Abstract
Mortality differences between peritoneal dialysis (PD) and hemodialysis (HD) are widely debated. In this study, mortality was compared between patients treated with PD and HD (including home HD) using data from 27,015 patients in the Australia and New Zealand Dialysis and Transplant Registry, 25,287 of whom were still receiving PD or HD 90 d after entry into the registry. Overall mortality rates were significantly lower during the 90- to 365-d period among those being treated with PD at day 90 (adjusted hazard ratio [HR] 0.89; 95% confidence interval [CI] 0.81 to 0.99]; P < 0.001). This effect, however, varied in direction and size with the presence of comorbidities: Younger patients without comorbidities had a mortality advantage with PD treatment, but other groups did not. After 12 mo, the use of PD at day 90 was associated with significantly increased mortality (adjusted HR 1.33; 95% CI 1.24 to 1.42; P < 0.001). In a supplementary as-treated analysis, PD treatment was associated with lower mortality during the first 90 d (adjusted HR 0.67; 95% CI 0.56 to 0.81; P < 0.001). These data suggest that the effect of dialysis modality on survival for an individual depends on time, age, and presence of comorbidities. Treatment with PD may be advantageous initially but may be associated with higher mortality after 12 mo.
The merits of peritoneal dialysis (PD) compared with hemodialysis (HD) therapy have been extensively debated.1 A key factor is the relative mortality between modalities. The ideal vehicle for comparison is a randomized, controlled trial. Previous attempts at such trials were complicated by lack of statistical power2 and poor recruitment.3
In the absence of trial evidence, large observational studies are of value, although they do not directly offer evidence of causality. Several registry-based or multicenter studies have been performed but with conflicting results4–17; however, these studies were performed in the United States or Europe, where rates of PD are generally lower than those in Australia and New Zealand.18
Across Australia and New Zealand, PD is used to treat between 20 and 40% of prevalent dialysis patients. Both modalities are available in all major treatment centers. Thus, selection bias toward PD either at a patient or a unit level is less likely than in countries with lower PD usage.
RESULTS
Between October 1, 1991, and December 31, 2005, 28,460 people commenced renal replacement therapy (RRT) in Australia or New Zealand. From this group were excluded recipients of preemptive kidney transplants (n = 695), those who were younger than 15 yr at RRT start (n = 370), those with a missing body mass index (BMI; n = 193), an extreme BMI (<15 [n = 89] or ≥50 kg/m2 [n = 98]), and those who died (n = 671) or received a transplant in the first 90 d of RRT (n = 301) or had <90 d of follow-up recorded (n = 692) or a missing dialysis modality at 90 d (n = 64). This left 25,287 people for analysis, with 68,020 person-years of follow-up (Table 1). This included 44,679 person-years of HD treatment (including 7525 yr of home HD) and 23,341 person-years of PD (including 4234 yr of ambulatory PD). There were 11,066 deaths, with an overall mortality rate of 16.3 (95% confidence interval [CI] 16.0 to 16.6) per 100 person-years. There were differences in the prevalence of several comorbidities between dialysis modalities. The group receiving PD treatment at 90 d (PD90) was older and more commonly obese. Coronary artery disease (CAD), type 2 diabetes, and peripheral vascular diseases wall ere more common among the PD90 group. Smoking was less common among the PD90 group, as was late referral. There was a temporal trend toward a decreasing frequency of PD90. The proportion of PD90 in each treating unit ranged from 0 to 76% with a median value of 43%.
Table 1.
Characteristics of cohort by dialysis modality at 90 d after first RRTa
| Characteristic | HD (n = 14,733; 39,163 person-years) | PD (n = 10,554; 28,857 person-years) | P |
|---|---|---|---|
| Person-years on PD (from day 90) | 2481 (6%) | 20,860 (72%) | |
| Modality at day 0 (among those on PD or HD at day 90) | 14,353 HD (97%) | 7042 (67%) PD | |
| Age (yr; median [IQR]) | 59.2 (46.6 to 69.9) | 61.1 (48.8 to 69.8) | <0.001 |
| BMI | 1461 (10%) | 1131 (11%) | <0.001 |
| underweight | 5326 (36%) | 4055 (38%) | |
| normal | 4605 (31%) | 3401 (32%) | |
| overweight | 3341 (23%) | 1967 (19%) | |
| obese | |||
| Male | 9189 (62%) | 5574 (53%) | <0.001 |
| CAD | 5416 (37%) | 4282 (41%) | <0.001 |
| Diabetes | <0.001 | ||
| type 1 | 484 (4%) | 595 (6%) | |
| type 2 | 4398 (30%) | 3380 (32%) | |
| Chronic lung disease | 2220 (15%) | 1568 (15%) | 0.600 |
| Current cigarette smoking (at time of RRT start) | 2082 (14%) | 1328 (13%) | <0.001 |
| Cerebrovascular disease | 1933 (13%) | 1641 (16%) | <0.001 |
| Peripheral vascular disease | 3480 (24%) | 3027 (29%) | <0.001 |
| Late referral | 3736 (25%) | 2420 (23%) | <0.001 |
| Indigenous | |||
| ATSI | 1275 (9%) | 567 (5%) | <0.001 |
| Maori/PI | 1093 (7%) | 1409 (13%) | |
| Serum creatinine (quartiles at RRT start)b | <0.001 | ||
| <581 | 2594 (27%) | 1883 (31%) | |
| 581 to 750 | 2475 (25%) | 1688 (28%) | |
| 751 to 950 | 2398 (25%) | 1362 (22%) | |
| ≥951 | 2321 (24%) | 1144 (19%) | |
| Serum creatinine (μmol/L)c | 673 (667 to 679) | 644 (638 to 651) | <0.001 |
| eGFR (ml/min per 1.73 m2)d | 7.10 (7.03 to 7.18) | 7.27 (7.17 to 7.37) | <0.010 |
ATSI, Aboriginal and Torres Strait Islander; eGFR, estimated GFR; IQR, interquartile range; PI, Pacific Islander.
Only for those who started RRT from April 1, 1998; n = 15865 with reported creatinine.
Harmonic mean.
Arithmetic mean.
Prognosis by Dialysis Modality at 90 D
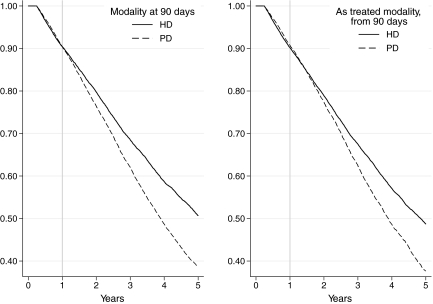
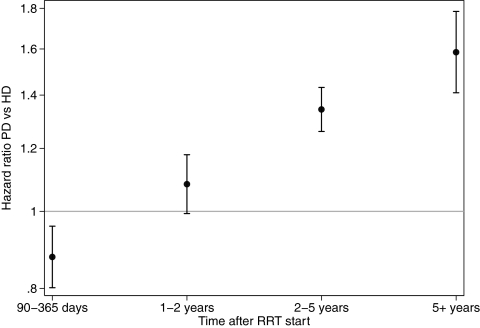
In univariate analysis, survival of the PD90 group was inferior to those receiving HD (P < 0.0001, log-rank test), but this risk was not constant over time (Figure 1). The follow-up period was divided into 90 to 365 d and 1 to 2, 2 to 5, and ≥5 years after the start of RRT. In univariate analyses, mortality was similar in the first 12 mo (P = 0.85, log-rank test). In the period beyond 12 mo, there was a significantly higher mortality among those treated with PD at 90 d (P < 0.0001, log-rank test).
Figure 1.
Kaplan-Meier survival curves, censored at transplantation, by RRT modality at 90 d (left) and as-treated modality, from 90 d. In the as-treated analyses, patients moved between groups at the date of treatment modality change. We then calculated the survivor function using the risk group at the point of each failure event.
In multivariate analyses, there was lower mortality risk in the first year among those treated with PD at 90 d; however, from 1 yr, the PD group had an increased mortality risk (Table 2). This increase was similar across all periods after 12 mo. A subanalysis (data not shown) restricted to those with serum creatinine at commencement (i.e., commenced RRT from April 1, 1998) showed similar results.
Table 2.
HR for mortality associated with PD compared with HD using various methods
| Approach | HR for PD Compared with HD
|
|||
|---|---|---|---|---|
| 90 to 365 d
|
≥366 d
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Multivariate Cox model: Treatment at 90 d | 0.80 (0.81 to 0.96) | 0.004 | 1.32 (1.26 to 1.38) | <0.001 |
| Multivariate Cox model: Treatment modality as time-varying factor | 0.82 (0.75 to 0.81) | <0.001 | 1.29 (1.23 to 1.35) | <0.001 |
| Propensity score: Matched cohort | 0.99 (0.89 to 1.10) | 0.800 | 1.35 (1.27 to 1.42) | <0.001 |
| Weighted multivariate Cox model: inverse probability of treatment weight | 0.93 (0.83 to 1.03) | 0.180 | 1.31 (1.24 to 1.38) | <0.001 |
Two-Way Interactions
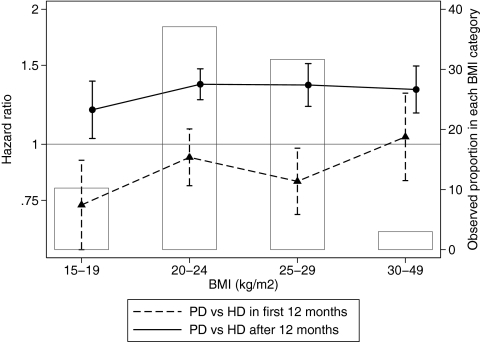
In view of reports of interactions between BMI and dialysis modality, we stratified the analysis by BMI group. There was no interaction with 90- to 365-d mortality (P = 0.2 for interaction). A statistically significant interaction was observed with ≥12 mo mortality (P = 0.0018), although the effect size was clinically similar across all BMI categories (Figure 2). There was no interaction between the presence of diabetes at RRT start and the adjusted hazard ratio (HR) for PD:HD in either time period (P = 0.6 and 0.4, respectively).
Figure 2.
HR for mortality during PD versus during HD, by modality at 90 d, stratified by BMI at RRT start. ▴, Risk (95% CI) for death within 12 mo of RRT start; •, risk for death ≥12 mo after dialysis start. Also shown is the proportion of the cohort in each BMI category.
There was a statistically significant interaction between vintage and the multivariate adjusted HR in the 90- to 365-d period (P = 0.03) and the adjusted HR from 1 yr (P = 0.01); however, this variation was of little clinical significance (Figure 3). There was also an interaction between age and the risk of PD for mortality in the 90- to 365-d period (P < 0.001) but not in the >1-yr period (P = 0.7). There was no statistically significant interaction of either the 90- to 365-d mortality or the ≥12 mo mortality with the proportion of PD treatment (at 90 d, divided at the median) within a treating renal unit.
Figure 3.
HR for PD versus HD by year of starting dialysis. ▴, Risk (95% CI) for death within 12 mo of RRT start; •, risk for death ≥12 mo after dialysis start. The numbers above the x axis indicate the number in each subcohort.
Three-Way Interactions
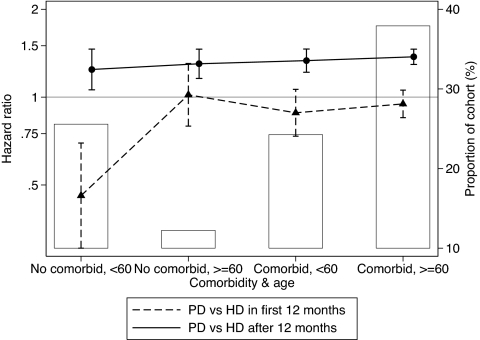
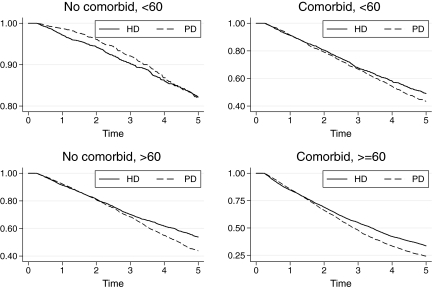
Three-way interactions were specifically sought of the PD risk with age and diabetes and with age and the presence of any comorbidity (including diabetes). These were explored by combining age categories (split at 60 yr) with diabetes or any comorbidity as appropriate. There was no three-way interaction between dialysis modality and age and diabetes either in the first 12 mo (P = 0.3) or subsequently (P = 0.4); however, there was a statistically and clinically significant interaction among PD risk, age, and comorbidity: The benefit of PD in the first 12 mo was particularly great in the <60-yr group without comorbidities (Figure 4). The effect of this interaction is illustrated in Figure 5. The balance of risks in the first and later years leads to differing “crossover” points in each group.
Figure 4.
Risk of PD compared with HD stratified by age and the presence of any comorbidity. ▴, Risk (95% CI) for death within 12 mo of RRT start; •, risk for death ≥12 mo after dialysis start. There is statistically significant variation in the risk for death in the 90- to 365-d group (P = 0.005 for interaction) but not for the >1-yr group (P = 0.5 for interaction).
Figure 5.
Kaplan-Meier graph by modality of treatment at 90 d, by presence of any comorbidity and age group at RRT start. ▴, Risk (95% CI) for death within 12 mo of RRT start; •, risk for death ≥12 mo after dialysis start. Graphs are adjusted for vintage (to year 2000) and gender (to equal male and female numbers). Note that the y axes differ between panels.
Interactions with indigenous racial origin were also examined within each group. The only statistical significant variation was between the HR for death within 12 mo among thegroup with comorbidity <60 yr and with diabetes <60 yr; however, neither of these was clinically significant (data not shown).
Prognosis by “As Treated” Dialysis Modality
When examined using the dialysis modality actually delivered as a time-dependent variable, there was a progressive increase in the risk associated with PD over time (Figure 6). Sensitivity analyses in which analyses were repeated with modality changes occurring within 30 or 60 d of death ignored and death attributed to the previous modality showed a similar pattern of results.
Figure 6.
HR for mortality for patients treated with PD compared with HD, treating modality as a time-dependent covariate (i.e., analyses of modality as treated). HR are adjusted for all measured covariates and derived from shared frailty Cox model.
Propensity Score Approaches
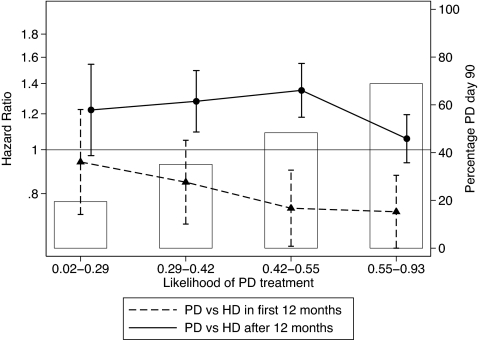
The logistic regression models used covariates for BMI, age, gender, race (nonindigenous/Aboriginal and Torres Strait Islander/Maori and Pacific Islander), comorbidities (CAD, peripheral vascular disease, chronic lung disease, diabetes), late referral, country of initial treatment (Australia/New Zealand), and vintage (in 2-yr blocks). This logistic regression model initially had only modest fit: The area under the receiver operating characteristic was 0.64 but improved to 0.71 with inclusion of treating center. The overall predicted probability of PD was only slightly different between those treated with PD and those treated with HD. The HR for mortality with PD did not vary across quartiles of predicted PD probability in the 90–365 day period; from 366 days onward there was some statistical although not clinical significant variation between quartiles groups (P = 0.67 for interaction with mortality 90 to 365 d and P = 0.03 for interaction with ≥12 mo mortality; Figure 7).
Figure 7.
HR for mortality associated with PD treatment compared with HD, by quartile of predicted probability of PD treatment at 90 d. ▴, Risk (95% CI) for death within 12 mo of RRT start; •, risk for death ≥12 mo after dialysis start. The bars indicate the observed proportion of people who received PD at 90 d in each quartile.
Using nearest-neighbor matching, we conducted analyses among a matched cohort of 16,791 people (10,542 PD at 90 d and 6249 HD at 90 d). There was no difference in mortality over 90 to 365 d, but after 12 mo, there was excess mortality in the PD group, similar to the other approaches (Table 2).
Inverse Probability of Treatment Weights
Weighting of observations by the inverse probability of treatment led to similar results as other techniques (Table 2).
Outcomes among Modality “Switchers”
The group with the lowest mortality during the 90- to 365-d period were those who were treated with PD at both day 0 and day 90; the groups who started with HD had similar results in this period regardless of whether they stayed on HD or switched to PD, whereas those who began on PD and switched to HD had a higher mortality in the 90- to 365-d period (Table 3). In contrast, after 1 yr, the group who were treated with HD at both day 0 and day 90 had the lowest mortality, with the other three combinations similar to each other (Table 3).
Table 3.
Adjusted HR for mortality by dialysis modality at first treatment and 90 d
| Parameter | HD at Day 0
|
PD at Day 0
|
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| 90 to 365 d | ||||
| HD at day 90 | 1.00 (referent) | 1.36 (1.04 to 1.78) | 0.030 | |
| PD at day 90 | 1.09 (0.97 to 1.23) | 0.140 | 0.87 (0.78 to 0.97) | 0.010 |
| ≥366 d | ||||
| HD at day 90 | 1.00 (referent) | 1.13 (0.95 to 1.34) | 0.160 | |
| PD at day 90 | 1.34 (1.26 to 1.43) | <0.010 | 1.28 (1.22 to 1.31) | <0.001 |
Among those who survived to 1 yr, subsequent mortality risk was examined by modality at 90 and 365 d. Compared with those who were on HD treatment both at 90 and 365 d, all other groups had an increased risk, particularly those who changed from HD at 90 d to PD by 365 d (Table 4).
Table 4.
Adjusted HR for mortality by dialysis modality at 90 and 366 da
| Parameter | HD at Day 90
|
PD at Day 90
|
Totals | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| HD at 1 yr | HR = 1.0 (reference) | HR = 1.24 (1.13–1.37) | n = 11,424 | ||
| n = 10,466 | n = 958 | <0.001 | |||
| PD at 1 yr | HR = 1.56 (1.41–1.87) | HR = 1.38 (1.31–1.44) | n = 8375 | ||
| n = 856 | <0.001 | n = 7519 | <0.001 | ||
| Total | n = 11,322 | n = 8477 | n = 19,799 | ||
Total n = 19,799, because only 1-yr survivors are included. HR are relative to people who were receiving HD at day 90 and 1 yr. All of theses risks significantly differed from each other in addition to the HD90/HD365 group. These risks did not significantly interact with BMI categories (P = 0.2 for interaction) or with comorbidity and age (P = 0.14 for interaction).
The First 90 D
In a further supplementary analysis, we also examined the relationship between “as treated” modality and outcome in the first 90 d. A total of 27,015 people fit the other inclusion criteria and commenced RRT; among whom there were 671 deaths before 90 d at a rate of 10.3 (95% CI 9.6 to 11.2) per 100 person-years. In this period, PD treatment was associated with lower mortality (adjusted HR 0.67; 95% CI 0.56 to 0.81; P < 0.001), consistent with the trends shown over later time periods (Figure 6).
DISCUSSION
In a large registry-based data set, we showed mortality rates are lower in some groups in the 90- to 365-d period in the PD90 group than those receiving HD, especially when they were treated with PD from day 0. From 12 mo after dialysis start, however, the mortality risk was consistently higher among the PD group. This was shown in an environment that has a higher prevalence of PD treatment and where PD-treated patients tend to have more comorbidities, in contrast to most other studies. Among younger patients (<60 yr) without comorbidity or diabetes, the PD90 group experienced better survival over 90 to 365 d. Of those who survived the first year, all comorbidity/age groups showed a higher mortality rate among those treated with PD from 12 mo, although the size of this effect was lesser among the patients who were younger than 60 yr at RRT start and had no comorbidity. The balance of these effects was such that, among the patients who were younger than 60 yr, had no comorbidities, and were treated with PD at 90 d, the initial survival advantage persisted until 4 yr. If the results of the supplementary analysis are valid, then this “crossover” would be further affected by the increased mortality among those treated with HD in the first 90 d.
Previous publications in this field have shown varying and conflicting results. Three articles, based on US Renal Data System (USRDS) data sets, also showed interactions with comorbidities and follow-up time.5,19,20 An area that has received particular attention is the interaction between obesity and RRT modality: Analysis of a large incident US cohort showed higher mortality rates among PD patients within the upper three BMI quintiles, apparent from 6 to 24 mo after dialysis initiation.19 In contrast, in our analysis, the association of PD at 90 d with poorer survival was seen across all BMI groups from 12 mo. Comorbidities were also studied in a similar USRDS cohort, with similar results to those seen here. Ganesh et al.20 found higher mortality risk associated with PD among patients who had diabetes with and without CAD and patients who did not have diabetes and had CAD but not in patients who did not have diabetes or CAD. Despite the similarities in results to those of our study, there were substantial differences in the composition of the US cohort (including a lower PD prevalence of 13%).19,20 Similar conclusions were drawn from an analysis of a smaller cohort, also based on USRDS.21 A higher mortality risk with PD in the 7- to 12-mo period was seen among patients with but not without diabetes in an inception cohort of ≥65-yr-old people in New Jersey, although the study power did not exclude an effect size similar to ours.22 A different approach to confounding—restriction—was taken by Inrig et al.,23 whose cohort was limited to those accepted onto the transplant waiting list. Not surprising, the absolute mortality rates were lower, but worse outcomes remained among people treated with PD, again with an interaction with obesity. In a separate (non-USRDS) study, Jaar et al.14 examined a cohort of 1041 patients in 81 clinics in the United States and showed no mortality difference in the first year of treatment but an excess mortality from that point among those treated with PD.
Comparisons in other countries have shown varying results. A study of 2284 patients in Romania did not show any mortality difference among older patients but did show greater mortality among younger patients who had diabetes and were treated with HD.24 Others have shown a similar pattern of progressive increase over time in PD compared with HD. A Danish registry study showed improved mortality in the first 2 yr among those treated with PD, with equivalent results thereafter.25 An earlier Canadian registry–based study showed lower mortality rates among PD- compared with HD-treated patients among an incident cohort of 11,970 people.13 A recent Dutch registry study16 showed a substantial benefit to PD in early treatment among younger people without diabetes, with lesser benefit among other groups, with a subsequent change to increased longer term mortality associated with PD. Other groups have attempted comparisons but were limited by small numbers.7,26,27
Many studies have shown a changing risk over time, with an increase in the mortality risk of PD compared with HD. Several factors may influence this; better preservation of residual renal function associated with PD may explain this.28 Residual renal function has been associated with a lower risk for death among PD29,30 and HD patients.31 In our data, the better survival among the PD90/HD365 group compared with the PD90/PD365 group was not consistent with this.
Another factor might be catheter use among HD patients; use of both tunneled and nontunneled catheters is associated with a higher mortality rate.32 Rates of catheter use among incident and prevalent patients in Australia and New Zealand have increased in recent years but are still low by international standards.33 Lower catheter rates than in other studies might explain an advantage for HD over PD and also a trend toward HD over time since RRT start (as catheter use falls). This would be consistent with the higher mortality rate of the group who began RRT on HD then switched to PD at 90 d compared with those who began and stayed on PD.
A third possible factor is the start point of the cohort: Exclusion of patients who did not survive 90 d may exclude patients at risk for early mortality; if this group is more likely to be treated with HD, then this would tend to advantage HD. This is consistent with our supplementary analysis. Whether this is due to selection bias or technique issues (i.e., catheter use) is not clear; however, the Australian and New Zealand Dialysis and Transplant (ANZDATA) Registry does not collect details of residual renal function or dates of catheter use among people receiving HD to examine possible mechanisms.
Although we did examine the relationship between as-treated modality and outcomes in the first 90 d, there are substantial and important limitations to analysis of this period. Inclusion in the ANZDATA Registry is based on a clinical diagnosis of end-stage kidney disease (ESKD) with an “intent” of long-term RRT. Thus, situations of prolonged acute renal failure may be included or cases of true ESKD not included (e.g., if they present as acute on chronic renal failure and die) and bias comparisons in the first 90 d in this data set. The lower reported mortality in this period emphasizes the difficulty in interpreting outcomes during this early period in this data set. In addition, there was substantial instability of modality—people who commence HD and then change to PD by 90 d. Examination of this early period is an important issue but one that will require more detailed cohort definition and data collection than that available from the ANZDATA Registry to ensure an unbiased comparison. The difference in renal function at the commencement of dialysis suggests those treated with PD started RRT earlier in their disease course, suggesting “lead-time bias”34; however, this would tend to favor better survival among the PD group and thus does not explain our findings.
Like all cohort studies, our study is subject to biases that affect the comparability of our results with others. Treated ESKD incidence rates in Australia are approximately 100 per million per year, substantially lower than in the United States and slightly lower than many European countries.35 The nature of our cohort does reduce some sources of bias: We included all patients who began RRT in either country during the time period, including centers with varying rates of PD and transplantation. Ultimately, statistical techniques can adjust only for measured factors, and the presence of unmeasured confounders in registry-based studies with limited comorbidity ascertainment is possible.
The results from the propensity score analyses were similar to those from the principal analyses. This is similar to other instances in which the use of propensity scores seems to have little effect on inferences drawn from observational studies.36 The modest fit of the logistic model emphasizes that there are likely to be other unmeasured factors relevant to the outcome; however, the absence of an interaction of the dialysis modality–associated risk with the predicted probability of PD lends a clinical interpretation that the difference in outcomes applies to all types of patients: Those for whom PD is commonly used and those “harder” patients, for whom it is less used.
There are many reasons behind choice of dialysis modality, including quality of life, patient satisfaction, local practice and expertise, funding, and geography; however, mortality is a key consideration. From this perspective, for young patients without comorbidities, PD is a reasonable short- and medium-term therapy that seems to provide a survival advantage compared with HD during the first few years. For other groups, no initial survival advantage of PD relative to HD is apparent, and PD was associated with survival disadvantage after the first 1 to 2 yr. Whether this is causal cannot be inferred from an observational study, but our data suggest caution in the use of PD in many patients, particularly when this therapy is continued beyond 1 to 2 yr. The potential for bias in analyses of observational cohorts underlies the need for randomized trials. A further attempt at such a trial is in early stages.37
Despite their weaknesses, large registry-based cohort studies are likely to remain the best available form of evidence to guide dialysis modality choice for some time. A more detailed data collection and more precise definitions, likely part of a prospectively designed cohort study, will be needed to address more accurately issues of mortality within the first 90 d of commencing RRT.
CONCISE METHODS
We examined outcomes for all patients in the ANZDATA Registry who commenced dialysis from October 1, 1991 to October 1, 2005, comprising all incident patients with ESKD in Australia and New Zealand during that time. The registry includes all those who receive RRT in Australia and New Zealand with the intention of chronic treatment.
RRT modality was classified into HD (including hospital-, satellite-, and home-based HD), PD (including continuous ambulatory PD and automated PD), and transplantation. The dates of commencement and cessation of each modality were retrieved, with information available to December 31, 2005. Brief changes from PD to HD as dialysis modality (<30 d) are not recorded, because they are assumed to be temporary interruptions related to peritonitis or technical events. Other information available included mortal status at December 31, 2005, and comorbid conditions at commencement of RRT (reported as the presence of CAD, peripheral vascular disease, cerebrovascular disease, and chronic lung disease and presence and type of diabetes) together with cigarette smoking. Late nephrologist referral (<6 mo before dialysis start for those who commenced from April 1, 1994, to March 31, 1997, and <3 mo before dialysis for those who started after April 1, 1997) was collected for those who commenced from April 1, 1994. From height and weight at commencement of dialysis, BMI was calculated and categorized into underweight (<20 kg/m2), normal (20 to 24.9 kg/m2), overweight (25 to 29.9 kg/m2), and obese (≥30 kg/m2). Serum creatinine concentration at commencement of RRT was collected prospectively only for people commencing RRT after April 1, 1998. A subanalysis was performed for those commencing after this date.
Patients who survived <90 d from the date of initial treatment were excluded from the main analyses. Survival analyses used Cox regression. Models were built using backward stepwise elimination and a threshold of 0.10 for retention. Unmeasured variation between treating centers was addressed by using a shared frailty Cox model, clustered by center. Records were censored at the date of first transplantation or December 31, 2005.
Six approaches to analysis were used. For the first two, we considered dialysis modality in two ways: As the modality in use at 90 d after initial treatment and as a time-dependent variable. These are analogous an intention-to-treat and as-treated analyses. For approaches 3 and 4, we used a propensity score method. We created a multiple logistic regression model with dialysis modality at 90 d as the dependent variable. All of the covariates available were used in creating this model.38 We then calculated a predicted probability of PD treatment for each person (PS). We created models with and without the treating center as a covariate. For the third approach, we compared HD and PD groups (using modality in use at 90 d) in Cox models within PS quartiles on the basis of the predicted probabilities. In the fourth approach to analysis, we created a matched data set using the PS using “nearest neighbor” matching (where the PS was within 0.05). In approach 5, we performed comparisons between the PD and HD groups after the weighting by the inverse probability of treatment (at 90 d) for each individual.39 This was implemented using the “iweight” facility of Stata. Finally, in approach 6, we compared outcomes among those whose dialysis modality at 12 mo differed from that at 90 d and those who started and remained on PD with those who remained on HD at both time points. In a supplementary analysis, we examined outcomes among the cohort in the first 90 d using the as-treated approach; the other inclusion criteria and methods were unchanged for this analysis.
DISCLOSURES
S.P.M. has received speaking honoraria from AMGEN Australia, Fresenius Australia, and Solvay Pharmaceuticals and travel grants from AMGEN Australia, Genzyme Australia, and Jansen-Cilag; D.W.J. has received speaker's honoraria, advisor's fees, research funding, and travel grants from the dialysis companies Fresenius Medical Care and Baxter Healthcare Pty Limited; M.R.M. has received honoraria as an advisor to Abbott Australia Pty Ltd and travel grants from Roche Products (NZ) Ltd, Novartis (NZ) Ltd, and Fresenius Medical Care–Australia Pty Ltd.; and K.P. has received speaking honoraria from AMGEN Australia and Jansen-Cilag and travel grants from AMGEN Australia. The ANZDATA Registry receives funding from the Australian Government Department of Health and Ageing, the New Zealand Ministry of Health and Kidney Health Australia. General support for registry activities has been received from AMGEN Australia Pty Ltd., Novartis Pharmaceuticals Australia Pty Ltd., Janssen-Cilag Pty Ltd., Fresenius Medical Care Australia, Roche products Pty Ltd., and Wyeth Australia Pty Ltd. There was no specific funding for this study.
Acknowledgments
All authors contributed to concept and study design, manuscript writing and editing, and final approval; S.P.M. and K.P. were responsible for statistical methods; S.P.M. was responsible for statistical analysis and had full access to all data and takes responsibility for the integrity of the data and accuracy of the analysis.
The ANZDATA Registry exists because of the tireless work of hundreds of people in renal units throughout Australia and New Zealand in collecting the information.
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Vonesh EF, Snyder JJ, Foley RN, Collins AJ: Mortality studies comparing peritoneal dialysis and hemodialysis: What do they tell us? Kidney Int 70: S3, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Gutman RA, Blumenkrantz MJ, Chan YK, Barbour GL, Gandhi VC, Shen FH, Tucker T, Murawski BJ, Coburn JW, Curtis FK: Controlled comparison of hemodialysis and peritoneal dialysis: Veterans Administration multicenter study. Kidney Int 26: 459–470, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Korevaar JC, Feith GW, Dekker FW, van Manen JG, Boeschoten EW, Bossuyt PM, Krediet RT: Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: A randomized controlled trial. Kidney Int 64: 2222–2228, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Burton PR, Walls J: Selection-adjusted comparison of life-expectancy of patients on continuous ambulatory peritoneal dialysis, haemodialysis, and renal transplantation. Lancet 1: 1115–1119, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Vonesh EF, Moran J: Mortality in end-stage renal disease: A reassessment of differences between patients treated with hemodialysis and peritoneal dialysis. J Am Soc Nephrol 10: 354–365, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Gokal R, Baillod R, Bogle S, Hunt L, Jakubowski C, Marsh F, Ogg C, Oliver D, Ward M, Wilkinson R: Multi-centre study on outcome of treatment in patients on continuous ambulatory peritoneal dialysis and haemodialysis. Nephrol Dial Transplant 2: 172–178, 1987 [PubMed] [Google Scholar]
- 7.Maiorca R, Vonesh E, Cancarini GC, Cantaluppi A, Manili L, Brunori G, Camerini C, Feller P, Strada A: A six-year comparison of patient and technique survivals in CAPD and HD. Kidney Int 34: 518–524, 1988 [DOI] [PubMed] [Google Scholar]
- 8.Maiorca R, Vonesh EF, Cavalli P, De Vecchi A, Giangrande A, La Greca G, Scarpioni LL, Bragantini L, Cancarini GC, Cantaluppi A, et al.: A multicenter, selection-adjusted comparison of patient and technique survivals on CAPD and hemodialysis. Perit Dial Int 11: 118–127, 1991 [PubMed] [Google Scholar]
- 9.Nelson CB, Port FK, Wolfe RA, Guire KE: Comparison of continuous ambulatory peritoneal dialysis and hemodialysis patient survival with evaluation of trends during the 1980s. J Am Soc Nephrol 3: 1147–1155, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Held PJ, Port FK, Turenne MN, Gaylin DS, Hamburger RJ, Wolfe RA: Continuous ambulatory peritoneal dialysis and hemodialysis: Comparison of patient mortality with adjustment for comorbid conditions. Kidney Int 45: 1163–1169, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Foley RN, Parfrey PS, Harnett JD, Kent GM, O'Dea R, Murray DC, Barre PE: Mode of dialysis therapy and mortality in end-stage renal disease. J Am Soc Nephrol 9: 267–276, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Bloembergen WE, Port FK, Mauger EA, Wolfe RA: A comparison of mortality between patients treated with hemodialysis and peritoneal dialysis. J Am Soc Nephrol 6: 177–183, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Fenton SS, Schaubel DE, Desmeules M, Morrison HI, Mao Y, Copleston P, Jeffery JR, Kjellstrand CM: Hemodialysis versus peritoneal dialysis: A comparison of adjusted mortality rates. Am J Kidney Dis 30: 334–342, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Jaar BG, Coresh J, Plantinga LC, Fink NE, Klag MJ, Levey AS, Levin NW, Sadler JH, Kliger A, Powe NR: Comparing the risk for death with peritoneal dialysis and hemodialysis in a national cohort of patients with chronic kidney disease. Ann Intern Med 143: 174–183, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Termorshuizen F, Korevaar JC, Dekker FW, Van Manen JG, Boeschoten EW, Krediet RT: Hemodialysis and peritoneal dialysis: Comparison of adjusted mortality rates according to the duration of dialysis—Analysis of The Netherlands Cooperative Study on the Adequacy of Dialysis 2. J Am Soc Nephrol 14: 2851–2860, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Liem YS, Wong JB, Hunink MG, de Charro Fort, Winkelmayer WC: Comparison of hemodialysis and peritoneal dialysis survival in the Netherlands. Kidney Int 71: 153–158, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Murphy SW, Foley RN, Barrett BJ, Kent GM, Morgan J, Barre P, Campbell P, Fine A, Goldstein MB, Handa SP, Jindal KK, Levin A, Mandin H, Muirhead N, Richardson Room, Parfrey PS: Comparative mortality of hemodialysis and peritoneal dialysis in Canada. Kidney Int 57: 1720–1726, 2000 [DOI] [PubMed] [Google Scholar]
- 18.US Renal Data System: International comparisons. In: USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2006, pp 223–234
- 19.Stack AG, Murthy BV, Molony DA: Survival differences between peritoneal dialysis and hemodialysis among “large” ESRD patients in the United States. Kidney Int 65: 2398–2408, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Ganesh SK, Hulbert-Shearon T, Port FK, Eagle K, Stack AG: Mortality differences by dialysis modality among incident ESRD patients with and without coronary artery disease. J Am Soc Nephrol 14: 415–424, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Abbott KC, Glanton CW, Trespalacios FC, Oliver DK, Ortiz MI, Agodoa LY, Cruess DF, Kimmel PL: Body mass index, dialysis modality, and survival: Analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int 65: 597–605, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Winkelmayer WC, Glynn RJ, Mittleman MA, Levin R, Pliskin JS, Avorn J: Comparing mortality of elderly patients on hemodialysis versus peritoneal dialysis: A propensity score approach. J Am Soc Nephrol 13: 2353–2362, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Inrig JK, Sun JL, Yang Q, Briley LP, Szczech LA: Mortality by dialysis modality among patients who have end-stage renal disease and are awaiting renal transplantation. Clin J Am Soc Nephrol 1: 774–779, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Mircescu G, Garneata L, Florea L, Cepoi V, Capsa D, Covic M, Gherman-Caprioara M, Gluhovschi G, Golea OS, Barbulescu C, Rus E, Santimbrean C, Mardare N, Covic A: The success story of peritoneal dialysis in Romania: Analysis of differences in mortality by dialysis modality and influence of risk factors in a national cohort. Perit Dial Int 26: 266–275, 2006 [PubMed] [Google Scholar]
- 25.Heaf JG, Lokkegaard H, Madsen M: Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant 17: 112–117, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Choi SR, Lee SC, Kim BS, Yoon SY, Park HC, Kang SW, Choi KH, Kim YS, Ha SK, Park KI, Han DS, Lee HY: Comparative study of renal replacement therapy in Korean diabetic end-stage renal disease patients: A single center study. Yonsei Med J 44: 454–462, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Iqbal MM, Islam MN, Mansur MA, Naeem GM, Sattar H, Hossain RM, Mohsin M, Rahman MH, Rashid HU: Outcome of peritoneal dialysis and hemodialysis in elderly patients with diabetes: Early experience from Bangladesh. Adv Perit Dial 21: 85–89, 2005 [PubMed] [Google Scholar]
- 28.Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, Hulbert-Shearon T, Jones CA, Bloembergen WE: Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol 11: 556–564, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Rocco M, Soucie JM, Pastan S, McClellan WM: Peritoneal dialysis adequacy and risk of death. Kidney Int 58: 446–457, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Diaz Buxo JA, Lowrie EG, Lew NL, Zhang SM, Zhu X, Lazarus JM: Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis 33: 523–534, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Maiorca R, Brunori G, Zubani R, Cancarini GC, Manili L, Camerini C, Movilli E, Pola A, d'Avolio, G, Gelatti U: Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients: A longitudinal study. Nephrol Dial Transplant 10: 2295–2305, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG: Vascular access and all-cause mortality: A propensity score analysis. J Am Soc Nephrol 15: 477–486, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Moist LM, Chang SH, Polkinghorne KR, McDonald SP: Trends in hemodialysis vascular access from the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) 2000 to 2005. Am J Kidney Dis 50: 612–621, 2007 [DOI] [PubMed] [Google Scholar]
- 34.McDonald S, McCredie M, Williams S, Stewart J: Factors influencing reported rates of treated end-stage renal disease. Adv Chronic Kidney Dis 12: 32–38, 2005 [DOI] [PubMed] [Google Scholar]
- 35.US Renal Data System: International comparisons. In: USRDS 2004 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2005, pp 215–226
- 36.Shah BR, Laupacis A, Hux JE, Austin PC: Propensity score methods gave similar results to traditional regression modeling in observational studies: A systematic review. J Clin Epidemiol 58: 550–559, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Baxter Healthcare Corporation: A Prospective, Randomized, Multicenter, Open Label, Interventional Pilot Study to Compare Mortality, Morbidity and QOL in Hemodialysis Versus Peritoneal Dialysis Subjects, Washington, DC, National Library of Medicine, 2007. Available at: http://clinicaltrials.gov, trial identifier: NCT00510549
- 38.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T: Variable selection for propensity score models. Am J Epidemiol 163: 1149–1156, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robins JM, Hernan MA, Brumback B: Marginal structural models and causal inference in epidemiology. Epidemiology 11: 550–560, 2000 [DOI] [PubMed] [Google Scholar]