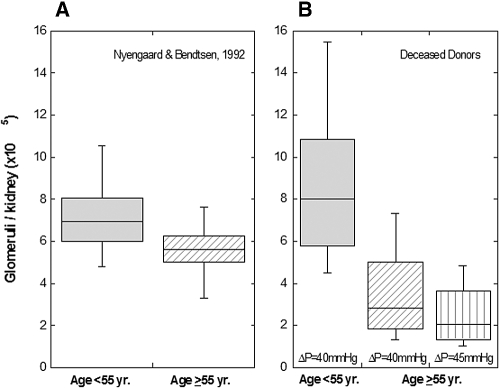
To approximate the NFG in vivo, we divided the whole allograft Kf by the SNKf. This quotient yielded a number of glomeruli in youthful donors that is very similar to that reported at autopsy using optimal, stereologic techniques11 (Figure 3). The corresponding number of nonsclerotic glomeruli in aging individuals >55 yr in the aforementioned autopsy study was depressed by only 20% below that in the youthful individuals (Figure 3). In this study, however, we estimated from allograft Kf/SNKf that the number of glomeruli in our aging donor group was substantially more depressed below corresponding youthful levels, on average by 44 to 62% depending on the value assumed for ΔP in calculating Kf (Table 3, Figures 2 and 3). We speculate that the more profound glomerulopenia likely reflects our use of expanded criteria donors. Our donors had a high incidence of primary hypertension (64%), which has been shown at autopsy by accurate stereologic techniques to be associated with substantial glomerulopenia.17 The hypertension may have resulted from congenital glomerulopenia with ensuing sodium overload17; however, the prevalent glomerulosclerosis and extensive interstitial fibrosis observed in our deceased donors is typical of hypertensive nephrosclerosis,18 which is thought to be a consequence of hypertension-induced renal injury.
Figure 3.
Box plots of NFG below and above age 55 yr estimated by stereologic methods11 (left) and in allograft recipients of youthful versus aging donors (Kf/SNKf; right). Estimation of NFG in aging donors were calculated assuming that ΔP = 40 mmHg (middle) or ΔP = 45 mmHg (right).
The potential accuracy of our estimate of NFG from the quotient of Kf/SNKf is limited by our suboptimal study design, inasmuch as our morphologic and physiologic evaluations were not simultaneous. GFR could not be determined before Tx in the deceased donors because we receive the overwhelming majority of our deceased-donor kidneys from hospitals that are remote from our transplant center. Although we have attempted to time our physiologic study so as to capture the optimal, post-Tx GFR, we cannot exclude the possibility that compensatory hypertrophy and adaptive hyperfiltration of a single kidney on the one hand and calcineurin inhibitor therapy on the other hand could have respectively increased or decreased the allograft GFR (and Kf) above or below that usually prevailing premortem in the deceased donor. An additional limitation of our study is that the accuracy of the quotient allograft Kf/SNKf (equation 5) is limited by several assumptions. These include the assumption that ΔP = 40 or 45 mmHg to calculate Kf. Similarly, the computation of SNKf assumes that the Darcy permeability of the glomerular basement membrane (GBM) in vivo is the same as that determined ex vivo in isolated GBM.19,20
A third category of limiting factors is that related to the phenomenon of heterogeneity of glomerular injury. For example, the use of whole-kidney values of GFR, RPF, and Kf suggests that the foregoing are representative of all glomeruli and ignores that each quantity could vary substantially among nephrons in chronic senescent injury. Another example is that the 20 to 90 glomeruli assessed at the light microscopic level and, more particularly, the three glomeruli studied by electron microscopy may not be representative of all patent glomeruli in a given individual. The same potential for sampling error is, of course, true of the study by micropuncture of disease models in the rat. In such studies, GFR, ΔP, πA, and RPF are estimated and Kf computed in two or three glomeruli on the surface of the kidney. Sampling error is thus inherent in all techniques for assessing progressive glomerular disease, be they structural or physiologic.
A final limitation is that our morphologic assessment of SNKf assumes that all glomeruli in the biopsy tissue contribute to filtration. In fact, some glomeruli may become detached from their proximal tubules and, thus, nonfiltering in a variety of chronic renal injuries.21 Such atubular glomeruli have also been reported in a very low incidence (<1%) in the aging kidney.22 Although atubular glomeruli tend to have smaller tufts,21 their appearance is not pathognomic, and we may have inadvertently included them in our determination of SNKf. Given their low prevalence in renal senescence, however,22 such inclusion should have a negligible effect on our computation of SNKf and, hence, NFG.
Notwithstanding the aforementioned limitations, allograft Kf/SNKf seems to provide a reasonable estimate of NFG. It is similar not only to the corresponding value in the aforementioned autopsy studies but also to a recent estimate, using a novel in vivo technique by Fulladosa et al.23 They estimated NFG in allograft recipients approximately 4 mo after Tx. They determined renocortical volume by magnetic resonance imaging along with glomerular density in a routine, protocol renal biopsy. From the product of renocortical volume and glomerular density, they estimated allograft NFG to average 7.3 ± 3.3 × 105, a value that is in the same general range estimated by us (Table 3). Other findings in the study by Fulladosa et al.23 that are in accordance with those in this study are a direct relationship between patent glomerular volume and donor age and an inverse one between NFG and donor age.
Our estimates of NFG in aging and youthful donors and those of Nyengaard and Bendsten11 in aging and youthful normotensive individuals at autopsy are illustrated as box plots in Figure 3. As shown, each youthful group has a median value of 7 to 8 × 105 glomeruli per kidney (i.e., approximately 1.5 million glomeruli in healthy binephric individuals). In contrast, the bottom quartile of NFG in our aging, deceased donors provided the recipient with a single allograft containing <2 × 105 or 1.3 × 105 glomeruli, depending on the value assumed for ΔP (Figure 3). We submit that such extreme glomerulopenia could lead to a “remnant kidney” phenomenon with progressive allograft failure. In keeping with this possibility is our recent observation that recipients of both kidneys (so-called dual transplantation) from expanded criteria donors have longer allograft survival.24 We recommend that dual-, rather than single-, organ Tx be considered in expanded criteria donors who exhibit findings that point to marked glomerulopenia. Findings suggestive of such glomerulopenia would include a marked reduction in renal volume, prevalent glomerulosclerosis and interstitial fibrosis, and hypertrophy of remaining nonsclerosed glomeruli.
CONCISE METHODS
Protocol
Forty three consecutive recipients of a deceased-donor renal allograft were recruited into a longitudinal study immediately before Tx. They met the criterion of having either aging (n = 20) or youthful (n = 23) allografts on the basis of a donor age of >55 or <40 yr, respectively.
A wedge biopsy was taken from the outer cortex of the donor kidney by the surgeon in the operating room before Tx into the recipient. After discharge from the hospital, post-Tx, the serum creatinine level was monitored at frequent (1 to 2 wk) intervals until it reached a stable nadir level, a phenomenon that was equated with the attainment of optimal allograft GFR. The time (median, range) to post-Tx nadir creatinine level was 43 (25 to 114) and 39 d (13 to 72 d) in the groups with aging and youthful donors, respectively. After an additional interval of approximately 1 mo to demonstrate postnadir stability of the serum creatinine, a physiologic evaluation of GFR and its determinants was undertaken. On average, the interval between Tx and the physiologic evaluation of “optimal” allograft GFR was 106 ± 67 and 87 ± 12 d in recipients of aging and youthful allografts, respectively. Control values for “optimal” single-kidney GFR in postnephrectomy patients (n = 51) were established by performing an identical evaluation of GFR in living donors 6 mo after kidney donation.
Physiologic Evaluation of GFR
Each recipient was subjected to a detailed evaluation of post-Tx“optimal” GFR and its determinants. Studies were performed in the General Clinical Research Center at the Stanford Medical School. Cold iothalamate and p-amino-hippuric acid (PAH) were given by constant infusion during a state of water diuresis. Four 20-min urine collections and bracketing plasma were analyzed to determine the GFR and RPF from the urinary clearance of each respective marker. RPF was corrected for an assumed PAH extraction ratio of 0.9, as described elsewhere.25 Mean arterial pressure was determined by Dynamap, and oncotic pressure in blood was determined by membrane osmometry at the commencement of the clearance study.
An HPLC system with a sensitive ultraviolet light detector was used to assay iothalamate and PAH at 236 nm (Instrumentation Shimadzu 6A, Kyoto, Japan). Ultrafiltrates of plasma and diluted urine were injected onto a reverse-phase column (C18, 5μ Ultrasphere; Beckman, San Ramon, CA). The mobile phase was 3.5% acetonitrile in 10 mM triethylamine at a pH of 3.5, and the flow rate was 1.0 ml/min. Iothalamate and PAH concentrations were determined from the peak area of each solute, corresponding to column retention times of 14 and 10 min, respectively.26 The oncotic pressure in venous plasma is taken to be the same as that in plasma entering the glomerular tuft (πA) and was measured in a Wescor 4400 colloid osmometer (Logan, UT).13 The semipermeable membrane of the osmometer has a molecular weight cutoff of 30 kD so as to exclude proteins the size of albumin and larger. The instrument was calibrated with a 5% albumin solution. The interassay coefficient of variation for πA for by this method is 1.8%.13
Morphometric Evaluation of Glomeruli
The biopsy of outer cortex (>15 mm in length) taken before Tx from the donor kidney was cut in 1-μm sections longitudinally, mounted on glass, and stained with toluidine blue. The median number (and range) of glomerular cross-sections available for determination of the tuft planar area at the light microscopic level was 53 (21 to 254) and 24 (16 to 222) in aging and youthful donor kidneys, respectively. Toluidine blue sections were used to choose three patent glomerular profiles at random. Thin sections of these were then cut from the blocks and collected on 3-mm copper grids, stained with lead citrate and uranyl nitrate, and examined in a JEOL 1230 transmission electron microscope (Peabody MA) located at the Beckman Microscopy Center at the Stanford University School of Medicine.
To assess S, we used only Epon-embedded sections of the core so as to circumvent the substantial glomerular shrinkage associated with paraffin embedding.27 The cross-sectional areas of glomerular tufts contained within the core were determined under a light microscope at ×600, using a standard morphometry system (Sigma Scan Pro V 5; Systat Software, Point Richmond, CA). Glomerular volume (VG) was calculated as follows:
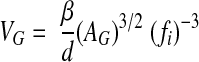 |
(1) |
where β is a dimensionless “shape coefficient” (β = 1.38 for spheres), d is a “size distribution” coefficient (d = 1.1; for caliper diameter coefficient of variation approximately 25%),28 AG is the average tuft cross-sectional area, and ƒi is a correction factor (ƒi = 0.85), which allows for the tuft shrinkage associated with immersion fixation as opposed to perfusion fixation.29 The last factor is used to simulate conditions in vivo when computing SNKf. Thin sections (70 nm) of the three randomly chosen glomeruli from each biopsy were then examined by transmission electron microscopy. Montage photomicrographs of whole glomerular profiles (×2820) were prepared electronically for determination of filtering surface density (Sv) of the peripheral glomerular capillary wall by line-intercept methods. S provided by the peripheral capillary wall per glomerulus was then calculated from the product of surface density (Sv) and Vg.28
An additional cause of declining filtration S in senescence is obliteration of entire glomerular tufts by global sclerosis. The percentage of glomeruli exhibiting global sclerosis was examined in the toluidine blue–stained, Epon-embedded material at the light microscopic level (×600). The number of globally sclerotic glomeruli in each randomly selected section was counted. An equation that takes into account the smaller diameter of sclerotic glomeruli and the consequent difference in the probability of encountering a glomerulus of either type in a random cross-section was used to calculate the percentage of sclerosed glomeruli.30 Finally, the fractional interstitial area was also examined at the light microscopic level (×600). An 11- × 11-point grid was superimposed on nine light microscopic fields of the biopsy, and the fraction of total area occupied by interstitium was determined by point counting. Interstitial area is defined as that outside tubular and vascular structures other than obvious peritubular capillaries and includes interstitial cells.
The assessment of hydraulic permeability (k) was performed entirely at the ultrastructural level. Dimensions of the podocyte layer that accounts for 50% resistance to water flow were determined at high magnification (×11,200). These included the width and the frequency of the filtration slits, where the latter is determined by counting the total number of epithelial filtration slits and dividing it by the total length of the peripheral glomerular capillary wall captured on the electron photomicrographs.19 The GBM accounts for the remaining resistance to water flow, and its thickness, an important determinant of k, was reported as the harmonic mean basement membrane thickness (δBM) using the method of orthogonal intercepts.31
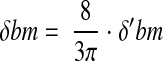 |
(2) |
where δ′bm is the apparent harmonic mean basement membrane thickness and 8/3π corrects for the angle of sectioning.
Mathematical Modeling and Calculations
Computation of SNKf
SNKf was calculated from the product of filtration S and an estimate of the hydraulic permeability of the walls of glomerular capillaries (k) in the three glomeruli examined ultrastructurally. The k was estimated from the filtration slit frequency (FSF), slit diaphragm width (Ws), and the δBM, using the ultrastructural-hydrodynamic model of viscous flow of Drumond and Deen as described by us previously.19,20,32 In the model, the normal capillary wall is represented as consisting of a large number of repeating structural units, each of which is based on a single filtration slit. The width of a structural unit (W) is calculated as follows:
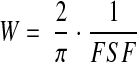 |
(3) |
where 2/π is a correction factor that accounts for the random angle of sectioning. The overall hydraulic permeability is related to the permeabilities of the individual layers, which are calculated from the foregoing quantities as described by us in detail previously.20
Computation of the Whole-Kidney Kf
We computed whole-kidney Kf from GFR and its hemodynamic determinants, using a modification of the mathematical model of glomerular ultrafiltration of Deen et al.33 Whereas πA and RPF can be determined with reasonable precision in the human kidney,13,15 the ΔP, the outward driving force for filtrate formation, cannot be measured because of the inaccessibility of human glomeruli. We inferred that an approximate value for ΔP in the healthy human is 40 mmHg. Because there is no reabsorption along glomerular capillaries, the corresponding lower bound cannot be less than efferent oncotic pressure (πE), which we have estimated from πA and the filtration fraction to be approximately 30 mmHg13; however, we have repeatedly shown GFR to remain constant during hypervolemia-induced elevations of RPF by 15 to 25%.34–36 Such independence of GFR from RPF is typical of filtration pressure disequilibrium,33 from which we infer that ΔP is substantially higher than πE. We accordingly selected a ΔP of 40 mmHg as the “best guess” value for ΔP at the time of optimal function in our recipients of youthful allografts.37 Because arterial pressure was similar in recipients from aging and youthful donors, we assumed further that glomerular capillary pressure and, hence, corresponding ΔP was likely also 40 mmHg in recipients of aging allografts. To allow for the possibility that senescence-related glomerulopenia might result in glomerular hypertension in recipients of aging allografts, however, we also attempted to set a lower limit on Kf by assuming that ΔP might be elevated by 5 to 45 mmHg in this group. We next used measured values of GFR, RPF, πA, and the aforementioned assumed ΔP values to compute a corresponding range of values for whole-kidney Kf.
The modification of the Deen ultrafiltration model used assumes that, for the range of plasma protein concentrations observed in humans, the plasma oncotic pressure (π) is approximately proportional to the total protein concentration (C), as suggested by a previous study from our laboratory.13 That is, to the extent that protein loss is negligible, conservation of mass gives CE = CA/(1 − FF), where subscripts E and A refer to efferent and afferent arterioles. If, in addition, π = αC, where α is a constant for a given subject or sample, then it is also true that πE = πA /(1 − FF).13 If this is the case, then Kf can be estimated without knowing the value of α. With πA a linear function of C and ΔP a constant, a derivation like that in Deen et al.33 leads to
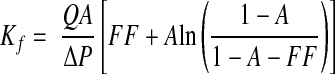 |
(4) |
where A = πA/ΔP. For whole-kidney inputs, QA is RPF rate and FF = GFR/QA. Thus, the procedure for estimating Kf is (1) assume a value of 40 or 45 mg for ΔP; (2) calculate A from ΔP and the measured πA; and (3) calculate Kf from A, the measured FF, the measured QA, and ΔP.
Computation of NFG
In an effort to compute the NFG in each allograft, we used the following equation:
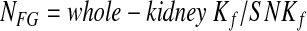 |
(5) |
where NFG is defined as the number of glomeruli that are not sclerosed, whole-kidney Kf is calculated using equation (4), and SNKf is calculated from the product of filtration S and glomerular hydraulic permeability (k) as described already. Because of uncertainty about the precision of the values assumed for ΔP in the calculation of whole-kidney Kf and of several assumptions used in the calculation of SNKf,22 our estimate of NFG should be regarded as an approximation only.
Statistical Analysis
Linear regression analysis was used to examine possible relationships between the GFR and its determinants on the one hand and age on the other. The t test or the Wilcoxon test was used to assess the difference in the GFR and its determinants between recipients of kidneys from youthful (<40 yr) and aging (>55 yr) deceased donors. Results are reported as the mean ± SD or, where distribution of findings was non-Gaussian, as median and range.
DISCLOSURES
None.
Acknowledgments
This study was supported by National Institutes of Health grants R01DK064697 and General Clinical Research Center grant M01-RR-00070 and by a grant from the John M. and Abby Sobrato Foundation. B.W.'s fellowship was supported by National Institutes of Health training grant 2 T32 DK07357.
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.U.S. Transplantation Data, United Network for Organ Sharing (UNOS), 2008
- 2.Davies DF, Shock NW: Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest 29: 496–507, 1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoang K, Tan JC, Derby G, Blouch KL, Masek M, Ma I, Lemley KV, Myers BD: Determinants of glomerular hypofiltration in aging humans. Kidney Int 64: 1417–1424, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Wesson LG: Renal hemodynamics in physiological states. In: Physiology of the Human Kidney, edited by Wesson LG, New York, Grune & Stratton, 1969, pp 96–108
- 5.Lindeman RD: Overview: Renal physiology and pathophysiology of aging. Am J Kidney Dis 16: 275–282, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Kappel B, Olsen S: Cortical interstitial tissue and sclerosed glomeruli in the normal human kidney, related to age and sex: A quantitative study. Virchows Arch A Pathol Anat Histol 387: 271–277, 1980 [DOI] [PubMed] [Google Scholar]
- 7.Kasiske BL: Relationship between vascular disease and age-associated changes in the human kidney. Kidney Int 31: 1153–1159, 1987 [DOI] [PubMed] [Google Scholar]
- 8.Ljungqvist A, Lagergren C: Normal intrarenal arterial pattern in adult and ageing human kidney: A microangiographical and histological study. J Anat 96: 285–300, 1962 [PMC free article] [PubMed] [Google Scholar]
- 9.Kasiske BL, Ma JZ, Louis TA, Swan SK: Long-term effects of reduced renal mass in humans. Kidney Int 48: 814–819, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Takazakura E, Sawabu N, Handa A, Takada A, Shinoda A, Takeuchi J: Intrarenal vascular changes with age and disease. Kidney Int 2: 224–230, 1972 [DOI] [PubMed] [Google Scholar]
- 11.Nyengaard JR, Bendtsen TF: Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 232: 194–201, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Pesce C: Glomerular number and size: Facts and artefacts. Anat Rec 251: 66–71, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Canaan-Kuhl S, Venkatraman ES, Ernst SI, Olshen RA, Myers BD: Relationships among protein and albumin concentrations and oncotic pressure in nephrotic plasma. Am J Physiol 264: F1052–F1059, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Saxena AB, Myers BD, Derby G, Blouch KL, Yan J, Ho B, Tan JC: Adaptive hyperfiltration in the aging kidney after contralateral nephrectomy. Am J Physiol Renal Physiol 291: F629–F634, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Samuel T, Hoy WE, Douglas-Denton R, Hughson MD, Bertram JF: Determinants of glomerular volume in different cortical zones of the human kidney. J Am Soc Nephrol 16: 3102–3109, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF: A stereological study of glomerular number and volume: Preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl S31–S17, 2003 [DOI] [PubMed]
- 17.Keller G, Zimmer G, Mall G, Ritz E, Amann K: Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Fogo A, Breyer JA, Smith MC, Cleveland WH, Agodoa L, Kirk KA, Glassock R: Accuracy of the diagnosis of hypertensive nephrosclerosis in African Americans: A report from the African American Study of Kidney Disease (AASK) Trial. AASK Pilot Study Investigators. Kidney Int 51: 244–252, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Drumond MC, Deen WM: Structural determinants of glomerular hydraulic permeability. Am J Physiol 266: F1–F12, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Drumond MC, Kristal B, Myers BD, Deen WM: Structural basis for reduced glomerular filtration capacity in nephrotic humans. J Clin Invest 94: 1187–1195, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chevalier RL, Forbes MS: Generation and evolution of atubular glomeruli in the progression of renal disorders. J Am Soc Nephrol 19: 197–206, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Gibson IW, Downie TT, More IA, Lindop GB: Atubular glomeruli and glomerular cysts: A possible pathway for nephron loss in the human kidney? J Pathol 179: 421–426, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Fulladosa X, Moreso F, Narvaez JA, Grinyo JM, Seron D: Estimation of total glomerular number in stable renal transplants. J Am Soc Nephrol 14: 2662–2668, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Tan JC, Alfrey EJ, Dafoe DC, Millan MT, Scandling JD: Dual-kidney transplantation with organs from expanded criteria donors: A long-term follow-up. Transplantation 78: 692–696, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Battilana C, Zhang HP, Olshen RA, Wexler L, Myers BD: PAH extraction and estimation of plasma flow in diseased human kidneys. Am J Physiol 261: F726–F733, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Alejandro V, Scandling JD Jr, Sibley RK, Dafoe D, Alfrey E, Deen W, Myers BD: Mechanisms of filtration failure during postischemic injury of the human kidney: A study of the reperfused renal allograft. J Clin Invest 95: 820–831, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW: Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weibel ER: Practical methods of biological morphometry. In: Sterological Methods, London, Academic Press, 1979, pp 1–415
- 29.Miller PL, Meyer TW: Effects of tissue preparation on glomerular volume and capillary structure in the rat. Lab Invest 63: 862–866, 1990 [PubMed] [Google Scholar]
- 30.Hladunewich MA, Lemley KV, Blouch KL, Myers BD: Determinants of GFR depression in early membranous nephropathy. Am J Physiol Renal Physiol 284: F1014–F1022, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Jensen EB, Gundersen HJ, Osterby R: Determination of membrane of thickness distribution from orthogonal intercepts. J Microsc 115: 19–33, 1979 [DOI] [PubMed] [Google Scholar]
- 32.Lafayette RA, Druzin M, Sibley R, Derby G, Malik T, Huie P, Polhemus C, Deen WM, Myers BD: Nature of glomerular dysfunction in pre-eclampsia. Kidney Int 54: 1240–1249, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Deen WM, Robertson CR, Brenner BM: A model of glomerular ultrafiltration in the rat. Am J Physiol 223: 1178–1183, 1972 [DOI] [PubMed] [Google Scholar]
- 34.Loon N, Chagnac A, Parra L, Schmidt K, Deen WM, Myers BD: Filtration dynamics and natriuretic response to volume expansion in humans. Am J Physiol 263: F284–F292, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Myers BD, Peterson C, Molina C, Tomlanovich SJ, Newton LD, Nitkin R, Sandler H, Murad F: Role of cardiac atria in the human renal response to changing plasma volume. Am J Physiol 254: F562–F573, 1988 [DOI] [PubMed] [Google Scholar]
- 36.Shemesh O, Deen WM, Brenner BM, McNeely E, Myers BD: Effect of colloid volume expansion on glomerular barrier size-selectivity in humans. Kidney Int 29: 916–923, 1986 [DOI] [PubMed] [Google Scholar]
- 37.Ramaswamy D, Corrigan G, Polhemus C, Boothroyd D, Scandling J, Sommer FG, Alfrey E, Higgins J, Deen WM, Olshen R, Myers BD: Maintenance and recovery stages of postischemic acute renal failure in humans. Am J Physiol Renal Physiol 282: F271–F280, 2002 [DOI] [PubMed] [Google Scholar]