Abstract
The role of dendritic cells (DC) that accumulate in the renal parenchyma of non–immune-mediated proteinuric nephropathies is not well understood. Under certain circumstances, DC capture immunologically ignored antigens, including self-antigens, and present them within MHC class I, initiating an autoimmune response. We studied whether DC could generate antigenic peptides from the self-protein albumin. Exposure of rat proximal tubular cells to autologous albumin resulted in its proteolytic cleavage to form an N-terminal 24–amino acid peptide (ALB1-24). This peptide was further processed by the DC proteasome into antigenic peptides that had binding motifs for MHC class I and were capable of activating syngeneic CD8+ T cells. In vivo, the rat five-sixths nephrectomy model allowed the localization and activation of renal DC. Accumulation of DC in the renal parenchyma peaked 1 wk after surgery and decreased at 4 wk, concomitant with their appearance in the renal draining lymph nodes. DC from renal lymph nodes, loaded with ALB1-24, activated syngeneic CD8+ T cells in primary culture. The response of CD8+ T cells of five-sixths nephrectomized rats was amplified with secondary stimulation. In contrast, DC from renal lymph nodes of five-sixths nephrectomized rats treated with the proteasomal inhibitor bortezomib lost their capacity to stimulate CD8+ T cells in primary and secondary cultures. These data suggest that albumin can be a source of potentially antigenic peptides upon renal injury and that renal DC play a role in processing self-proteins through a proteasome-dependent pathway.
Dendritic cells (DC), which reside in most tissues in immature state, represent a double-edged sword in the immune system controlling immunity and tolerance.1,2 Acting as a network of sentinel cells, they capture antigens and present them as peptides within MHC classes I and II (MHC-I and MHC-II). Subsequent T cell activation and differentiation determine the outcome of infectious and autoimmune diseases. Studies have found that immunologically ignored antigens, including self-antigens, can be taken up and transported by DC under special circumstances, initiating an autoimmune response.3–5 Separate processing pathways for presentation of exogenous and endogenous antigen by antigen presenting cells (APC) have been described. Exogenous antigens are loaded on MHC-II in a post-Golgi compartment, whereas peptides derived from intracellular synthesized proteins are presented within MHC-I after proteasome processing. This strict dichotomy was recently challenged by studies showing that DC can present exogenous proteins on MHC-I, through a process called “cross presentation.”6,7 Antigen presentation leads to either the initiation of immune response or tolerance, depending on the availability of co-stimulatory signals that are highly expressed upon DC activation and maturation.8,9 Inflammatory stimuli also favor DC immunogenic capacity.8,9
Here we took as a pertinent paradigm the case of proteinuric nephropathy, whereby excess filtration of albumin upon breakdown of the glomerular barrier promotes tubular damage and release of chemokines into the interstitium.10 The ensuing inflammatory environment might convert the tolerogenic capacity of resident DC into an immunogenic one, favoring recruitment of T cells. Actually, the significance of the presence of immune system cells as DC and CD8+ T cells in the renal parenchyma of proteinuric nephropathies, even in the absence of an immune insult,11,12 has never been explored before. The major APC population of the healthy kidney are DC, which, being in close contact with the tubular epithelium, sense and respond to molecules that cross the tubule layer and diffuse away.13,14 Upon injury, immunogenic DC might cross-present normally ignored self-antigens, namely albumin, triggering T cell effector response. Here we demonstrate that proteolysis of the self-protein albumin by proximal tubular cells provides the substrate to DC for the generation—via a proteasome-dependent pathway—of antigenic peptides recognized by CD8+ T cells.
RESULTS
N-Terminal Truncation of Albumin by a Proximal Epithelial Cell Protease
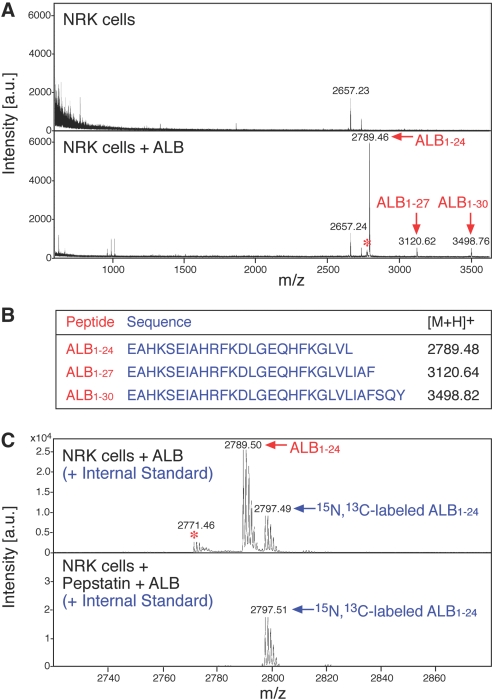
We first investigated the profile of the peptides present in the supernatant of rat proximal tubular cells (NRK) after incubation with 0.5 mg/ml fatty acid–free albumin, a concentration measured in the glomerular ultrafiltrate of proteinuric rats.15,16 Mass spectrometry profiles by matrix-assisted laser desorption ionization mass spectrometry (MALDI-TOF-MS; mass range 600 to 3600 Da), obtained from NRK supernatants acidified to pH 2.5 and extracted on C18 ZipTip, showed the presence of three prominent peptides (Figure 1A). Figure 1B shows the amino acid sequence of the three peptides, named here ALB1-24, ALB1-27, ALB1-30. These cleavage products, identified by liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) with Mascot database search engine, derive from N-terminal truncation of albumin at position 24, 27, and 30. ALB1-24 was by far the most abundant product (Figure 1A, Supplemental Figure 1). Identity of ALB1-24 was confirmed by comparison with the synthetic peptide ALB1-24 prepared by F-moc chemistry (Supplemental Figures 2 through 4). Further experiments with NRK cell supernatant using stable isotope–labeled ALB1-24 as internal standard, showed that the formation of ALB1-24 occurs in an acidic environment (pH 2.5 to 5.0; data not shown) and is completely suppressed by pepstatin A (Figure 1C).
Figure 1.
Formation of N-terminal albumin fragments in NRK cell supernatants. (A) MALDI-TOF-MS analysis of supernatants from NRK cells exposed or not to rat serum albumin (0.5 mg/ml). Samples were acidified to pH 2.5 and extracted on C18 ZipTip. The most abundant newly formed compounds were identified as ALB1-24, ALB1-27, and ALB1-30. *Corresponds to a modified form of ALB1-24, with pyroglutamic acid at position 1 (see Supplemental Figure 1). (B) Sequence and monoisotopic molecular mass of the [M+H]+ ions of ALB1-24, ALB1-27, and ALB1-30. (C) MALDI-TOF-MS analysis of supernatants from NRK cells exposed to albumin in the presence or absence of pepstatin A (10 μg/ml). The two peak groups refer to the isotopic cluster of ALB1-24 and to the internal standard 15N, 13C-ALB1-24, respectively (labels refer to the monoisotopic peak). In the presence of pepstatin A, signals of ALB1-24 but not those of the internal standard disappeared, indicating complete suppression of ALB1-24 formation (n = 3).
DC Proteasome Processes ALB1-24 into CD8+ T Cell–Recognized Antigenic Peptides
Given that albumin degradation fragments are predominantly exocytosed to the basolateral side of the proximal tubular cell17 in a manner that in vivo allows further processing in the interstitial compartment, we next asked whether DC—abundantly present in the renal interstitium in proteinuric diseases—could process albumin fragments and present them to T cells. Studies were also undertaken to document the pathways of albumin peptide processing. Bone marrow DC from Brown Norway (BN) rats were incubated overnight with synthetic ALB1-24 and then used to prime syngeneic CD8+ T cells. CD8+ T cell stimulation, measured as IFN-γ release by enzyme-linked immunospot (ELISPOT) assay, was not evident at the first encounter (Figure 2A). When ALB1-24-primed CD8+ T cells were restimulated by syngeneic pulsed DC, they showed a significant activation (P < 0.05 versus unpulsed DC), disclosing the antigenic nature of the self-peptide (Figure 2B). To assess further the intracellular pathways involved in ALB1-24 processing and presentation by DC, we performed experiments in the presence of amiloride analog 5-(N-ethyl-N-isopropyl) amiloride (EIPA), which inhibits antigen uptake by macropinocytosis,18 or brefeldin A (BfA), which interferes with the egress of newly synthesized MHC molecules.19 Activation of CD8+ T cells was completely prevented by both EIPA and BfA (Figure 2B). We then determined the possible involvement of proteasome, known to produce most antigenic peptides presented by MHC-I,20 in CD8+ T cell activation by DC. The proteasome inhibitor lactacystin21 completely prevented the stimulation of CD8+ T cells by DC preexposed to ALB1-24 (Figure 2B). These findings indicate that, through the proteasome, DC are effective in processing protein fragments into smaller peptides, which are presented to CD8+ T cells, leading to their activation.
Figure 2.
Proteasome-dependent processing by DC of ALB1-24 stimulates syngeneic CD8+ T cells. (A) Primary mixed leukocyte reaction (MLR). Bone marrow–derived DC from BN rats were incubated with (▒) or without (  ) the synthetic ALB1-24 (35 μg/ml) and used to stimulate syngeneic CD8+ T cells. CD8+ T cell activation was evaluated by IFN-γ ELISPOT assay. As positive control, unpulsed DC were cultured with allogeneic LW T cells (□). (B) Secondary MLR. After 3-d primary MLR, primed CD8+ T cells were harvested and cultured again in a 2-d secondary stimulation with DC previously pulsed with or without ALB1-24. As positive control, unpulsed DC were cultured in secondary MLR with allogeneic LW T cells recovered at the end of primary MLR (□). The inhibitors EIPA (50 μM), BfA (0.5 μg/ml), or lactacystin (Lac; 10 μM), were added to DC 30 min before pulsing with ALB1-24. Data are means ± SEM (n = 5 independent experiments). *P < 0.05 versus syngeneic CD8+ T cells re-incubated with unpulsed DC or pulsed DC in presence of inhibitors.
) the synthetic ALB1-24 (35 μg/ml) and used to stimulate syngeneic CD8+ T cells. CD8+ T cell activation was evaluated by IFN-γ ELISPOT assay. As positive control, unpulsed DC were cultured with allogeneic LW T cells (□). (B) Secondary MLR. After 3-d primary MLR, primed CD8+ T cells were harvested and cultured again in a 2-d secondary stimulation with DC previously pulsed with or without ALB1-24. As positive control, unpulsed DC were cultured in secondary MLR with allogeneic LW T cells recovered at the end of primary MLR (□). The inhibitors EIPA (50 μM), BfA (0.5 μg/ml), or lactacystin (Lac; 10 μM), were added to DC 30 min before pulsing with ALB1-24. Data are means ± SEM (n = 5 independent experiments). *P < 0.05 versus syngeneic CD8+ T cells re-incubated with unpulsed DC or pulsed DC in presence of inhibitors.
Proteasome Generates Predicted MHC-I Ligands from ALB1-24
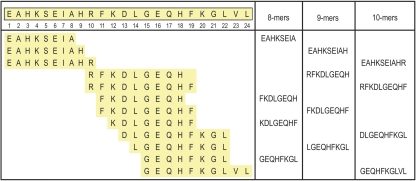
To assess whether the proteasome is able to cleave from ALB1-24 peptide fragments possibly responsible for its antigenicity, we incubated ALB1-24 with highly purified 20S proteasome for 0 to 8 h at 37°C and identified the most relevant cleavage products by mass spectrometry (MALDI-TOF-MS and LC-ESI-MS/MS). At 0 h, only the intact peptide was observed. The intact substrate was still present at 2 and 4 h but exhausted after 8 h. After 4 h, the main products observed by MALDI-TOF-MS were from C-terminal trimming of ALB1-24 (1 to 22, 1 to 19, and 1 to 18 fragments). These products then tended to disappear, whereas shorter peptides (8 to 10 amino acid size) accumulated in the 2- to 8-h interval (data not shown). Figure 3 lists the MS/MS-identified proteasome-cleaved fragments of ALB1-24 with a size compatible with MHC-I binding (8 to 10 amino acids). Supplemental Figures 5 and 6 show the interpreted MS/MS spectra and peptide fragmentation for two peptides, EAHKSEIAHR and GEQHFKGL.
Figure 3.
Cleavage of ALB1-24 by proteasome. ALB1-24 was incubated for 0 to 8 h with purified proteasome, and the fragments were identified by MS. Only peptides with a size compatible with MHC-I binding (8 to 10 amino acids) are listed here. The sequence of ALB1-24 is reported, with the position of the amino acids.
To investigate the presence of a potential binder for MHC-I within the 1 to 24 domain of albumin, we submitted the entire sequence of rat serum albumin precursor (SwissProt accession no. P02770) to database search for potential MHC ligands, using three bioinformatics tools (SYFPEITHI, ProPred-I, and MAPPP). Among all of the 8- to 10-mers in the entire albumin sequence, a number of peptides belonging to the 1 to 24 domain indeed ranked within the five best binders on various murine MHC-I alleles (Table 1). For example, GEQHFKGLV (15 to 23) was consistently ranked as the number 1 among 9-mer binders for H2-Kk and GEQHFKGL (15 to 22) as a top-ranked 8-mer ligand for H2-Kb. Interestingly, we identified GEQHFKGL as a product of in vitro proteasomal degradation of ALB1-24 (Figure 3, Supplemental Figure 6).
Table 1.
In silico predicted peptide binding to MHC-Ia
| MHC-I Allele | Sequence
|
Predicted Bindersb | Rank of Relative Binding Score
|
|||
|---|---|---|---|---|---|---|
| Start | End | SYFPEITHI | PRO-PRED I | MAPPP | ||
| H2-Kb | 6 | 14 | EIAHRFKDL | 2 | ||
| H2-Kb | 7 | 14 | IAHRFKDL | 1 | ||
| H2-Dd | 14 | 22 | LGEQHFKGL | 5 | 4 | |
| H2-Kb | 15 | 22 | GEQHFKGL | 4 | 1 | |
| H2-Kk | 15 | 23 | GEQHFKGLV | 1 | 1 | 1 |
In silico prediction of binding strength between all albumin peptides and murine MHC-I alleles using bioinformatic tools (SYFPEITHI, PRO-PRED I, and MAPPP; see Concise Methods section for details).
Only peptides belonging to the N-terminal sequence 1 to 24 and with binding scores ranked 1 to 5 are reported here.
Proteasome Inhibition Reduces Renal Inflammation and T Cell Stimulatory Capacity of DC in Proteinuric Nephropathy
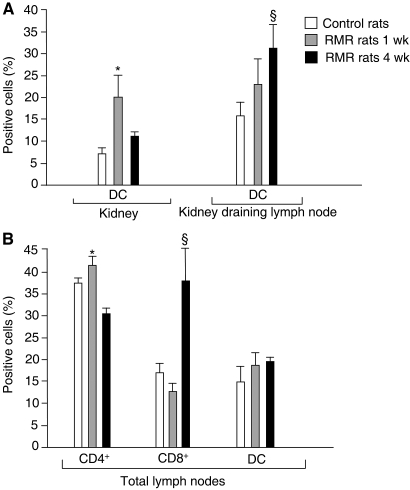
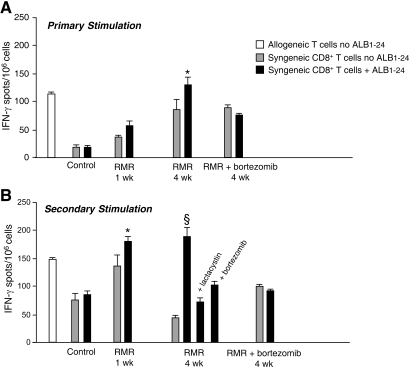
To assess the in vivo functional contribution of proteasome activity to the formation of self-antigens, we evaluated the effect of the proteasome inhibitor bortezomib on the pattern and activation of inflammatory cells infiltrating renal interstitium in a rat model of proteinuric progressive renal disease as a result of surgical ablation of renal mass. Despite no primary immune insult in this model, the accumulation of DC and CD8+ T cells in the interstitium was found to be effectively controlled by an antiproteinuric therapy, indicating that the upstream mechanism of protein traffic is critical to initiate and maintain inflammation and immune injury.11 Rats underwent five-sixths nephrectomy and were given bortezomib or vehicle from days 1 to 28. As reported in Table 2, no significant changes in systolic BP and renal functional parameters were found after bortezomib treatment. Morphologic evaluation of the kidneys by light microscopy showed that the incidence of focal glomerulosclerosis, affecting on average 22 ± 5% of glomeruli in the vehicle-treated rats with renal mass reduction (RMR), was reduced by bortezomib treatment to 11 ± 2%, although the difference did not reach statistical significance. Hematocrit and white blood cell count were also comparable between the two groups (hematocrit: RMR + vehicle, 45 ± 1.2%, RMR + bortezomib, 41 ± 1.15%; white blood cell: RMR + vehicle, 11,067 ± 694 cells/μl, RMR + bortezomib, 13,520 ± 528 cells/μl). All animals developed interstitial inflammation. The nature of interstitial infiltrates examined by immunohistochemistry revealed the presence of macrophages (ED1+ cells), CD4+ T cells, CD8+ T cells, and DC (OX62+ cells; Table 3). Bortezomib significantly prevented the extent of infiltration of both inflammatory and immune cells (Table 3). To characterize renal DC better, we performed immunophenotypic analysis by FACS by double labeling renal cells with anti-OX62 and anti-CD11c antibodies. As shown in Supplemental Figure 7, the majority of OX62+ cells (approximately 80%) also expressed CD11c. FACS analysis of CD11c+ DC in total kidney infiltrating cells confirmed increased content of these cells in remnant kidneys as compared with control kidneys, thereby validating immunohistochemistry findings (Figure 4A). The comparison of CD11c+ DC isolated from kidneys and renal draining lymph nodes (LN) of RMR rats, both at 1 and 4 wk after surgical ablation, allowed us to document dynamic changes in renal DC at different stages of the disease (Figure 4A). The percentage of CD11c+ DC among renal cells peaked at 1 wk. At 4 wk, a decrease in the percentage of DC in the kidney was paralleled by an enhancement of these cells in the renal LN, suggesting migration of DC from the renal interstitium. These changes were associated with a significant increase in CD8+ T cells in total LN at 4 wk preceded by a marked increase in CD4+ T cells at 1 wk (Figure 4B). Importantly, DC sorted at 4 wk from renal LN of RMR rats and exposed in vitro to ALB1-24 significantly activated CD8+ T cells isolated at the same time points from RMR rats (syngeneic CD8+ T cells) already in a primary mixed leukocyte reaction, suggesting an in vivo cross-priming of CD8+ T cells by the albumin peptide (Figure 5A). Of note, CD8+ T cells isolated from RMR rats at 1 wk were not significantly stimulated by ALB1-24–loaded DC in primary cultures, indicating that in vivo CD8+ T cell cross-priming was a chronic progressive event. Upon secondary stimulation in vitro by ALB1-24–loaded DC, CD8+ T cells isolated at both 1 and 4 wk after RMR showed an amplified activation as compared with CD8+ T cells from control rats stimulated in the same condition. Addition of either lactacystin or bortezomib almost completely prevented RMR CD8+ T cell activation by ALB1-24–loaded DC (Figure 5B). Consistently, treatment of RMR rats with bortezomib for 4 wk reduced the capacity of ALB1-24–loaded DC from renal LN to stimulate CD8+ T cells in both primary and secondary cultures (Figure 5B).
Table 2.
SBP, renal functional parameters, and histology in rats with renal mass reduction treated with vehicle or bortezomiba
| Parameter | RMR + Vehicle | RMR + Bortezomib |
|---|---|---|
| SBP (mmHg) | 182 ± 9 | 165 ± 9 |
| Proteinuria (mg/24 h) | 251 ± 43 | 201 ± 67 |
| Serum creatinine (mg/dl) | 0.95 ± 0.06 | 1.07 ± 0.09 |
| Sclerotic glomeruli (%) | 22 ± 5 | 11 ± 2 |
Data are means ± SEM. RMR + vehicle (n = 11); RMR + bortezomib (n = 10). SBP, systolic blood pressure.
Table 3.
Effect of proteasome inhibition on interstitial infiltrates in RMRa
| Parameter | Control | RMR + Vehicle (No. of Cells/HPF) | RMR + Bortezomib (No. of Cells/HPF) |
|---|---|---|---|
| ED1 | 5.70 ± 1.20 | 21.87 ± 8.84b | 8.73 ± 2.97c |
| OX62 | 0.33 ± 0.26 | 7.11 ± 1.89b | 2.24 ± 1.02d |
| W3/25 | 6.13 ± 1.65 | 54.24 ± 8.90b | 23.99 ± 9.41d |
| OX8 | 3.16 ± 0.74 | 11.08 ± 5.54b | 3.74 ± 1.91c |
Data are means ± SEM. Control (n = 4); RMR + vehicle (n = 6); RMR + bortezomib (n = 5). ED1, monocytes/macrophages; HPF, high-power field; OX62, DC; OX8, CD8 T cells; W3/25, CD4 T cells/macrophages.
P < 0.05 versus control.
P < 0.05 versus RMR + vehicle.
P < 0.01 versus RMR + vehicle.
Figure 4.
Immunophenotypic analysis in RMR rats. (A) Cells obtained from kidneys and renal draining LN of RMR rats (at 1 wk [n = 4] and 4 wk [n = 5] after surgical ablation) and control rats (n = 5) were labeled with FITC-conjugated mouse anti-rat CD11c and FACS analyzed. (B) Cells obtained from total LN of RMR rats (at 1 wk [n = 4] and 4 wk [n = 5] after surgical ablation) and control rats (n = 5) were labeled with PE-conjugated mouse anti-rat CD4, FITC-conjugated mouse anti-rat CD8, and FITC-conjugated mouse anti-rat CD11c. Labeled cells were analyzed using FACSAria. Data are means ± SEM. *P < 0.05 versus control rats and RMR rats at 4 wk; §P < 0.05 versus control rats and RMR rats at 1 wk.
Figure 5.
Primary and secondary MLR in RMR rats. (A) Primary MLR. CD11c+ DC sorted from renal draining LN of vehicle or bortezomib-treated RMR rats and splenic CD11c+ DC from control rats were incubated with (▪) or without (  ) the synthetic ALB1-24 peptide (35 μg/ml) and used to stimulate syngeneic CD8+ T cells. CD8+ T cell activation was evaluated by IFN-γ ELISPOT assay. As positive control, unpulsed DC were cultured with allogeneic LW T cells (□). (B) Secondary MLR. After 3-d primary MLR, primed CD8+ T cells were harvested and cultured in a 2-d secondary stimulation with DC previously pulsed with or without ALB1-24 peptide. As positive control, unpulsed DC were cultured in secondary MLR with allogeneic LW T cells recovered at the end of primary MLR (□). The inhibitors lactacystin (10 μM) or bortezomib (20 ng/ml) were added 30 min before peptide pulsing. Data are means ± SEM (n = 4 independent experiments). *P < 0.05 versus no ALB1-24; §P < 0.05 versus no ALB1-24 and + ALB1-24 in the presence of inhibitors.
) the synthetic ALB1-24 peptide (35 μg/ml) and used to stimulate syngeneic CD8+ T cells. CD8+ T cell activation was evaluated by IFN-γ ELISPOT assay. As positive control, unpulsed DC were cultured with allogeneic LW T cells (□). (B) Secondary MLR. After 3-d primary MLR, primed CD8+ T cells were harvested and cultured in a 2-d secondary stimulation with DC previously pulsed with or without ALB1-24 peptide. As positive control, unpulsed DC were cultured in secondary MLR with allogeneic LW T cells recovered at the end of primary MLR (□). The inhibitors lactacystin (10 μM) or bortezomib (20 ng/ml) were added 30 min before peptide pulsing. Data are means ± SEM (n = 4 independent experiments). *P < 0.05 versus no ALB1-24; §P < 0.05 versus no ALB1-24 and + ALB1-24 in the presence of inhibitors.
DISCUSSION
The presence of DC and T cells in the interstitium, a common finding of non–immunologically induced nephropathies, indicates activation of the immune system. The present study documents the generation of antigenic peptides from a self-protein, namely albumin, through the concerted action of renal proximal tubules and DC. Exposure of proximal tubular cells to excess autologous albumin, as in the case of proteinuric nephropathies, results in the formation of the N-terminal 24–residue fragment of albumin. This peptide is acquired by DC, where it is further processed by proteasome into antigenic peptides that activate CD8+ T cells.
An acid protease with pepsin-like activity is responsible for the N-terminal truncation of albumin as documented by the inhibitory effect of pepstatin. These results are in line with previously reported studies showing selective truncation of albumin at position 24.22,23 The N-terminal 24–amino acid peptide has been described in various species as a major product of albumin digestion by pepsin at acidic pH,24 a condition that makes albumin more susceptible to proteolytic cleavage.25
Peptides of 8 to 10 residues in length, generated by proteasome of APC and presented on MHC-I, are normally recognized by CD8+ T cells. Proteasome digestion of ALB1-24 in a cell-free system indeed produced a number of peptides 8 to 10 amino acids long. Database search for potential peptide binders to MHC-I indicates that, within the products of in vitro proteosomal degradation of ALB1-24, a possible candidate for CD8+ T cell cross-priming is GEQHFKGL.
The crucial role of proteasome in the activation of immune cells is further supported here by in vivo findings showing that the proteasome inhibitor bortezomib prevents infiltration of inflammatory and immune cells in the interstitium in the rat model of progressive nephropathy caused by renal mass ablation. Accumulation of DC in the peritubular interstitium is a constant although unexplained finding of proteinuric nephropathies. Recent observations indicate that interstitial DC, positioned to serve as a major interface between innate and adaptive immunity, express on their surface the fractalkine receptor CX3CR1.26 Expression of fractalkine is increased in the renal interstitium of mice with protein overload proteinuria, which would explain DC recruitment into the renal interstitium.27 The limited capacity of lysosomal proteolysis of DC allows internalized antigens to persist and enhances the chance for them to be degraded by proteasome and cross-presented on MHC-I, thereby enhancing immunogenic T cell response.28
Our findings that DC were increased in kidney draining LN from RMR rats at the late stage of the disease suggests transport of the antigen taken up by DC from the renal interstitium to the T cell area of the renal LN. Renal DC are important participants of renal injury by virtue of their capacity to mediate antigen transfer from the renal parenchyma to renal LN, where Ag-specific T cell activation takes place.13 Furthermore, DC from RMR rats can cross-present albumin peptides to CD8+ T cells. Processing of ALB1-24 by DC likely occurs through the proteasome-dependent pathway as suggested by the in vitro inhibitory effect of both bortezomib and lactacystin on T cell activation. Of note, that DC from renal LN of bortezomib-treated RMR rats lose their capacity to stimulate T cells further supports a role for the proteasome in immunologic and inflammatory processes that concur to parenchymal damage. It has been proposed that rat CD4+/SIRPα+ DC exhibit strong CD8+ T cell stimulatory capacity.29 We have identified the presence of this subset in the rat remnant kidney (see Supplemental Figure 8). Whether such a population acts as cross-presenting cell in this model of proteinuric nephropathy merits further investigations.
In the kidney, the tubular acidic milieu provides the environment for proteolytic cleavage of albumin, which, upon sieving dysfunction of the glomerular barrier, is filtered in abnormally high amounts resulting in excess protein loading of proximal tubular cells. The N-terminal 24-residue fragment of albumin can be acquired by interstitial DC that have the ability to uptake molecules filtered into the urinary space.13 Extracellular acidosis, which is usually associated with inflammatory responses, increases the uptake of exogenous antigens and MHC-I–restricted presentation of derived peptides by DC stimulating CD8+ T cell immunity.30 Acidic regions were also detected in the renal cortical interstitium by using a low pH–sensitive membrane peptide construct that targets acidic tissues in vivo.31 Thus, the high acidic milieu related to inflammation is likely to facilitate strongly albumin peptide acquisition by interstitial DC and its recognition by CD8+ T cells.
DC were recently described to form an immune sentinel network throughout the entire kidney, where they probe the environment in search of antigens.26 Cross-presentation by DC is a major mechanism for the immune surveillance of tissues against foreign antigens. Its outcome toward immunity depends on the availability of immunostimulatory signals that are acquired together with the antigen.32 The current view that immunity is controlled by internal communication between the tissue and immune-competent cells suggests that tolerance is maintained by healthy organs, whereas immunity can be stimulated by a distressed tissue.33 Conceivably, inflammatory stimuli released from damaged tubules represent “danger” signals that, once in the presence of the albumin peptide, alert DC to promote local immunity via CD8+ T cells. Theoretically, in humans, any chronic condition characterized by both the nephrotic syndrome and the presence of the interstitial inflammatory infiltrates may provide the environment for the DC processing of albumin peptide to occur in areas surrounding the injured nephron. Our data indicate that a self-protein that is normally ignored by immune competent cells becomes a reservoir of potentially antigenic peptides upon organ injury. It is plausible that the mechanism underlying the formation of self-antigens in conditions of kidney injury also operates for other proteins and in other circumstances of organ damage. One notable situation to which this paradigm may apply is the processing of unique pathogenic epitopes that are present in normal cardiac myosin in experimental myocarditis.34 Proteasomal degradation of self-proteins into antigenic peptides that drive the outcome of cross-presentation toward immunity deserves further investigation as a novel trigger of disease states and break of immune tolerance in chronic inflammatory diseases.
CONCISE METHODS
Proximal Tubular Epithelial Cell Culture and Incubation
NRK-52E cells (a rat proximal tubular epithelial cell line) obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany) were cultured in DMEM supplemented with 5% FBS, l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml). Confluent cells were maintained 48 h in serum–free DMEM before experiments and then exposed for 19 h to essentially fatty acid and globulin-free rat albumin (0.5 mg/ml; Sigma-Aldrich, St. Louis, MO) or medium alone. At the end of the incubation, supernatants (100 μl) were acidified to pH 2.5 before extraction and analysis by mass spectrometry, as described in the next section. In additional experiments, NRK cells were preincubated with or without the aspartic protease inhibitor pepstatin A (10 μg/ml; Sigma-Aldrich) 30 min before and during the addition of rat albumin (0.5 mg/ml) for 19 h. Supernatants (50 μl) were enriched with internal standard 15N, 13C-labeled ALB1-24 (2 pmol/μl), acidified to pH 2.5, and incubated for 24 h at 37°C before extraction and analysis by mass spectrometry (see below).
Peptide Extraction
Peptides were extracted from NRK cells supernatant (acidified to pH 2.5 with 2.5% trifluoroacetic acid) on ZipTip C18 pipette tips (Millipore Corp., Bedford, MA). Peptides were identified by MALDI-TOF-MS and LC-ESI-MS/MS (see Supplemental Information for details).
Proteasome Digestion
Synthetic ALB1-24 peptide (2.4 nmol) was incubated with purified rabbit 20S proteasome (Sigma-Aldrich) at a molar ratio of 800:1 (peptide:proteasome) at 37°C in 10 mM Tris-HCl (pH 7.5).35 Aliquots were taken at different times of incubation (0, 2, 4, and 8 h) and analyzed by MS in comparison with incubated reaction blanks (peptide in buffer or buffer alone).
In Silico Predicted Peptide Binding to MHC-I
Bioinformatic tools (SYFPEITHI at http://www.syfpeithi.de/Scripts/MHCServer.dll/EpitopePrediction.htm, PRO-PRED I at http://www.imtech.res.in/raghava/propred1/index.html, and MAPPP at http://www.mpiib-berlin.mpg.de/MAPPP/) were used to search possible binders to MHC-I within the sequence of rat serum albumin. Mouse MHC-I alleles were tested, because rat alleles were scantly represented. All possible peptides formed in the entire rat serum albumin were ranked according to their relative binding scores for each allele.
Immunophenotypic Analysis
Immunophenotypic analysis was performed using FACS (FACSAria; Becton Dickinson, Franklin Lakes, NJ). Cells obtained from kidneys and kidney draining LN of RMR (both at 1 and 4 wk after surgical ablation) and sham-operated rats were labeled with FITC-conjugated mouse anti-rat CD11c (clone 8A2, AbD; Serotec, Oxford, UK). Cells from total LN of RMR and sham-operated rats were labeled with anti-CD11c, FITC-conjugated mouse anti-rat CD8 (clone OX8, AbD), and PE-conjugated mouse anti-rat CD4 (clone W3/25, AbD). In a selected experiment, renal DC were analyzed for Sirpα (CD172a, clone OX41, AbD) CD4 coexpression (see Supplemental Information for details). Negative controls were performed using control isotype FITC- and PE-conjugated antibodies.
Primary and Secondary CD8+ T Cell Stimulation
For primary and secondary stimulation, DC were used as stimulators and syngeneic CD8+ T cells as responders. Preparation of DC and CD8+ T cells is described in supplemental materials. DC were bone marrow–derived CD11c+ DC from BN rats, splenic FACS-sorted CD11c+ DC from Sprague-Dawley (SD) control sham-operated rats, renal draining LN FACS-sorted CD11c+ DC from SD RMR rats, and bortezomib-treated RMR rats. Because SD is an outbred strain, to maintain syngeneic condition, we obtained co-cultured DC and CD8+ T cells from the same animals (both for RMR rats and for control sham-operated rats).
DC were incubated (105 cells/100 μl RPMI/5% rat serum, 37°C, 5% CO2) for 18 h with or without the synthetic ALB1-24 (35 μg/ml) and used as stimulators in a 3-d primary stimulation with syngeneic CD8+ T cells (DC-to-responders ratio: 1:100 for BN and 1:40 for SD). Allogeneic Lewis (LW, RT1l) T cells were used as positive control to verify DC allostimulatory capacity in each experiment. After primary stimulation, CD8+ T cells were harvested and cultured again in a 2-d secondary stimulation with DC previously pulsed with or without ALB1-24 (at the same DC-to-responder ratio of primary stimulation). CD8+ T cell activation was evaluated by IFN-γ ELISPOT assay (Euroclone, Ltd., Paington, UK). For experiments with BN cells, inhibitors EIPA (50 μM; Sigma-Aldrich), BfA (0.5 μg /ml; Sigma-Aldrich), or lactacystin (10 μM; Sigma-Aldrich) were added to DC 30 min (37°C, 5% CO2) before pulsing with ALB1-24. For experiments with SD cells, proteasome inhibitors such as lactacystin or bortezomib (20 ng/ml) were added to DC 30 min (37°C, 5% CO2) before pulsing with ALB1-24. DC were washed with RPMI before adding CD8+ T cells to avoid possible inhibitors’ toxic effect. DC viability, evaluated by Trypan blue dye exclusion, accounted for 70% on average in all settings.
Experimental Model of RMR in Rat
RMR was induced in male SD rats (320 to 340 g; Charles River Italia, Calco, Italy) as described previously.11 Animals received vehicle (saline; n = 11) or bortezomib36 (JANSSEN-CILAG SpA, Milan, Italy; n = 10, 0.3 mg/kg intravenously twice a week from days 1 to 28). An additional group of untreated RMR rats (n = 4) were killed 1 wk after surgical ablation. Nine sham-operated rats served as controls. At the end of the study, kidneys, LN, and spleens were removed under anesthesia for immunophenotypic characterization of DC and T cells by FACS (see also Supplemental Information) and cross-presentation studies. Kidney specimens were also used for the evaluation of glomerular sclerosis and interstitial infiltrates by immunohistochemistry (see Supplemental Information). Systolic BP, proteinuria, serum creatinine, and glomerular damage were determined as described previously.11 Animal care and treatment were conducted in conformity with the institutional guidelines that are in compliance with national and international laws and policies (EEC Council Directive 86/609, OJL 358, Dec 12 1987; Guide for the Care and Use of Laboratory Animals, US National Research Council, 1996).
Statistical Analysis
Results are expressed as means ± SEM. Data on IFN-γ spots and FACS analysis of DC and T cells were compared by ANOVA and Bonferroni post hoc analysis. Systolic BP, renal functional parameters, and histology in RMR rats treated with vehicle or bortezomib were analyzed by unpaired t test. Results of renal interstitial infiltrates were analyzed by nonparametric Kruskal-Wallis test for multiple comparisons. Statistical significance was defined as P < 0.05.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
L.C. and S.B. are recipients of a fellowship of Fondazione ART (Milan, Italy) and Fondazione Aiuti per la Ricerca sulle Malattie Rare (ARMR; Bergamo, Italy), respectively.
We are profoundly indebted to Dr. Alfred L. Goldberg for thoughtful discussion of the rationale of this investigation. We thank Drs. Mauro Abbate and Marina Morigi for helpful discussion; Dr. Mario Salmona for the synthesis of ALB1-24; Dr. Francesco Gregis for providing bortezomib; and Anna Pezzotta, Federica Rocchetta, Marilena Mister, and Marta Todeschini for technical assistance.
Published online ahead of print. Publication date available at www.jasn.org.
D.M. and C.C. contributed equally to this work.
Supplemental information for this article is available online at http://www.jasn.org/.
See related editorial, “Cross Dendritic Cells Anger T Cells after Kidney Injury,” on pages 3–5.
REFERENCES
- 1.Banchereau J, Steinman RM: Dendritic cells and the control of immunity. Nature 392: 245–252, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Raimondi G, Turnquist HR, Thomson AW: Frontiers of immunological tolerance. Methods Mol Biol 380: 1–24, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Ludewig B, Odermatt B, Ochsenbein AF, Zinkernagel RM, Hengartner H: Role of dendritic cells in the induction and maintenance of autoimmune diseases. Immunol Rev 169: 45–54, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B: Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med 11: 328–334, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM, Banchereau J: Taking dendritic cells into medicine. Nature 449: 419–426, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA: Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev 199: 9–26, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Wilson NS, Villadangos JA: Regulation of antigen presentation and cross-presentation in the dendritic cell network: Facts, hypothesis, and immunological implications. Adv Immunol 86: 241–305, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Huang FP, MacPherson GG: Continuing education of the immune system: Dendritic cells, immune regulation and tolerance. Curr Mol Med 1: 457–468, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC: Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol 157: 1406–1414, 1996 [PubMed] [Google Scholar]
- 10.Zoja C, Benigni A, Remuzzi G: Cellular responses to protein overload: Key event in renal disease progression. Curr Opin Nephrol Hypertens 13: 31–37, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Remuzzi G, Zoja C, Gagliardini E, Corna D, Abbate M, Benigni A: Combing an antiproteinuric approach with mycophenolate mofetil fully suppresses progressive nephropathy of experimental animals. J Am Soc Nephrol 10: 1542–1549, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Remuzzi A, Gagliardini E, Donadoni C, Fassi A, Sangalli F, Lepre MS, Remuzzi G, Benigni A: Effect of angiotensin II antagonism on the regression of kidney disease in the rat. Kidney Int 62: 885–894, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD: Antigen presentation by dendritic cells in renal lymph nodes is linked to systemic and local injury to the kidney. Kidney Int 68: 1096–1108, 2005 [DOI] [PubMed] [Google Scholar]
- 14.John R, Nelson PJ: Dendritic cells in the kidney. J Am Soc Nephrol 18: 2628–2635, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Landwehr DM, Carvalho JS, Oken DE: Micropuncture studies of the filtration and absorption of albumin by nephrotic rats. Kidney Int 11: 9–17, 1977 [DOI] [PubMed] [Google Scholar]
- 16.Lewy JE, Pesce A: Micropuncture study of albumin transfer in aminonucleoside. Pediatr Res 7: 553–559, 1973 [DOI] [PubMed] [Google Scholar]
- 17.Gudehithlu KP, Pegoraro A, Dunea G, Arruda JA, Singh AK: Degradation of albumin by the renal proximal tubule cells and the subsequent fate of its fragments. Kidney Int 65: 2113–2122, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Nakase I, Niwa M, Takeuchi T, Sonomura K, Kawabata N, Koike Y, Takehashi M, Tanaka S, Ueda K, Simpson JC, Jones AT, Sugiura Y, Futaki S: Cellular uptake of arginine-rich peptides: Roles for macropinocytosis and actin rearrangement. Mol Ther 10: 1011–1022, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Yewdell JW, Bennink JR: Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science 244: 1072–1075, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL: Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78: 761–771, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Dick LR, Cruikshank AA, Grenier L, Melandri FD, Nunes SL, Stein RL: Mechanistic studies on the inactivation of the proteasome by lactacystin: A central role for clasto-lactacystin beta-lactone. J Biol Chem 271: 7273–7276, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Gorevic PD, Levo Y, Chatpar PC, Frangione B, Franklin EC: Some effects of the administration of endotoxin in mice: Specific cleavage of serum albumin by an acid protease and the generation of amyloid serum component. J Clin Invest 63: 254–261, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaiser VL, Sifri ZC, Senthil M, Dikdan GS, Lu Q, Xu DZ, Deitch EA: Albumin peptide: A molecular marker for trauma/hemorrhagic-shock in rat mesenteric lymph. Peptides 26: 2491–2499, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Peters T Jr, Hawn C: Isolation of two large peptide fragments from the amino- and carboxyl-terminal positions of bovine serum albumin. J Biol Chem 242: 1566–1573, 1967 [PubMed] [Google Scholar]
- 25.Weber G, Young LB: Fragmentation of bovine serum albumin by pepsin: I. The origin of the acid expansion of the albumin molecule. J Biol Chem 239: 1415–1423, 1964 [PubMed] [Google Scholar]
- 26.Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ: CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int 70: 591–596, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Donadelli R, Zanchi C, Morigi M, Buelli S, Batani C, Tomasoni S, Corna D, Rottoli D, Benigni A, Abbate M, Remuzzi G, Zoja C: Protein overload induces fractalkine upregulation in proximal tubular cells through nuclear factor kappaB- and p38 mitogen-activated protein kinase-dependent pathways. J Am Soc Nephrol 14: 2436–2446, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES: Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 307: 1630–1634, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Voisine C, Hubert FX, Trinite B, Heslan M, Josien R: Two phenotypically distinct subsets of spleen dendritic cells in rats exhibit different cytokine production and T cell stimulatory activity. J Immunol 169: 2284–2291, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Vermeulen M, Giordano M, Trevani AS, Sedlik C, Gamberale R, Fernandez-Calotti P, Salamone G, Raiden S, Sanjurjo J, Geffner JR: Acidosis improves uptake of antigens and MHC class I-restricted presentation by dendritic cells. J Immunol 172: 3196–3204, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Andreev OA, Dupuy AD, Segala M, Sandugu S, Serra DA, Chichester CO, Engelman DM, Reshetnyak YK: Mechanism and uses of a membrane peptide that targets tumors and other acidic tissues in vivo. Proc Natl Acad Sci U S A 104: 7893–7898, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rock KL, Shen L: Cross-presentation: Underlying mechanisms and role in immune surveillance. Immunol Rev 207: 166–183, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Matzinger P: The danger model: A renewed sense of self. Science 296: 301–305, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Cunningham MW: Cardiac myosin and the TH1/TH2 paradigm in autoimmune myocarditis. Am J Pathol 159: 5–12, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vigneron N, Stroobant V, Chapiro J, Ooms A, Degiovanni G, Morel S, van der Bruggen P, Boon T, Van den Eynde BJ: An antigenic peptide produced by peptide splicing in the proteasome. Science 304: 587–590, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Sinn DI, Lee ST, Chu K, Jung KH, Kim EH, Kim JM, Park DK, Song EC, Kim BS, Yoon SS, Kim M, Roh JK: Proteasomal inhibition in intracerebral hemorrhage: Neuroprotective and anti-inflammatory effects of bortezomib. Neurosci Res 58: 12–18, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.