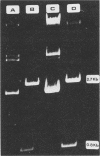
Abstract
We used the Salmonella typhi galactose epimerase (galE) mutant strain Ty21a, shown to be a safe, effective, living, attenuated oral typhoid vaccine, as a recipient for a recombinant plasmid containing the gene for production of the nontoxic B subunit of the heat-labile enterotoxin of Escherichia coli. The S. typhi derivative, strain SE12, produced heat-labile enterotoxin subunit B that was structurally and immunologically indistinguishable from heat-labile enterotoxin subunit B produced by strains of E. coli harboring the same plasmid. Tests in mice and guinea pigs showed that strain SE12 was safe when given orally and was capable of inducing a significant antitoxic antibody response when injected parenterally. Moreover, it retained the galactose sensitivity of the parent strain, preserving its utility as a typhoid vaccine. This strain may prove to be a useful live oral bivalent vaccine strain for typhoid fever and cholera-E. coli-related diarrheas.
Full text
PDF

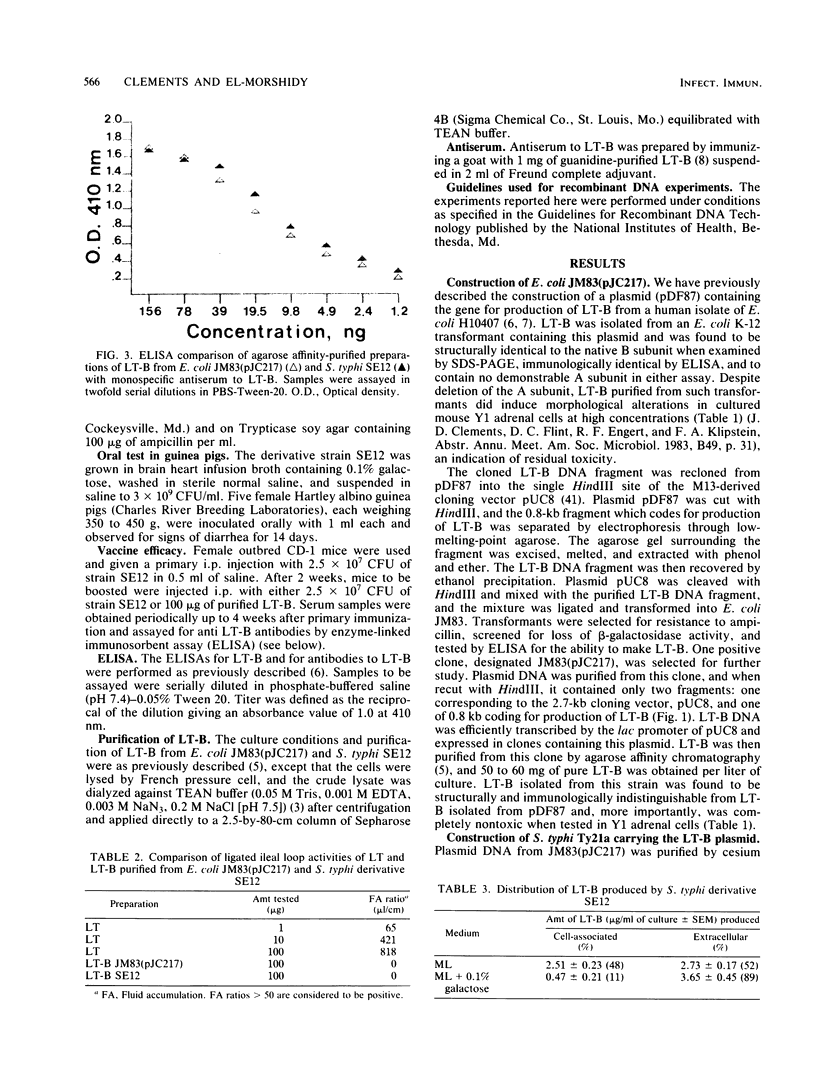
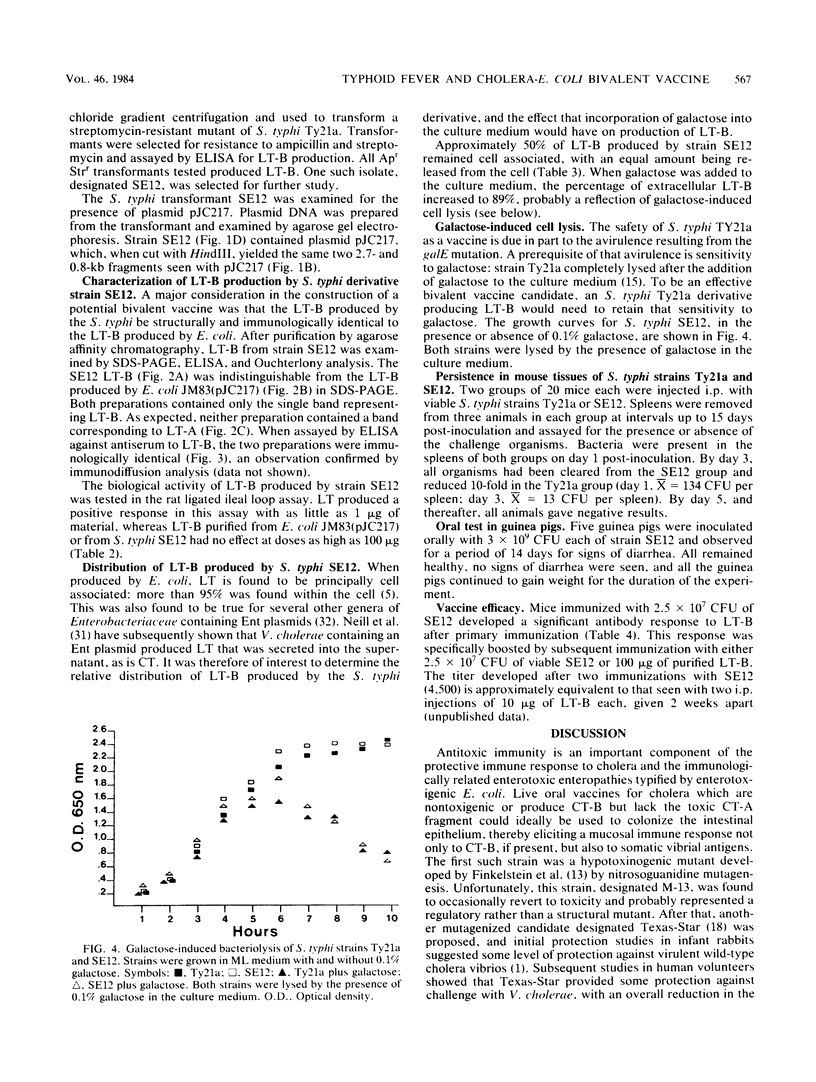


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boesman-Finkelstein M., Finkelstein R. A. Protection in rabbits induced by the Texas Star-SR attenuated A-B+ mutant candidate live oral cholera vaccine. Infect Immun. 1982 Apr;36(1):221–226. doi: 10.1128/iai.36.1.221-226.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Demonstration of shared and unique immunological determinants in enterotoxins from Vibrio cholerae and Escherichia coli. Infect Immun. 1978 Dec;22(3):709–713. doi: 10.1128/iai.22.3.709-713.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Immunological cross-reactivity between a heat-labile enterotoxin(s) of Escherichia coli and subunits of Vibrio cholerae enterotoxin. Infect Immun. 1978 Sep;21(3):1036–1039. doi: 10.1128/iai.21.3.1036-1039.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979 Jun;24(3):760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Flint D. C., Engert R. F., Klipstein F. A. Cloning and molecular characterization of the B subunit of Escherichia coli heat-labile enterotoxin. Infect Immun. 1983 May;40(2):653–658. doi: 10.1128/iai.40.2.653-658.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Flint D. C., Klipstein F. A. Immunological and physicochemical characterization of heat-labile enterotoxins isolated from two strains of Escherichia coli. Infect Immun. 1982 Nov;38(2):806–809. doi: 10.1128/iai.38.2.806-809.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Yancey R. J., Finkelstein R. A. Properties of homogeneous heat-labile enterotoxin from Escherichia coli. Infect Immun. 1980 Jul;29(1):91–97. doi: 10.1128/iai.29.1.91-97.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Vasil M. L., Holmes R. K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- Formal S. B., Baron L. S., Kopecko D. J., Washington O., Powell C., Life C. A. Construction of a potential bivalent vaccine strain: introduction of Shigella sonnei form I antigen genes into the galE Salmonella typhi Ty21a typhoid vaccine strain. Infect Immun. 1981 Dec;34(3):746–750. doi: 10.1128/iai.34.3.746-750.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanier R., Füer E. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975 May;131(5):553–558. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- Gilman R. H., Hornick R. B., Woodard W. E., DuPont H. L., Snyder M. J., Levine M. M., Libonati J. P. Evaluation of a UDP-glucose-4-epimeraseless mutant of Salmonella typhi as a liver oral vaccine. J Infect Dis. 1977 Dec;136(6):717–723. doi: 10.1093/infdis/136.6.717. [DOI] [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- Honda T., Finkelstein R. A. Selection and characteristics of a Vibrio cholerae mutant lacking the A (ADP-ribosylating) portion of the cholera enterotoxin. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2052–2056. doi: 10.1073/pnas.76.4.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Levine M. M. Cloned cholera enterotoxin genes in study and prevention of cholera. Lancet. 1981 Nov 21;2(8256):1162–1163. doi: 10.1016/s0140-6736(81)90605-x. [DOI] [PubMed] [Google Scholar]
- Kaper J. B., Lockman H., Baldini M. M., Levine M. M. Recombinant nontoxinogenic Vibrio cholerae strains as attenuated cholera vaccine candidates. Nature. 1984 Apr 12;308(5960):655–658. doi: 10.1038/308655a0. [DOI] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Clements J. D. Arousal of mucosal secretory immunoglobulin A antitoxin in rats immunized with Escherichia coli heat-labile enterotoxin. Infect Immun. 1982 Sep;37(3):1086–1092. doi: 10.1128/iai.37.3.1086-1092.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Clements J. D. Development of a vaccine of cross-linked heat-stable and heat-labile enterotoxins that protects against Escherichia coli producing either enterotoxin. Infect Immun. 1982 Aug;37(2):550–557. doi: 10.1128/iai.37.2.550-557.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Clements J. D., Houghten R. A. Differences in cross-protection in rats immunized with the B subunits of cholera toxin and Escherichia coli heat-labile toxin. Infect Immun. 1984 Mar;43(3):811–816. doi: 10.1128/iai.43.3.811-816.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Clements J. D., Houghten R. A. Vaccine for enterotoxigenic Escherichia coli based on synthetic heat-stable toxin crossed-linked to the B subunit of heat-labile toxin. J Infect Dis. 1983 Feb;147(2):318–326. doi: 10.1093/infdis/147.2.318. [DOI] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Clements J. D. Immunization of rats with heat-labile enterotoxin provides uniform protection against heterologous serotypes of enterotoxigenic Escherichia coli. Infect Immun. 1981 Jun;32(3):1100–1104. doi: 10.1128/iai.32.3.1100-1104.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Nalin D. R., Hoover D. L., Bergquist E. J., Hornick R. B., Young C. R. Immunity to enterotoxigenic Escherichia coli. Infect Immun. 1979 Mar;23(3):729–736. doi: 10.1128/iai.23.3.729-736.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Neill R. J., Ivins B. E., Holmes R. K. Synthesis and secretion of the plasmid-coded heat-labile enterotoxin of Escherichia coli in Vibrio cholerae. Science. 1983 Jul 15;221(4607):289–291. doi: 10.1126/science.6857285. [DOI] [PubMed] [Google Scholar]
- Neill R. J., Twiddy E. M., Holmes R. K. Synthesis of plasmid-coded heat-labile enterotoxin in wild-type and hypertoxinogenic strains of Escherichia coli and in other genera of Enterobacteriaceae. Infect Immun. 1983 Sep;41(3):1056–1061. doi: 10.1128/iai.41.3.1056-1061.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrup R. S., Chisari F. V. Response of monkeys to immunization with cholera toxoid, toxin, and vaccine: reversion of cholera toxoid. J Infect Dis. 1972 May;125(5):471–479. doi: 10.1093/infdis/125.5.471. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Chen M. E., Holmes R. K., Kaper J., Levine M. M. Environmental and human isolates of Vibrio cholerae and Vibrio parahaemolyticus produce a Shigella dysenteriae 1 (Shiga)-like cytotoxin. Lancet. 1984 Jan 14;1(8368):77–78. doi: 10.1016/s0140-6736(84)90006-0. [DOI] [PubMed] [Google Scholar]
- Peterson J. W., Hejtmancik K. E., Markel D. E., Craig J. P., Kurosky A. Antigenic specificity of neutralizing antibody to cholera toxin. Infect Immun. 1979 Jun;24(3):774–779. doi: 10.1128/iai.24.3.774-779.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack D. A., Sack R. B. Test for enterotoxigenic Escherichia coli using Y-1 adrenal cells in miniculture. Infect Immun. 1975 Feb;11(2):334–336. doi: 10.1128/iai.11.2.334-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Ahrén C. Immune protection against enterotoxinogenic E. coli: search for synergy between antibodies to enterotoxin and somatic antigens. Acta Pathol Microbiol Immunol Scand C. 1982 Feb;90(1):1–6. doi: 10.1111/j.1699-0463.1982.tb01409.x. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M., Sack D. A., Holmgren J., Bardhan P. K. Intestinal antibody responses after immunisation with cholera B subunit. Lancet. 1982 Feb 6;1(8267):305–308. doi: 10.1016/s0140-6736(82)91568-9. [DOI] [PubMed] [Google Scholar]
- Tokunaga E., Cray W. C., Jr, Pierce N. F. Compared colonizing and immunizing efficiency of toxinogenic (A+ B+) Vibrio cholerae and an A- B+ mutant (Texas Star-SR) studied in adult rabbits. Infect Immun. 1984 May;44(2):364–369. doi: 10.1128/iai.44.2.364-369.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C., Chung R., Berman S., Keren D., Kapfer C., Formal S. B. Safety and antigenicity of typhoid-Shigella sonnei vaccine (strain 5076-1C). J Infect Dis. 1984 Feb;149(2):133–136. doi: 10.1093/infdis/149.2.133. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wahdan M. H., Serie C., Germanier R., Lackany A., Cerisier Y., Guerin N., Sallam S., Geoffroy P., el Tantawi A. S., Guesry P. A controlled field trial of liver oral typhoid vaccine Ty21a. Bull World Health Organ. 1980;58(3):469–474. [PMC free article] [PubMed] [Google Scholar]