Abstract
Imatinib is a receptor tyrosine kinase inhibitor that blocks the activity of c-Abl, c-Kit, and PDGF receptors. We tested the protective effects of imatinib in thymic stromal lymphopoietin transgenic mice, a model of cryoglobulinemia and associated membranoproliferative glomerulonephritis (MPGN), in which some glomerular manifestations likely result from PDGF receptor activation. Surprising, administration of imatinib beginning at weaning suppressed production of cryoglobulin, attenuating both the renal injury and systemic features of cryoglobulinemia. Flow cytometry suggested that inhibition of B cell development in the bone marrow likely caused the reduction in cryoglobulin production. In addition, administration of imatinib to thymic stromal lymphopoietin transgenic mice with established MPGN also diminished cryoglobulin production and reversed the renal and systemic lesions. These data suggest that treatment with imatinib may be a novel therapeutic approach for cryoglobulinemia and MPGN in humans.
Thymic stromal lymphopoietin (TSLP) is a four-helix bundle cytokine first identified as having growth-promoting properties for pre-B cells.1–3 Recent studies have shown that TSLP is a critical factor for the induction of Th2-type immune responses and inflammation, and several hematopoietically derived cell populations have been shown to be TSLP responsive, including CD4 T cells, B cells, CD11c+ dendritic cells, monocytes, and mast cells.4–7 In addition to its role in allergic inflammatory responses, TSLP has been shown to affect B cell responses in vivo. Through still undetermined mechanisms, transgenic mice that express TSLP under the control of the proximal lck promoter have been shown to develop reliably a disorder in which B cells produce abnormal Ig that result in a mixed cryoglobulinemia and a cryoglobulinemic membranoproliferative glomerulonephritis (MPGN) that closely resembles human MPGN.8 Humans afflicted with mixed cryoglobulinemia (a disorder in which complexes of Ig precipitate in the cold) develop various manifestations, including small vessel vasculitis affecting the skin and visceral organs, and MPGN. In severely affected patients, this disorder can be fatal. Currently, there is no reliably effective therapy for human cryoglobulinemia or its associated MPGN. The predictability and early development of the disease in the TSLP model makes these mice particularly well suited to study pathogenetic mechanisms and specific interventions designed to alter the development of cryoglobulinemia and its manifestations.
Imatinib (Gleevec; Novartis Pharmaceuticals, East Hanover, NJ), a selective tyrosine kinase inhibitor that inhibits c-Abl, c-Kit, and PDGF receptors (PDGFR), has therapeutic efficacy in patients with chronic myeloid leukemia and gastrointestinal stromal tumors.9,10 The therapeutic benefit of imatinib in animal models of kidney diseases was reported and has largely been attributed to its effect on PDGFR.11–16 It is well established that the PDGF-B and -D isoforms induce glomerular mesangial cell proliferation through engagement of the PDGFR-β.17–20 Furthermore, it has been shown that glomerular cell proliferation and extracellular matrix expansion in TSLP-transgenic (TSLP-Tg) mice are closely associated with enhanced expression of PDGF-B and PDGFR-β.21 These data indicate that the PDGF ligand/receptor system is an attractive therapeutic target to ameliorate the glomerular injury in the TSLP-Tg MPGN model. Lacking suitable highly specific reagents (e.g., neutralizing antibodies to PDGF) to test this strategy in mice, an obvious consideration for this approach was imatinib.
Our studies demonstrated remarkable and unexpected protective and therapeutic effects of imatinib on the renal injury of cryoglobulinemic MPGN as well as the systemic injuries of cryoglobulinemia, resulting from a profound suppression of cryoglobulin production. A likely mechanism responsible for these unanticipated results is direct inhibition of early B cell development by imatinib. Several reports have shown that c-Kit or c-Abl is involved in B cell development.22–29 These reports, together with our findings, suggest that imatinib might prevent cryoglobulin production through inhibition of one of these tyrosine kinases, in addition to its effects on PDGFR. Treatment with a receptor tyrosine kinase inhibitor is a novel therapeutic approach for attenuation of disease manifestations of cryoglobulinemia that may specifically benefit patients with cryoglobulinemic MPGN.
RESULTS
Imatinib Markedly Reduces Cryoglobulin Production and Alters the Serum Ig Profile in TSLP-Tg Mice
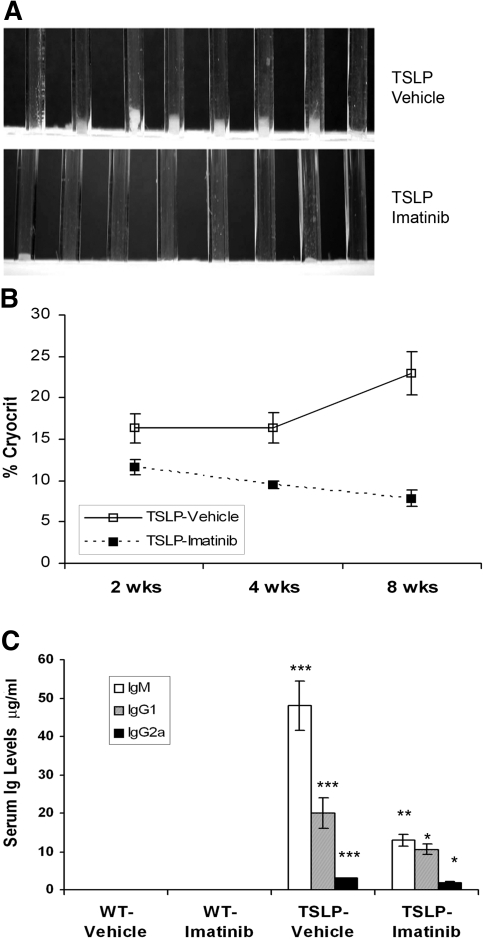
TSLP-Tg mice treated with vehicle developed marked cryoglobulinemia, progressive MPGN, and systemic inflammatory injuries to lung, liver, and skin identical to our previous descriptions of this model.8,30 In mice treated beginning at weaning, the total amount of circulating cryoglobulins, as determined by volume of serum cryoprecipitate, was markedly reduced and frequently undetectable in imatinib-treated TSLP-Tg mice compared with untreated control mice, assessed by both visual examination (Figure 1A) and measured cryocrit (Figure 1B). The serum IgM, IgG1, and IgG2a concentrations in TSLP-Tg mice were markedly increased compared with those of wild-type (WT) mice. Imatinib treatment resulted in significantly decreased levels of each of these Ig in TSLP-Tg mice (Figure 1C). Similarly, treatment of mice with established MPGN resulted in reduction of cryoglobulins (six of six untreated mice had visible cryoglobulins versus one of six imatinib-treated Tg mice) and serum Ig (Supplemental Table 4).
Figure 1.
Imatinib decreases cryoglobulin levels and serum Ig repertoire in TSLP-Tg mice. (A) The sera from vehicle-treated TSLP-Tg mice demonstrate visible cryoprecipitates but are dramatically reduced after 4 wk of treatment with imatinib. (B) Compared with vehicle-treated TSLP-Tg mice, a significant decrease of cryocrit is observed at each time point. Data are means ± SEM; n = 8 mice per group. Mann-Whitney test: *P < 0.05, **P < 0.01, ***P < 0.001: vehicle-treated TSLP-Tg mice versus imatinib-treated TSLP-Tg mice. (C) A significant decrease of serum Ig repertoire is seen after 4 wk of treatment with imatinib in TSLP-Tg mice. Data are means ± SEM; n = 6 to 8 mice per group. ANOVA, followed by Tukey-Kramer test: *P < 0.05, **P < 0.01, ***P < 0.001: vehicle-treated TSLP-Tg mice versus imatinib-treated TSLP-Tg mice; †P < 0.001: vehicle-treated WT mice versus vehicle-treated TSLP-Tg mice.
Imatinib Reduces Renal Injury in Cryoglobulinemic Mice
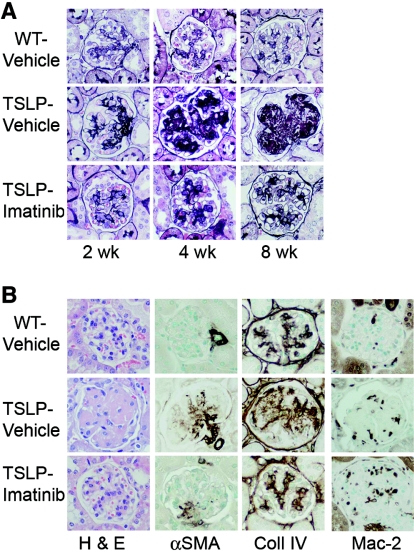
Treatment with imatinib reduced immune complex (IC) deposition in glomeruli. Figure 2A depicts histologic sections of glomeruli. In control TSLP-Tg mice, the mesangium was widened and some of the capillary lumina were occupied by IC deposits, features that increased over time. After 8 wk of treatment with vehicle in TSLP-Tg mice, the majority of glomeruli showed prominent glomerular lobulation and occlusion of capillary lumina as a result of massive IC deposition. In contrast, imatinib treatment dramatically reduced the widening of the mesangial area and the extent of IC deposits, with preservation of the patency of capillary lumina.
Figure 2.
Imatinib attenuates glomerulonephritis in TSLP-Tg mice. (A) TSLP-Tg mice treated with vehicle (middle) develop progressive accumulation of matrix and deposits of IC compared with WT mice (top). Treatment with imatinib dramatically reduces extracellular matrix expansion and IC deposits in TSLP-Tg mice at each time point (bottom), and depicted graphically in Supplemental Figure 7. (B) Treatment with imatinib attenuates renal injury in TSLP-Tg mice with decreased glomerular matrix deposition (collagen type IV expression) and mesangial cell activation (α-SMA expression), despite increased glomerular macrophage influx (Mac-2 expression), shown graphically in Supplemental Figure 7. Glomerular cellularity is not statistically affected by imatinib treatment (hematoxylin and eosin [H & E] stain), shown graphically in Supplemental Figure 7. Magnification, ×400.
The glomerular tuft area (GTA) in TSLP-Tg mice was significantly reduced after both early and late imatinib treatment (Figure 2A, Supplemental Figure 7). In histologic sections of mice treated early, the percentage of GTA occupied by silver-stained matrix in control TSLP-Tg mice was significantly reduced after imatinib treatment (Figure 2A, Supplemental Figure 7). A second measure of extracellular matrix expansion, accumulation of collagen IV, was significantly decreased by imatinib treatment compared with vehicle-treated TSLP-Tg mice (Figure 2B, Supplemental Figure 7). Glomerular cellularity, assessed in glomerular cross-sections, trended toward an increase in imatinib-treated TSLP-Tg mice compared with controls, but this trend did not achieve statistical significance (Figure 2B, Supplemental Figure 7). Imatinib treatment was associated with a dramatic reduction of mesangial cell activation as assessed by the percentage of GTA occupied by α-smooth muscle actin (α-SMA)-expressing cells (Figure 2B, Supplemental Figure 7). Figure 2B depicts the typically increased glomerular Mac-2–positive monocyte/macrophage infiltration in TSLP-Tg mice compared with WT mice, which was further increased in imatinib-treated TSLP-Tg mice (Figure 2B, Supplemental Figure 7).
Mice that had established MPGN and were treated for 30 d with imatinib also demonstrated a reduction in both mesangial matrix and α-SMA–positive cells (Table 1, Supplemental Figure 8). TSLP-Tg control mice had significantly more glomerular Mac-2+ macrophages than WT mice, and this was increased in imatinib-treated TSLP-Tg mice (Table 1, Supplemental Figure 8).
Table 1.
Morphometric data from mice receiving 4 wk of imatinib treatment beginning at day 90a
| Parameter | WT | TSLP-Control | TSLP-Imatinib |
|---|---|---|---|
| % GTA occupied by silver-stained matrix | 6.25 ± 0.95 | 14.27 ± 0.11b | 6.79 ± 0.66d |
| Cell number/glomerular cross-section | 32.70 ± 1.34 | 38.13 ± 0.69c | 38.70 ± 1.91c |
| % GTA occupied by collagen type IV–stained matrix | 10.47 ± 0.43 | 17.06 ± 1.48b | 13.46 ± 0.68e |
| % GTA occupied by α-SMA–expressing cells | 0.65 ± 0.06 | 1.76 ± 0.17b | 1.26 ± 0.24f |
| GTA occupied by Mac-2–expressing cells (μm2) | 4.98 ± 1.18 | 61.36 ± 11.89b | 142.33 ± 51.98c,e |
Data are means ± SEM.
P < 0.001,
P < 0.01 versus WT mice.
P < 0.001,
P < 0.05,
P < 0.01 versus control TSLP-Tg mice.
There was significantly increased albumin excretion (μg albumin/24 h) in vehicle-treated TSLP-Tg mice compared with WT controls, which was abolished by imatinib treatment (WT-Vehicle 4.2 +/− 0.8, WT-Imatinib 4.4 +/− 1.3, TSLP-Vehicle 16.6 +/− 4.7* and TSLP-Imatinib 4.9 +/− 1.6, *P < 0.05 vs. imatinib treated TSLP mice and WT mice). Analysis of spot urine obtained from the animals that received late treatment with imatinib also demonstrated significantly diminished albumin/creatinine ratios compared with TSLP-Tg controls (13.72 ± 5.36 versus 22.48 ± 5.36; P < 0.05). Serum creatinine levels in TSLP-Tg mice were essentially identical to that in WT mice (8 wk: 0.19 ± 0.01 versus 0.15 ± 0.01 mg/dl; NS, control WT mice versus control TSLP-Tg mice), which was not affected by treatment (8 wk: 0.15 ± 0.01 versus 0.17 ± 0.02 mg/dl; NS, control TSLP-Tg mice versus imatinib-treated TSLP-Tg mice).
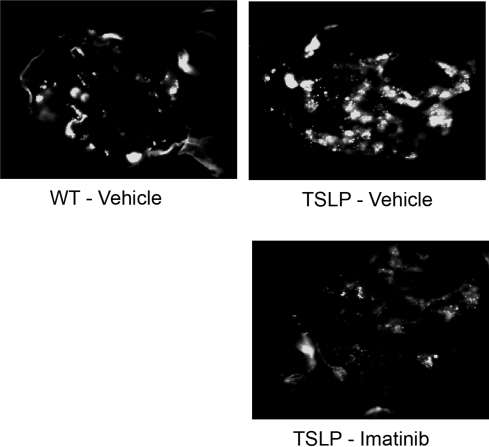
TSLP-Tg mice showed increased deposition of Ig and complement component C3 in the glomeruli compared with WT mice as detected by immunofluorescence microscopy (Figure 3, Supplemental Figure 9). The glomerular IgG and IgM deposition in TSLP-Tg mice trended toward a reduction after 8 wk treatment with imatinib, but this trend did not achieve statistical significance (Figure 3). Mice receiving late treatment with imatinib demonstrated a reduction in positive area stained with IgG and IgM, although the fluorescence intensity remained similar. Glomerular C3 deposition in imatinib-treated TSLP-Tg mice was reduced significantly compared with that in control mice (Figure 3, Supplemental Figure 9). Although late treatment with imatinib resulted in decreased C3 deposition (control WT 0.00 ± 0.00; control TSLP-Tg mice 1.63 ± 0.45; imatinib-treated TSLP-Tg mice 1.33 ± 0.21), this trend was not statistically significant.
Figure 3.
Imatinib reduces glomerular C3 and IC deposition. Representative C3 stained immunofluorescence pictures at 8 wk of treatment show a significant decrease in the imatinib-treated mouse.
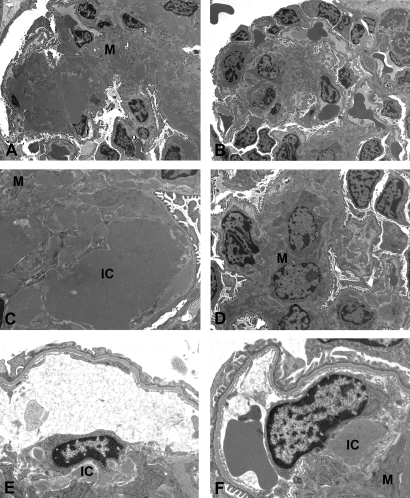
Electron micrographs of glomeruli of control TSLP-Tg mice showed an increase in the amount of matrix compared with WT mice. Extensive and focally massive electron-dense immune deposits were apparent in the mesangium as well as in the subendothelial spaces of glomerular capillary walls (Figure 4, A and C). The imatinib-treated TSLP-Tg mice showed lesser amounts of matrix and few electron-dense immune deposits (Figure 4, B and D). In electron micrographs from mice treated after disease was established, macrophages can be seen in close contact and apparently internalizing the immune deposits (Figure 4, E and F).
Figure 4.
Electron microscopic features of the glomerular lesions. (A through D) Whereas glomeruli from the vehicle-treated TSLP-Tg mice showed extracellular matrix expansion with massive electron-dense IC deposits in the mesangium as well as in the subendothelial space (A and C), 4 wk of treatment with imatinib results in dramatic reduction of an extracellular matrix expansion and IC deposits (B and D). (E and F) Mice that received imatinib treatment beginning at day 90 also showed a reduction of IC deposition, and macrophages can be seen apparently internalizing these complexes. M, mesangium. Magnifications: ×2400 in A and B; ×7100 in C; ×4400 in D; ×10,400 in E and F.
Imatinib Reduces Systemic Injuries in Cryoglobulinemic Mice
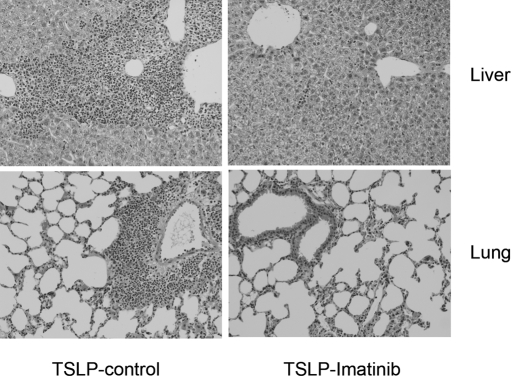
TSLP-Tg mice exhibited liver injury characterized by the presence of portal and periportal inflammation, foci of parenchymal injury, and fibrosis of portal tracts as described previously.31 Imatinib treatment reduced the infiltration of inflammatory cells, and the parenchymal injury and fibrosis were attenuated in a time-dependent manner and in the animals receiving late treatment (Figure 5, Table 2). TSLP-Tg mice develop severe lung involvement with mixed peribronchial and perivascular leukocyte infiltration. Treatment with imatinib reduced leukocyte infiltration in the lungs of TSLP-Tg mice, in a time-dependent manner, and in the late treatment animals, although mild peribronchial and perivascular inflammation persisted (Figure 5, Table 2). In TSLP-Tg mice, survival at day 77 (after 8 wk of treatment with vehicle commenced) was 66.7% (eight of 12 animals survived). Treatment with imatinib improved the survival rate to 87.5% (seven of eight animals survived).
Figure 5.
Imatinib reduces liver and lung injury in TSLP-Tg mice. Representative liver and lung sections stained with H & E of a control TSLP-Tg mouse and an imatinib-treated TSLP-Tg mouse receiving 4 wk of treatment beginning at age day 90. Imatinib dramatically reduces portal inflammatory cell infiltrates and lung infiltrates.
Table 2.
Grading of liver and lung inflammation and staging of liver fibrosis in vehicle-treated and imatinib-treated TSLP-Tg mice show significant reduction of inflammation and fibrosis in imatinib-treated TSLP-Tg mice in a time-dependent mannera
| Parameter | Time Course Study
|
Late Treatment Study
|
||
|---|---|---|---|---|
| TSLP-Vehicle | TSLP-Imatinib | TSLP-Control | TSLP-Imatinib | |
| Liver injury | ||||
| portal inflammation | ||||
| 2 wk | 2.17 ± 0.40 | 1.33 ± 0.21b | ||
| 4 wk | 2.71 ± 0.44 | 1.125 ± 0.125c | 1.56 ± 0.24 | 0.36 ± 0.14c |
| 8 wk | 3.14 ± 0.33 | 0.43 ± 0.17d | ||
| periportal injury | ||||
| 2 wk | 2.00 ± 0.26 | 1.33 ± 0.21b | ||
| 4 wk | 2.28 ± 0.52 | 0.25 ± 0.25c | 1.38 ± 0.25 | 0.29 ± 0.15b |
| 8 wk | 2.42 ± 0.40 | 0.14 ± 0.12d | ||
| parenchymal injury | ||||
| 2 wk | 1.83 ± 0.31 | 0.66 ± 0.21e | ||
| 4 wk | 1.42 ± 0.28 | 0.63 ± 0.26b | 1.06 ± 0.10 | 0.57 ± 0.20e |
| 8 wk | 1.71 ± 0.40 | 0.28 ± 0.15c | ||
| fibrosis | ||||
| 2 wk | 2.00 ± 0.36 | 0.50 ± 0.17e | ||
| 4 wk | 2.42 ± 0.53 | 0.25 ± 0.16b | 0.94 ± 0.18 | 0.43 ± 0.17e |
| 8 wk | 2.29 ± 0.24 | 0.14 ± 0.12d | ||
| Lung injury | ||||
| inflammation | ||||
| 2 wk | 2.50 ± 0.19 | 2.50 ± 0.19 | ||
| 4 wk | 2.13 ± 0.21 | 1.38 ± 0.25e | 2.19 ± 0.18 | 0.93 ± 0.20c |
| 8 wk | 2.36 ± 0.23 | 1.29 ± 0.17b | ||
The late treatment study, with mice treated for 4 wk beginning at day 90, also showed significant reductions in liver and lung inflammation. Data are means ± SEM; n = 6 to 8 mice per group;
Mann-Whitney test:
P < 0.01,
P < 0.001,
P < 0.0001,
P < 0.05: vehicle-treated TSLP tg mice vs imatinib-treated TSLP tg mice.
Imatinib Leads to a Reduction in Both Immature and Mature Splenic B Cells in TSLP-Tg Mice
We recently showed that both lck-TSLP-Tg mice and mice Tg for the TSLP under the control of a doxycycline-inducible keratin-5 promoter skin specific (K5-TSLP) exhibit a marked increase in bone marrow (BM) B cell lymphopoiesis.32 This change occurs through a direct effect of TSLP via promotion of pre-B cell clonal expansion. Both strains exhibit an increase in the exodus of immature, B220+AA4.1+ B cells from the BM and accumulation of large numbers of immature transitional 1 (T1) and naive follicular mature (FM) B cells within the spleen. In parallel, sustained elevation in systemic levels of TSLP also leads to a near complete disappearance of splenic marginal zone (MZ) and marginal zone precursor (T2/MZP) B cells.32
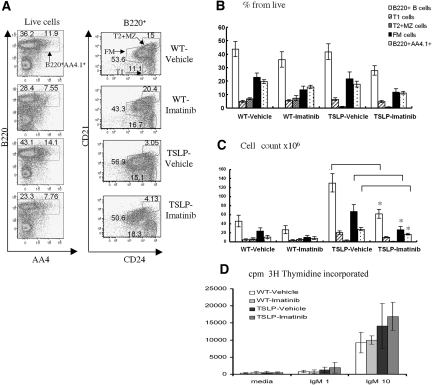
We isolated spleens from control vehicle-treated versus imatinib-treated WT or TSLP-Tg mice and analyzed the composition of the splenic B cell compartment including total B220+AA4+ immature, FM, and MZ B cells by FACS. In WT mice, imatinib-treatment led to a modest reduction in the total number of splenic B cells, and this change reflected a numerical decrease in both immature, AA4+ and mature, FM B cells. These numerical changes, however, did not achieve statistical significance in the limited number of mice evaluated (Figure 6).
Figure 6.
Imatinib inhibits B cell development in TSLP-Tg mice. Splenic B cell subsets were identified on the basis of seven-color FACS analysis after 4 wk of treatment in each study group. B cell subsets were defined as follows: Immature B cells as B220hi AA4.1+; transitional 1 (T1) B cells as B220 hi CD21lowCD24hi; FM B cells as B220hi CD21intCD24int; and transitional 2 and marginal zone (T2/MZ) B cells as B220hi CD21hiCD24hi. (A, left) Percentage of cells within live cell gate that fall within B220hiAA4.1+ immature B versus remaining B220hi B cell population. (Right) Percentage of cells within live, B220+ cell gate that comprise each designated B cell subset. (B and C) Relative and absolute numbers of each splenic B cell subset from designated study groups. Treatment with imatinib significantly reduced the absolute numbers of B220+ B cells, B220hi AA4.1+ immature cells, and FM B cells in TSLP-Tg mice (*P < 0.05). (D) B cell proliferation assay. Purified splenic B cells from each study group were stimulated with either 1 or 10 μg/ml anti-IgM or left unstimulated, and proliferation was assessed by [3H]thymidine incorporation. Data are means ± SEM. ANOVA, followed by Tukey-Kramer test: *P < 0.05: vehicle-treated TSLP-Tg mice versus imatinib-treated TSLP-Tg mice.
The spleen weight was reduced by 24 to 32% in imatinib-treated TSLP-Tg mice compared with vehicle-treated animals, at weeks 4 and 8 of therapy, whereas no significant difference was present in treated versus untreated WT mice. As evident in Figure 6 (and also reported in reference29), imatinib treatment in both control and TSLP-Tg mice led to a decrease in the percentage and absolute number of BB20+AA4.1+ and FM B cells. Although the percentage of total B220+ cells was not significantly different between imatinib-treated and untreated control mice (Figure 6B), imatinib treatment in TSLP-Tg mice led to a significant reduction (approximately 50%) in the absolute number of B220+ cells (Figure 6C). This difference reflected a numerical decrease in both immature, B220+AA4.1+ cells (approximately 40% decrease) and CD21intC24int FM B cells (approximately 60% decrease; Figure 6C). We also observed a numerical decrease in early transitional (T1) B cells, but this was not statistically significant. Both treated and untreated Tg mice exhibited a reduction in the relative number of cells committed to the MZ lineage, and this did not change significantly after treatment (T2-MZP and MZ B cells; Figure 6C).
Taken in concert with the described inhibitory effect of imatinib on early BM B lymphopoiesis,29 these data suggest that imatinib functioned to limit the generation of BM B cells capable of responding to TSLP. This blockade thereby reduced TSLP-driven pre-B cell expansion,32 leading, ultimately, to an overall reduction in the exodus of B220+AA4.1+ B cells from the marrow and subsequent generation of FM B cells.
Imatinib Treatment In Vivo Does not Affect In Vitro B Cell Proliferative Responses
On the basis of recent findings that imatinib can inhibit both B cell proliferation and Ig secretion in vitro,33 we also determined whether B cells from mice treated with imatinib manifest overt functional defects. We stimulated isolated B splenocytes from control or imatinib-treated mice with anti-IgM (without the addition of imatinib into the culture medium). Imatinib treatment in vivo did not appreciably alter in vitro B cell receptor (BCR)-induced proliferation of cells derived from either WT or TSLP-Tg mice (Figure 6D). B cells from TSLP-Tg mice proliferated to a slightly greater degree than control cells, likely reflecting the reduced number of BCR-nonresponsive MZ B cells within the total B cell pool; however, imatinib treatment in vivo did not blunt the BCR response in either WT or Tg mice. Although these data do not rule out the possibility that B cell responses might be affected by imatinib in vivo, our combined findings suggest that the primary therapeutic benefit of imatinib occurs via an overall reduction in the absolute number of antibody secreting cells.
Effect of Imatinib on Cryoglobulin Production and B Cell Maturation Is Independent of an Effect on Circulating TSLP Levels
Treatment with imatinib did not affect circulating TSLP levels in TSLP-Tg mice (2 wk: 23.10 ± 6.41 versus 34.54 ± 8.41 pg/ml [NS]; 4 wk: 8.81 ± 0.63 versus 9.09 ± 1.02 pg/ml [NS]; 8 wk: 17.03 ± 1.48 versus 13.22 ± 0.40 pg/ml [NS]; control TSLP-Tg mice versus imatinib-treated TSLP-Tg mice).
DISCUSSION
This study began as a focused intervention in PDGF/PDGFR-β–mediated glomerular cell proliferation in an experimental model of MPGN but yielded a surprising and striking clinical benefit in both the MPGN and systemic cryoglobulinemia of TSLP-Tg mice. This benefit included a dramatic reduction in key pathologic features of glomerular injury, reduction in IC deposition as revealed by histologic and ultrastructural analysis, and reduction of circulating cryoglobulins, frequently to the point of undetectability. This study demonstrates that the structural and pathologic alterations of MPGN, induced by IC deposition, can be reversed by effective therapy directed against the initiating stimulus. This improvement in renal injury was obtained whether mice were treated early in the disease course or when the disease was well established at the time therapy was initiated. These benefits were also demonstrable in other organs affected by cryoglobulin deposition, and these benefits led to reduced mortality in Tg mice. Such rapid improvement in MPGN with effective therapy directed against the stimulus for IC deposition suggests great promise for the resolution of human MPGN should comparably effective treatments directed toward the underlying stimulus be developed.
The dramatic improvement in cryoglobulinemia and MPGN conferred by imatinib treatment is likely the result of its effect on the overall size of the peripheral B cell compartment. Our studies demonstrated imatinib treatment significantly affected splenic B cell numbers with a marked reduction in both immature AA4.1+ and mature follicular B cells. In addition, although the changes observed did not reach statistical significance, we also observed a trend toward reduced peripheral B cell numbers in imatinib-treated WT animals. These combined data support the conclusion that imatinib inhibits B cell development at an early stage(s) within the BM and are consistent with a previous study indicating that imatinib therapy leads to increased apoptosis in BM B cell progenitors.26,29 We reported recently that TSLP mediates an increase in pre-B cell clonal expansion.32 Additional experiments are required to determine whether imatinib directly affects this pre-B cell expansion versus exerting its negative effects at an earlier stage in B lymphopoiesis. Our findings do not rule out a potential, concurrent role for imatinib in modulating the survival of immature and mature B cells upon entry into the peripheral lymphoid compartment.
The simplest explanation for the clinical benefit observed in this study is that the imatinib-mediated decrease in absolute numbers of antibody-secreting peripheral B cells in TSLP-Tg mice directly limits cryoglobulin production in treated animals, in turn limiting cryoglobulin deposition in the kidneys, rather than acting through postdevelopmental B cell functional inhibition. This interpretation is consistent with the overall reduction in total IgM, IgG1, and IgG2a observed in imatinib-treated TSLP-Tg animals and with our recent finding that peripheral B cells in the spleen are major producers of the cryoglobulin-forming IgG and IgM in TSLP-Tg mice.32 Although we failed to identify any effect of the levels of imatinib achieved in vivo on BCR-driven B cell proliferation, it remains possible that imatinib may partially inhibit T-dependent or -independent B cell activation signals in vivo, thereby affecting antibody production at additional steps in this cascade.
The mechanism by which imatinib modulates BM B cell development in TSLP-Tg mice remains to be fully elucidated. One likely candidate effect is via direct inhibition of c-Abl kinase activity. c-Abl–deficient mice exhibit elevated apoptosis and a reduction in early BM B cell progenitors and pre-B cells.26 Imatinib also directly inhibits c-Kit signaling. c-Kit–deficient mice display an age-dependent block in early BM B cell development,28 and mice expressing the mutant KitY567F allele, which fails to activate Src family kinase signaling, exhibit a similar developmental arrest. Consistent with these findings, treatment of WT mice with imatinib mimics this phenotype29 and leads to a reduction in the BM B cell populations that are specifically expanded in TSLP-Tg animals. Thus, the marked decrease in relative numbers of immature, AA4.1+ B cells in imatinib-treated versus control animals is consistent with alterations in either c-Abl or c-Kit function or, most likely, a combined effect on both signaling cascades.
A noteworthy finding was that the improvement in structural injury and reduced proteinuria resulting from imatinib therapy was accompanied by increased glomerular cellularity and increased influx of monocyte/macrophages, indicating that at least some monocyte populations may be beneficial in amelioration of GN. We are unable to ascertain the basis for the influx of monocytes; we hypothesize that the increased monocyte/macrophages represent a population of pro-reparative rather than proinflammatory leukocytes. Although there is growing evidence that circulating monocytes are heterogeneous and can be separated by both surface marker expression and their ability to migrate in or out of tissues, it has been more difficult to achieve consensus definitions of what constitutes a proinflammatory or pro-repair monocyte.34 Given this uncertainty, our hypothesized basis for the increased monocytes/macrophages remains untested.
In conclusion, our findings demonstrate that renal and systemic injuries in TSLP-Tg mice are dramatically attenuated by treatment with imatinib. This protective effect seems to be largely due to effects of imatinib on B cell development that, in turn, diminish cryoglobulin production. The mechanism of cryoglobulin induction and whether imatinib also directly acts on these events, however, still remains to be determined. It also remains unclear whether inhibition of PDGFR activity by imatinib has additional benefits in this process. Overall, our results strongly suggest that imatinib or related tyrosine kinase inhibitors may provide a novel means to achieve a significant therapeutic benefit in patients with cryoglobulinemia and MPGN.
CONCISE METHODS
Experimental Protocol
The experimental protocol for this study was reviewed and approved by the Animal Care Committee of the University of Washington in Seattle. Mice Tg for TSLP have been previously described in detail.8 Female mice were used in the first part of the study examining the effects of imatinib early in the disease course, because of the shorter time frame in which the MPGN develops in the female gender. Both TSLP-Tg and WT mice were randomly assigned to four groups: Treated TSLP-Tg mice, untreated TSLP-Tg mice, treated WT mice, and untreated WT mice. Treated groups received imatinib daily by intraperitoneal injection at a dosage of 50 mg/kg. Vehicle-treated groups received an equal volume of sterile water. Treatment was started after weaning at day 21 and was continued for 2, 4, or 8 wk, when mice were then killed.
In the second part of the study examining the effect of imatinib in mice with established disease, male TSLP-Tg mice at the age of 90 d, when chronic MPGN with glomerular matrix accumulation is well established, were treated with daily intraperitoneal injections of imatinib (50 mg/kg) for 4 wk and then killed. Untreated male TSLP-Tg mice and WT mice at the age of 120 d were used as controls. For each group, six to eight mice were analyzed. Body weight was examined every week after treatment until the mice were killed. Urine samples were collected in each mouse 1 d before being killed. At the end of the study, mice were killed and blood and organs were collected. Renal tissue was collected in neutral-buffered formalin and methyl Carnoy solution, then processed and embedded in paraffin, sectioned, and stained using standard methods. Portions of kidney tissue were snap-frozen for immunofluorescence study. Tissue was collected in half-strength Karnovsky fixative for electron microscopy as described previously.8
Immunohistochemical and Immunofluorescence Analysis
Mac-2, α-SMA, and collagen IV immunostaining were performed as described previously.30,35 We performed immunofluorescence analysis for IgM, IgG, IgA, and C3 as described previously.8
Flow Cytometry
Single-cell suspensions from spleens were obtained from control vehicle- and imatinib-treated WT and TSLP-Tg mice to determine B cell composition, as described previously.32 Suspensions were incubated with fluorescently labeled antibodies for 15 min at 4°C in staining buffer (PBS with 0.5% BSA of 2.5% FCS). Data were collected on FACSCalibur or LSR II flow cytometer (BD Biosciences, Mountain View, CA) and analyzed using FlowJo software (Treestar, Ashland, OR). For LSR II experiments, the data were analyzed using biexponential transformation function for complete data visualization. The following antibodies were used for staining: CD24-FITC and CD21-PE (BD Biosciences). Monoclonal antibodies to B220 and AA4.1 were obtained from eBioscience (San Diego, CA). For sorting, cells were labeled with specific antibodies in staining buffer and filtered through a 35-μM mesh before sorting. Cells were sorted with FACSAria cell sorter with Diva software (BD Biosciences).
[3H]Thymidine Uptake Proliferation Assay of Splenic B Cells
Purified splenic B cells were incubated at 5 × 104 cells per well in RPMI with 10% FCS plus supplement for 48 h in the presence of F(ab′)2 of goat anti-mouse IgM (μ-chain; Jackson ImmunoResearch Laboratories, West Grove, PA). Cells were pulsed with 1 μCi [3H]thymidine for the last 8 h before harvesting. Cells were harvested, and [3H]thymidine uptake was analyzed using a scintillation counter.
Laboratory Data
Timed urine was collected in the cohorts of mice treated beginning at weaning. Mice in the study group treated beginning at 90 d had proteinuria measured from spot urine samples. Albuminuria was measured using the Albuwell (Exocell, Philadelphia, PA) mouse albumin ELISA according to the manufacturer's protocols, and μg/albumin per 24 h was determined from the timed urine samples. Creatinine levels were assessed using the Creatinine Companion Kit (Exocell), and albumin/creatinine ratios were determined for spot urine. Serum TSLP levels were measured using the Quantikine (R&D Systems, Minneapolis, MN) mouse TSLP ELISA. Sera were analyzed by ELISA for total IgG1, IgG2a, and IgM. For total serum IgG1, IgG2a, and IgM, plates were coated overnight with 2 μg/ml goat anti-mouse Ig (H+L; Southern Biotechnology Associates, Birmingham, AL); blocked with PBS/1% BSA; and incubated sequentially with serial dilutions of mouse sera, 2 μg/ml alkaline phosphatase–conjugated rat anti-mouse IgG1/IgG2a/IgM (1:1000; Southern Biotechnology Associates, Birmingham, AL), and 1 mg/ml of the alkaline phosphatase substrate DNP phosphate (Sigma-Aldrich, St. Louis, MO). Plates were read on a microplate autoreader at 405 nm, and concentrations were calculated by plotting against standard curves generated from purified IgG1, IgG2a, and IgM (Southern Biotechnology Associates).
Analytical Methods
The degree of renal histopathologic alterations was quantified using the Image Pro Plus Software (Media Cybernetics, Silver Spring, MD) as described previously.8 Serum samples were stored at 4°C for >5 d, and cryocrits were photographed and quantified with this software. Fluorescence intensity of renal tissue stained for deposition of immune reactants was assessed on a scale from 0 (negative) to 3 (strong staining) as described previously.8 We used tissue sections stained with hematoxylin and eosin, periodic acid-Schiff, Masson's trichrome, and Sirius red stains for histologic assessment of liver injury. To assess the grade of inflammation and stage of the liver fibrosis, we evaluated the tissue sections according to the modified Histologic Activity Index of Knodell (AKA Ishak score).31 We used tissue sections stained with hematoxylin and eosin for histologic assessment of lung injury. The lung inflammation was scored blindly on a scale of 0 to 3 for inflammation as follows: 0, no inflammation; 1, mild; 2, moderate; and 3, severe.
Statistical Analysis
Data were recorded as means ± SEM. Differences between multiple groups were evaluated using ANOVA, followed when significant (P < 0.05) by the Tukey-Kramer test or, when only two groups were compared, the Mann-Whitney test.
DISCLOSURES
None.
Acknowledgments
This work was supported by grants DK68802 from the National Institutes of Health (to C.E.A.), AI68731 (to S.F.Z.), AI44259 (to S.F.Z. and D.J.R.), and HD37091 (to D.J.R.). M.I. is supported by a grant from the promotion and mutual aid corporation for private schools of Japan.
Portions of this work were presented at the 39th annual meeting of the American Society of Nephrology; November 14 through November 19, 2006; San Diego CA.
The imatinib (Gleevec) used in this study was a gift from Novartis, which did not otherwise participate in the design, execution, or funding of this study.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A: A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol 22: 321–328, 1994 [PubMed] [Google Scholar]
- 2.Levin SD, Koelling RM, Friend SL, Isaksen DE, Ziegler SF, Perlmutter RM, Farr AG: Thymic stromal lymphopoietin: A cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J Immunol 162: 677–683, 1999 [PubMed] [Google Scholar]
- 3.Ray RJ, Furlonger C, Williams DE, Paige CJ: Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur J Immunol 26: 10–16, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF: Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol 6: 1047–1053, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Ziegler SF, Liu YJ: Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol 7: 709–714, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, Comeau MR, Campbell DJ, Ziegler SF: Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med 202: 541–549, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Shami A, Spolski R, Kelly J, Fry T, Schwartzberg PL, Pandey A, Mackall CL, Leonard WJ: A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med 200: 159–168, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taneda S, Segerer S, Hudkins KL, Cui Y, Wen M, Segerer M, Wener MH, Khairallah CG, Farr AG, Alpers CE: Cryoglobulinemic glomerulonephritis in thymic stromal lymphopoietin transgenic mice. Am J Pathol 159: 2355–2369, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong S, Witte ON: The BCR-ABL story: Bench to bedside and back. Annu Rev Immunol 22: 247–306, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Druker BJ: Imatinib as a paradigm of targeted therapies. Adv Cancer Res 91: 1–30, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Gilbert RE, Kelly DJ, McKay T, Chadban S, Hill PA, Cooper ME, Atkins RC, Nikolic-Paterson DJ: PDGF signal transduction inhibition ameliorates experimental mesangial proliferative glomerulonephritis. Kidney Int 59: 1324–1332, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Lassila M, Jandeleit-Dahm K, Seah KK, Smith CM, Calkin AC, Allen TJ, Cooper ME: Imatinib attenuates diabetic nephropathy in apolipoprotein E-knockout mice. J Am Soc Nephrol 16: 363–373, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Savikko J, Taskinen E, Von Willebrand E: Chronic allograft nephropathy is prevented by inhibition of platelet-derived growth factor receptor: Tyrosine kinase inhibitors as a potential therapy. Transplantation 75: 1147–1153, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Zoja C, Corna D, Rottoli D, Zanchi C, Abbate M, Remuzzi G: Imatinib ameliorates renal disease and survival in murine lupus autoimmune disease. Kidney Int 70: 97–103, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Sadanaga A, Nakashima H, Masutani K, Miyake K, Shimizu S, Igawa T, Sugiyama N, Niiro H, Hirakata H, Harada M: Amelioration of autoimmune nephritis by imatinib in MRL/lpr mice. Arthritis Rheum 52: 3987–3996, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Wang S, Wilkes MC, Leof EB, Hirschberg R: Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J 19: 1–11, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Floege J, Eitner F, Van Roeyen C, Ostendorf T: PDGF-D and renal disease: Yet another one of those growth factors? J Am Soc Nephrol 14: 2690–2691, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Hudkins KL, Gilbertson DG, Carling MD, S T, Hughes SD, Holdren MS, Palmer TE, Topouzis S, Haran AC, Feldhaus AL, Alpers CE: Exogenous PDGF-D is a potent mesangial cell mitogen and causes a severe mesangial proliferative glomerulopathy. J Am Soc Nephrol 15: 286–298, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Iida H, Seifert R, Alpers CE, Gronwald RG, Phillips PE, Pritzl P, Gordon K, Gown AM, Ross R, Bowen-Pope DF, et al.: Platelet-derived growth factor (PDGF) and PDGF receptor are induced in mesangial proliferative nephritis in the rat. Proc Natl Acad Sci U S A 88: 6560–6564, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson RJ, Floege J, Couser WG, Alpers CE: Role of platelet-derived growth factor in glomerular disease. J Am Soc Nephrol 4: 119–128, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Taneda S, Hudkins KL, Cui Y, Farr AG, Alpers CE, Segerer S: Growth factor expression in a murine model of cryoglobulinemia. Kidney Int 63: 576–590, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Schwartzberg PL, Stall AM, Hardin JD, Bowdish KS, Humaran T, Boast S, Harbison ML, Robertson EJ, Goff SP: Mice homozygous for the ablm1 mutation show poor viability and depletion of selected B and T cell populations. Cell 65: 1165–1175, 1991 [DOI] [PubMed] [Google Scholar]
- 23.Hardin JD, Boast S, Schwartzberg PL, Lee G, Alt FW, Stall AM, Goff SP: Bone marrow B lymphocyte development in c-abl-deficient mice. Cell Immunol 165: 44–54, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC: Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65: 1153–1163, 1991 [DOI] [PubMed] [Google Scholar]
- 25.Hardin JD, Boast S, Schwartzberg PL, Lee G, Alt FW, Stall AM, Goff SP: Abnormal peripheral lymphocyte function in c-abl mutant mice. Cell Immunol 172: 100–107, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Lam QL, Lo CK, Zheng BJ, Ko KH, Osmond DG, Wu GE, Rottapel R, Lu L: Impaired V(D)J recombination and increased apoptosis among B cell precursors in the bone marrow of c-Abl-deficient mice. Int Immunol 19: 267–276, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Rolink A, Streb M, Nishikawa S, Melchers F: The c-kit-encoded tyrosine kinase regulates the proliferation of early pre-B cells. Eur J Immunol 21: 2609–2612, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Waskow C, Paul S, Haller C, Gassmann M, Rodewald HR: Viable c-Kit(W/W) mutants reveal pivotal role for c-kit in the maintenance of lymphopoiesis. Immunity 17: 277–288, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Agosti V, Corbacioglu S, Ehlers I, Waskow C, Sommer G, Berrozpe G, Kissel H, Tucker CM, Manova K, Moore MA, Rodewald HR, Besmer P: Critical role for Kit-mediated Src kinase but not PI 3-kinase signaling in pro T and pro B cell development. J Exp Med 199: 867–878, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muhlfeld AS, Segerer S, Hudkins K, Carling MD, Wen M, Farr AG, Ravetch JV, Alpers CE: Deletion of the fcgamma receptor IIb in thymic stromal lymphopoietin transgenic mice aggravates membranoproliferative glomerulonephritis. Am J Pathol 163: 1127–1136, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowalewska J, Muhlfeld AS, Hudkins KL, Yeh MM, Farr AG, Ravetch JV, Alpers CE: Thymic stromal lymphopoietin transgenic mice develop cryoglobulinemia and hepatitis with similarities to human hepatitis C liver disease. Am J Pathol 170: 981–989, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Astrakhan A, Omori M, Nguyen T, Becker-Herman S, Iseki M, Aye T, Hudkins K, Dooley J, Farr A, Alpers CE, Ziegler SF, Rawlings DJ: Local increase in thymic stromal lymphopoietin induces systemic alterations in B cell development. Nat Immunol 8: 522–531, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Paniagua RT, Sharpe O, Ho PP, Chan SM, Chang A, Higgins JP, Tomooka BH, Thomas FM, Song JJ, Goodman SB, Lee DM, Genovese MC, Utz PJ, Steinman L, Robinson WH: Selective tyrosine kinase inhibition by imatinib mesylate for the treatment of autoimmune arthritis. J Clin Invest 116: 2633–2642, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon S, Taylor PR: Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Muhlfeld AS, Segerer S, Hudkins K, Farr AG, Bao L, Kraus D, Holers VM, Quigg RJ, Alpers CE: Overexpression of complement inhibitor Crry does not prevent cryoglobulin-associated membranoproliferative glomerulonephritis. Kidney Int 65: 1214–1223, 2004 [DOI] [PubMed] [Google Scholar]