Abstract
Chronic kidney disease (CKD) guidelines recommend evaluating patients with GFR <60 ml/min per 1.73 m2 for complications, but little evidence supports the use of a single GFR threshold for all metabolic disorders. We used data from the NephroTest cohort, including 1038 adult patients who had stages 2 through 5 CKD and were not on dialysis, to study the occurrence of metabolic complications. GFR was measured using renal clearance of 51Cr-EDTA (mGFR) and estimated using two equations derived from the Modification of Diet in Renal Disease study. As mGFR decreased from 60 to 90 to <20 ml/min per 1.73 m2, the prevalence of hyperparathyroidism increased from 17 to 85%, anemia from 8 to 41%, hyperphosphatemia from 1 to 30%, metabolic acidosis from 2 to 39%, and hyperkalemia from 2 to 42%. Factors most strongly associated with metabolic complications, independent of mGFR, were younger age for acidosis and hyperphosphatemia, presence of diabetes for acidosis, diabetic kidney disease for anemia, and both male gender and the use of inhibitors of the renin-angiotensin system for hyperkalemia. mGFR thresholds for detecting complications with 90% sensitivity were 50, 44, 40, 39, and 37 ml/min per 1.73 m2 for hyperparathyroidism, anemia, acidosis, hyperkalemia, and hyperphosphatemia, respectively. Analysis using estimated GFR produced similar results. In summary, this study describes the onset of CKD-related complications at different levels of GFR; anemia and hyperparathyroidism occur earlier than acidosis, hyperkalemia, and hyperphosphatemia.
Since the National Kidney Foundation published its definition and classification of chronic kidney disease (CKD),1 evidence has accumulated showing that it is a common disease,2,3 associated with morbidity and mortality risks far broader and higher than those of simple progression to kidney failure.4–6 Early detection of CKD and its metabolic complications is now a priority for delaying disease progression and for primary prevention of many CKD-associated chronic diseases, including cardiovascular, mineral, and bone diseases5,7–9; however, data on the natural history of these complications according to reference methods are sparse, and there is little evidence about the most appropriate timing for their detection.
CKD metabolic complications, which include anemia, metabolic acidosis, and mineral and electrolyte disorders, may be asymptomatic for a long time.10–21 According to Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines,1 all patients at stage 3 CKD or above (i.e., those with a GFR <60 ml/min per 1.73 m2), should be evaluated for all complications. This threshold, however, was defined from clinical and population-based studies, all of which used equation-estimated GFR (eGFR),1 a method sensitive to both the choice of equation and serum creatinine (Scr) calibration, particularly for the highest GFR values.22,23 Population-based studies, with one exception,24 have also lacked the power to search for complication-specific GFR thresholds below 60 ml/min per 1.73 m2. Moreover, although a few studies showed the influence of some patient characteristics, such as ethnic origin and diabetes, on the prevalence of various complications,24–29 neither their potential impact nor the effect of clinical factors on metabolic disorders has been investigated systematically.
Our primary purpose, therefore, was to define GFR thresholds, measured with a reference method (mGFR: 51Cr-EDTA renal clearance), and factors associated with CKD-related metabolic complications in a clinical cohort of 1038 patients with stages 2 through 5 CKD. Because mGFR is rarely performed in clinical practice, we also estimated these thresholds with eGFR and studied how the results differed according to method.
RESULTS
Patient Characteristics
Of the 1038 cohort patients, 69% were men and 6% were black (Table 1). Mean age was 59 yr. The most frequent renal diagnoses were vascular and glomerular nephropathies. More than half of the latter—but <20% of the other diagnoses—were biopsy proven. Nearly all of these patients, who were referred to nephrology care before entering the cohort, were prescribed at least one antihypertensive drug and 75% an angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin receptor blocker (ARB). BP nonetheless exceeded therapeutic goals in nearly two thirds of patients. In all, 79% were classified as having stage 3 or 4 CKD on the basis of mGFR. Mean GFR and distribution of CKD stages did not differ significantly when we used mGFR or eGFR calculated with the Modification of Diet in Renal Disease (MDRD) study equation or when, for the latter, Scr values were calibrated according to the Cleveland Clinic Laboratory (eGFRcl)30 or standardized by mass spectrometry (eGFRms).23
Table 1.
Baseline characteristics of the 1038 cohort patientsa
| Characteristic | Value |
|---|---|
| Age (yr; mean ± SD) | 59 ± 15 |
| Men (%) | 69 |
| Black (%) | 6 |
| Renal disease (% biopsy-proven)a | |
| vascular nephropathy | 34 (10) |
| glomerulonephritis | 19 (55) |
| diabetic kidney disease | 13 (10) |
| tubulointerstitial nephritis | 11 (20) |
| polycystic kidney disease | 5 (0) |
| undetermined | 17 (5) |
| BMI (%; kg/m2) | |
| <25 | 43 |
| 25 to 30 | 37 |
| ≥30 | 20 |
| Diabetes (%) | 26 |
| BP ≥130/80 mmHg (%) | 65 |
| Any antihypertensive treatment (%) | 92 |
| ACEi or ARB treatment (%) | 77 |
| Albuminemia (g/L; mean ± SD) | 39.5 ± 5.1 |
| Proteinuria (%; g/g creatinine) | |
| <0.5 | 53 |
| 0.5 to 1.0 | 15 |
| ≥1.0 | 32 |
| mGFR (ml/min per 1.73 m2; mean ± SD) | 37 ± 17 |
| eGFRcl (ml/min per 1.73 m2; mean ± SD) | 38 ± 17 |
| eGFRms (ml/min per 1.73 m2; mean ± SD) | 36 ± 16 |
| CKD stages based on mGFR/eGFRcl/eGFRms (%) | |
| 2 (60 to 89 ml/min per 1.73 m2) | 12/10/7 |
| 3 (30 to 59 ml/min per 1.73 m2) | 48/55/53 |
| 4 (15 to 29 ml/min per 1.73 m2) | 31/28/31 |
| 5 (<15 ml/min per 1.73 m2, not on dialysis) | 9/7/9 |
Percentages in parentheses are those of biopsy-proven diagnoses among patients with each type of renal disease. mGFR, measured glomerular filtration rate; eGFRcl, estimated glomerular filtration rate, using the MDRD Study equation with serum creatinine values calibrated by the Cleveland Clinic Laboratory; eGFRms, eGFR using the MDRD equation with serum creatinine values standardized to mass spectrometry.
Overall Prevalence and Treatment of Metabolic Complications
Metabolic acidosis, hyperkalemia, and hyperphosphatemia were less frequent than hyperparathyroidism and anemia, defined as hemoglobin (Hb) <110 g/L according to K/DOQI-based criteria,31 but a higher percentage of patients with the former complications received treatment for them (Table 2). Of note, using World Health Organization (WHO) gender-specific thresholds,32 Hb <130 g/L in men and <120 g/L in women, the overall prevalence of anemia was 56%.
Table 2.
Prevalence of metabolic complicationsa in the cohort
| Prevalence of Complications
|
Prevalence of Treatment among Patients with Complications
|
|||
|---|---|---|---|---|
| n | % | n | % | |
| Hyperparathyroidism | 610 | 59 | 87 | 14 |
| Anemia | 210 | 20 | 37 | 18 |
| Acidosis | 160 | 15 | 35 | 22 |
| Hyperkalemia | 176 | 17 | 87 | 49 |
| Hyperphosphatemia | 84 | 8 | 32 | 38 |
Hyperparathyroidism was defined as a PTH >60 pg/ml or active vitamin D treatment; anemia was defined as Hb<110 g/L according to K/DOQI-based criteria or erythropoiesis-stimulating agent (ESA) treatment; acidosis was defined a tCO2 <22 mmol/L) or bicarbonate treatment; hyperkalemia was defined as plasma potassium concentration >5 mmol/L or ion exchange resin treatment; hyperphosphatemia was defined as plasma phosphate concentration >4.3 mg/dl (1.38 mmol/L) or phosphate binder treatment.
Prevalence of Metabolic Complications According to GFR Level
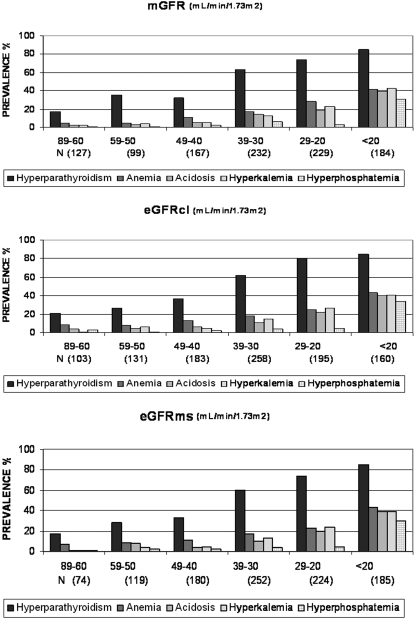
Analysis by strata of 10 ml/min per 1.73 m2 showed that the prevalence of each metabolic complication increased strongly with decreasing GFR. Trends were very similar with mGFR and both eGFR (all P < 0.001 for trend). Hyperparathyroidism was present in nearly 20% of patients with stage 2 CKD. In contrast, K/DOQI-defined anemia affected >10% of the patients only at a GFR of ≤49 ml/min per 1.73 m2 and other metabolic disorders at a GFR of ≤39 ml/min per 1.73 m2 (Figure 1). The prevalence of WHO-defined anemia by stratum followed that of hyperparathyroidism, increasing from 29 to 85% as mGFR decreased from 89 to 60 to <20 ml/min per 1.73 m2.
Figure 1.
Prevalence of metabolic complications according to GFR. Hyperparathyroidism was defined as PTH >60 pg/ml or active vitamin D treatment; anemia was defined as Hb <110 g/L according to K/DOQI-based criteria or erythropoiesis-stimulating agent treatment; acidosis was defined as a tCO2 <22 mmol/L or bicarbonate treatment; hyperkalemia was defined as a plasma potassium concentration >5 mmol/L or ion exchange resin treatment; hyperphosphatemia was defined as a plasma phosphate concentration >4.3 mg/dl (>1.38 mmol/L) or phosphate binder treatment. mGFR, measured glomerular filtration rate; eGFRcl, estimated glomerular filtration rate, using the MDRD Study equation with serum creatinine values calibrated by the Cleveland Clinic Laboratory; eGFRms, eGFR using the MDRD equation with serum creatinine values standardized to mass spectrometry.
Odds Ratios of Metabolic Complications According to Patient Characteristics
Multiple logistic regression models showed that the adjusted odds ratios (OR) for hyperparathyroidism increased significantly with black race and high BP, those for anemia with diabetic nephropathy, for acidosis with diabetes and younger age (<65 versus ≥65 yr), for hyperkalemia with male gender and ACEi or ARB treatment, and for hyperphosphatemia with younger age, independent of mGFR level and other patient characteristics (Table 3). Parathyroid hormone (PTH) also tended to increase with body mass index (BMI), but the trend was not statistically significant. In contrast, obesity was associated with a reduced OR for anemia. Adjustment for either eGFR instead of mGFR did not alter any of these associations (data not shown). As expected, when using WHO definition with gender-specific Hb threshold, gender was no longer related to anemia (OR 0.9; 95% confidence interval [CI] 0.6 to 1.7). Additional analysis showed that the adjusted OR for hyperphosphatemia increased significantly with active vitamin D therapy (OR 2.6; 95% CI 1.3 to 5.2), independent of PTH level and other patient factors. It is worth noting that urinary urea nitrogen, a marker of protein intake that may influence plasma phosphate and total CO2 (tCO2) levels, was higher in younger patients (1025 ± 375 mg/d in those <65 yr versus 963 ± 319 mg/d in those ≥65 yr; P < 0.01). Neither proteinuria nor the type of CKD was a risk factor for the complications studied, except for diabetic kidney disease with anemia.
Table 3.
Adjusted OR of metabolic complicationsa according to patient characteristics
| Parameter | Hyperparathyroidism (OR [95% CI]) | Anemia (OR [95% CI]) | Acidosis (OR [95% CI]) | Hyperkalemia (OR [95% CI]) | Hyperphosphatemia (OR [95% CI]) |
|---|---|---|---|---|---|
| Age <65 versus ≥65 yr | 1.0 (0.7 to 1.4) | 0.9 (0.6 to 1.4) | 1.7 (1.1 to 2.7) | 1.3 (0.8 to 2.0) | 4.2 (2.1 to 8.5) |
| Men versus women | 1.4 (0.9 to 1.8) | 0.3 (0.2 to 0.4) | 1.0 (0.6 to 1.5) | 2.0 (1.2 to 3.0) | 1.7 (0.9 to 3.2) |
| Black versus white | 2.8 (1.4 to 5.5) | 1.9 (0.9 to 4.0) | 0.5 (0.2 to 1.4) | 0.4 (0.1 to 1.1) | 0.3 (0.1 to 1.7) |
| Renal disease | |||||
| glomerulonephritis | Reference | Reference | Reference | Reference | Reference |
| vascular nephropathy | 1.4 (0.9 to 2.3) | 0.6 (0.3 to 1.3) | 1.3 (0.7 to 2.4) | 0.7 (0.4 to 1.2) | 0.9 (0.4 to 2.1) |
| diabetic kidney disease | 1.4 (0.7 to 2.8) | 2.6 (1.1 to 5.8) | 0.8 (0.4 to 1.9) | 1.4 (0.6 to 3.2) | 2.1 (0.6 to 7.6) |
| tubulointerstitial nephritis | 1.7 (0.9 to 3.2) | 1.0 (0.6 to 1.8) | 1.7 (0.9 to 3.4) | 1.1 (0.5 to 2.2) | 1.8 (0.7 to 4.4) |
| polycystic kidney disease | 1.3 (0.6 to 2.9) | 1.7 (0.8 to 3.2) | 1.4 (0.5 to 3.5) | 0.5 (0.2 to 1.4) | 0.9 (0.3 to 3.6) |
| undetermined | 1.0 (0.6 to 1.8) | 1.5 (0.6 to 3.5) | 0.6 (0.3 to 1.4) | 0.7 (0.3 to 1.4) | 1.3 (0.6 to 3.3) |
| Diabetes, yes versus no | 1.1 (0.6 to 1.7) | 1.2 (0.7 to 2.1) | 1.9 (1.1 to 3.5) | 0.9 (0.5 to 1.7) | 0.9 (0.3 to 2.6) |
| BMI (kg/m2) | |||||
| <25 | Reference | Reference | Reference | Reference | Reference |
| 25 to 30 | 1.1 (0.7 to 1.5) | 0.7 (0.5 to 1.0) | 0.8 (0.5 to 1.4) | 0.8 (0.5 to 1.3) | 0.9 (0.5 to 1.7) |
| >30 | 1.5 (0.9 to 2.3) | 0.6 (0.3 to 0.9) | 0.7 (0.4 to 1.3) | 1.0 (0.6 to 1.7) | 1.2 (0.6 to 2.6) |
| BP > versus ≤130/80 mmHg | 1.5 (1.1 to 2.1) | 1.2 (0.8 to 1.8) | 1.1 (0.7 to 1.7) | 0.9 (0.6 to 1.4) | 1.1 (0.6 to 1.9) |
| Proteinuriab | 0.9 (0.8 to 1.2) | 0.9 (0.7 to 1.1) | 0.8 (0.6 to 1.1) | 0.9 (0.7 to 1.4) | 0.7 (0.5 to 1.1) |
| ACEi/ARB, yes versus no | 1.1 (0.8 to 1.6) | 0.9 (0.6 to 1.5) | 0.8 (0.5 to 1.3) | 3.4 (1.8 to 6.2) | 1.1 (0.5 to 2.2) |
| mGFR (ml/min per 1.73 m2) | 0.4 (0.5 to 0.6) | 0.5 (0.4 to 0.6) | 0.5 (0.4 to 0.6) | 0.4 (0.3 to 0.5) | 0.3 (0.2 to 0.5) |
Hyperparathyroidism was defined as a PTH >60 pg/ml or active vitamin D treatment; anemia was defined as Hb <110 g/L according to K/DOQI-based criteria or ESA treatment; acidosis was defined as a tCO2 <22 mmol/L or bicarbonate treatment; hyperkalemia was defined as a plasma potassium concentration >5 mmol/L or ion exchange resin treatment; hyperphosphatemia was defined as a plasma phosphate concentration >4.3 mg/dl (>1.38 mmol/L) or phosphate binder treatment.
Proteinuria in three classes (<0.5, 0.5 to 1.0, ≥1.0 g/g) treated as a continuous variable.
ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; mGFR, measured glomerular filtration rate; eGFRcl, estimated glomerular filtration rate, using the MDRD Study equation with serum creatinine values calibrated by the Cleveland Clinic Laboratory; eGFRms, eGFR using the MDRD equation with serum creatinine values standardized to mass spectrometry.
GFR Thresholds and Specificity for Detection of CKD Complications with 90% Sensitivity
Using receiver operating characteristic (ROC) analysis with internal validation, we determined GFR thresholds associated with the detection of each complication with 90% sensitivity according to the various GFR assessment methods. On the basis of mGFR, these thresholds were approximately 50 ml/min per 1.73 m2 for hyperparathyroidism, 45 ml/min per 1.73 m2 for K/DOQI-defined anemia, and 40 ml/min per 1.73 m2 for the other three complications (Table 4). Diagnostic specificity for mGFR ranged from 30 to 45% according to the complication. Using eGFRcl increased anemia threshold by >5 ml/min per 1.73 m2 but altered the others only slightly. Thresholds with eGFRms were very similar to those based on mGFR. Except for hyperparathyroidism and hyperphosphatemia, diagnostic specificity was lower for both eGFR than for mGFR, particularly for anemia.
Table 4.
Measured GFR thresholds and specificity for metabolic complicationa diagnosis with 90% sensitivity
| Parameter | Hyperparathyroidism
|
Anemia
|
Acidosis
|
Hyperkalemia
|
Hyperphosphatemia
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| GFR (95% CI) | Sp (%) | GFR (95% CI) | Sp (%) | GFR (95% CI) | Sp (%) | GFR (95% CI) | Sp (%) | GFR (95% CI) | Sp (%) | |
| mGFR | 49.5 (49.4 to 49.6) | 30 | 44.2 (44.1 to 44.3) | 35 | 39.8 (39.7 to 39.9) | 40 | 39.1 (39.0 to 39.1) | 45 | 36.9 (36.8 to 37.0) | 45 |
| eGFRcl | 46.5 (46.3 to 46.6) | 42 | 51.7 (51.6 to 51.8) | 23 | 45.4 (45.3 to 45.5) | 36 | 41.4 (41.3 to 41.4) | 44 | 38.0 (37.9 to 38.1) | 49 |
| eGFRms | 46.8 (46.7 to 46.9) | 42 | 46.5 (46.3 to 46.6) | 27 | 42.4 (42.3 to 42.5) | 36 | 39.1 (39.0 to 39.2) | 43 | 36.2 (36.1 to 36.3) | 47 |
Hyperparathyroidism was defined as a PTH >60 pg/ml or active vitamin D treatment; anemia was defined as Hb <110 g/L according to K/DOQI-based criteria or ESA treatment; acidosis was defined as a tCO2 <22 mmol/L or bicarbonate treatment; hyperkalemia was defined as a plasma potassium concentration >5 mmol/L or ion exchange resin treatment; hyperphosphatemia was defined as a plasma phosphate concentration >4.3 mg/dl (>1.38 mmol/L) or phosphate binder treatment.
mGFR, measured glomerular filtration rate; eGFRcl, estimated glomerular filtration rate, using the MDRD Study equation with serum creatinine values calibrated by the Cleveland Clinic Laboratory; eGFRms, eGFR using the MDRD equation with serum creatinine values standardized to mass spectrometry.
DISCUSSION
Our data from a large cohort of nephrology patients show different thresholds of GFR at onset of the metabolic complications we studied. Whereas anemia and hyperparathyroidism were observed early in stage 3 CKD, other disorders appeared later in the course of the disease. Another original finding was the identification of several factors independently associated with these complications. The importance of this study lies especially in its reliance on a sample of >1000 patients with GFR both measured with a reference method and estimated and its simultaneous assessment of the principal metabolic complications of CKD. It is the first study to examine so large a sample with such diagnostic precision, and it has important clinical and research implications.
Comparison of our findings with others is limited by differences in the study populations (patients with CKD versus general population), the cutoffs chosen to define the metabolic complications and the use of different GFR assessment methods; however, we note that, with a few exceptions discussed here, using eGFR in this cohort provided very similar results to those obtained with mGFR. Because our purpose was to improve knowledge of renal function levels and factors influencing the onset of CKD complications, we focused on standard criteria rather than on therapeutic targets to define abnormal values. Nevertheless, in view of the widespread use of the K/DOQI target for clinical intervention for patients who have CKD and are not on dialysis, the analysis was primarily based on anemia defined with these criteria.
We found, as other studies10–13 did, that the prevalence of anemia increased sharply as GFR decreased. This prevalence, however, was much higher—5, 9, and 7% with mGFR, eGFRcl, and eGFRms, respectively—in patients with a GFR between 60 and 89 ml/min per 1.73 m2 than would have been anticipated from population-based studies, such as National Health and Nutrition Examination Survey (NHANES III), 1%, with K/DOQI criteria and eGFRcl.12 On the basis of WHO criteria, our prevalence of 29% for stage 2 CKD was also higher than the 5% in men and 6% in women from NHANES III,11 the 13% in participants of the New Opportunities for Early Renal Intervention by Computerised Assessment (NEOERICA) study,14 or the 15% in the targeted Kidney Early Evaluation Project (KEEP) CKD screening program cohort.33 With the exception of the last, these studies, however, did not take into account the presence of kidney damage, a key element in the definition of stage 2 CKD; this is likely to explain these discrepancies. The higher risk for anemia in black than in white patients here, although not statistically significant but this subgroup was very small, is consistent with that observed in a previous study.33 We also found, as others did, that diabetic nephropathy is related to a higher risk for anemia, independent of GFR level.29,33,34 We otherwise cannot rule out the possibility that the presence of other comorbidities in these patients with CKD may explain, at least in part, the relatively high prevalence of anemia observed in stage 2 and early stage 3, without demonstration of an erythropoietin-dependent mechanism linked to the moderate decrease in GFR. A new finding, to the best of our knowledge, is that obesity is associated with a lower risk for anemia. Although this association requires confirmation from other studies, we noted that some underlying mechanisms, such as better nutrition or less iron deficiency in obese compared with normal-weight patients, may be causal. Finally, we did not confirm the higher risk for anemia with ACEi/ARB treatment suggested by Kamper et al.35 The ROC analysis identified a diagnostic threshold for 90% sensitivity of approximately 45 ml/min per 1.73 m2 with either mGFR or eGFRms, which means that 90% of the patients with K/DOQI-defined anemia have a GFR lower than these values; it was >50 ml/min per 1.73 m2 with eGFRcl. As expected, GFR threshold for WHO-defined anemia was higher, close to that for hyperparathyroidism. These findings as a whole provide evidence that anemia screening should start in the upper range of GFR in patients with stage 3 CKD and possibly even earlier in those with diabetic kidney disease.
Regarding bone and mineral metabolism disorders, we confirmed the finding by Levin and colleagues1,17–19 that high PTH levels can be observed at stage 2 CKD in a number of patients (17%) and that the prevalence of hyperparathyroidism rises substantially when GFR falls to <60 ml/min per 1.73 m2. The detection threshold for 90% sensitivity was close to 50 ml/min per 1.73 m2 with either technique used to assess GFR. As reported elsewhere,24–26 black patients are at higher risk than white patients. A number of studies, however, showed that PTH concentration was higher in black than in white patients, both with and without CKD, at similar calcium intake and vitamin D levels.36,37 Because normal values may differ by race, it is difficult to define hyperparathyroidism in black patients.36 Although not statistically significant, the trend we observed between BMI and hyperparathyroidism is consistent with those from recent studies.38 We also found that BP >130/80 mmHg was independently related to higher PTH levels. Such a cross-sectional association is also reported in patients without CKD,39 but establishing the existence of causality would require a prospective investigation. For hyperphosphatemia, the GFR threshold at diagnosis was 37 ml/min per 1.73 m2, consistent with results from the SEEK study, which used a slightly higher cutoff (1.48 mmol/L).17 Others1,17 have reported that electrolyte disorders occur later in the course of CKD than does renal hormonal dysfunction.
An original finding of this study, however, was the existence of a higher risk for hyperphosphatemia at a younger age. One possible explanation is protein intake, which may affect plasma phosphate level in patients with CKD. This hypothesis is supported by the higher mean level of urinary urea nitrogen in the patients younger than 65. Moreover, phosphate levels were significantly higher in patients treated with active vitamin D than in those not treated, independent of PTH level and other characteristics. We therefore suggest that screening for hyperparathyroidism should start at a GFR between 50 and 60 ml/min per 1.73 m2. Because treatment of hyperparathyroidism will affect phosphatemia, it seems reasonable to measure phosphatemia together with PTH and not await a GFR <40 ml/min per 1.73 m2 before measuring it, as suggested by our data.
There is relatively little information about thresholds for acidosis and electrolyte disturbance in patients with CKD before ESRD. We found an mGFR threshold of approximately 40 ml/min per 1.73 m2 for detecting both metabolic acidosis and hyperkalemia. Using a similar cutoff, however, NHANES III found that metabolic acidosis increased significantly at a GFR <30 ml/min per 1.73 m2.12,16,20 A younger age was independently associated with acidosis. This may be explained, as for hyperphosphatemia, by higher protein intake among younger patients.40 As reported by Braden et al.,41 we observed that patients with tubulointerstitial nephritis had a higher odds ratio for metabolic acidosis, but it was not statistically significant. Men were also at higher risk for hyperkalemia. Importantly, our results did not confirm the previously reported excess risk for metabolic acidosis in patients treated with ACEi or ARB.35 We did confirm such a risk for hyperkalemia with these drugs: Risk more than tripled. Overall, this study suggests that screening for metabolic acidosis and hyperkalemia should begin when GFR reaches 40 ml/min per 1.73 m2, but this threshold may be higher in younger patients, in those with diabetes, and in those treated with ACEi or ARB. We, like Cirillo et al.,13 did not observe any association between proteinuria and a higher risk for metabolic complications.
The major strengths of our study include its large sample size and statistical power, its use of an unbiased measure of renal function, and the inclusion of patients with a wide range of GFR and all types of kidney disease. Moreover, because only minor differences in complication thresholds were observed between mGFR and eGFR, particularly when eGFR was based on mass spectrometry–calibrated creatinine, their relevance for clinical practice is high. The study also has several limitations. First, it enrolled nephrologist-referred patients, who may not be representative of the overall population with CKD. The percentage of diabetic nephropathy in our study was low, 13%. This is likely to reflect a combination of low prevalence of the disease in the Paris area (18% of the prevalent ESRD population has diabetic nephropathy)42 and the later referral to nephrologists, commonly at stage 5, of patients with diabetes compared with other patients with CKD, as well as recruitment bias from university hospitals. This may result in underestimating the prevalence of anemia, given its link with diabetic kidney disease. Moreover, patients at stage 1 CKD were excluded from this cohort, so we could not evaluate anemia and hyperparathyroidism at this earliest CKD stage. Nonetheless, an advantage of clinical over population-based studies is that the former consider individuals with confirmed CKD. When CKD is defined only as an eGFR <60 ml/min per 1.73 m2 or microalbuminuria, as in NHANES III,11,12,20 the presence of clinical CKD is uncertain for a large proportion of individuals.
Second, this cross-sectional analysis of the cohort baseline data cannot expressly demonstrate cause-and-effect relations between the clinical factors and the metabolic disorders. Finally, despite the large sample size, we may have lacked power for the study of some associations; therefore, some negative findings, such as that between tubulointerstitial nephritis and acidosis, should be interpreted with caution.
In conclusion, these findings do not support the use of a single GFR threshold (<60 ml/min per 1.73 m2) to begin screening for metabolic complications. Instead, they clearly show that testing for anemia and mineral metabolism disorders should start early during stage 3 CKD, as currently recommended, with particular attention to patients with diabetes, whereas regular screening for other metabolic disorders may await a GFR <40 ml/min per 1.73 m2. Their main clinical implications are that patient follow-up should be adjusted more closely to GFR level within stage 3. Because we did not use predefined GFR thresholds, this study provides reference values that may contribute to refining CKD staging, as suggested by others.5,43 Only clinical trials such as those carried out for anemia can determine whether treating these abnormalities at these stages would prevent the excess risks for morbidity and mortality associated with them, and our study provides useful information to help design such trials.
CONCISE METHODS
Patients and Study Design
The NephroTest study is a prospective hospital-based cohort that began in 2000, enrolling patients who had all diagnoses of stages 2 through 5 CKD and were referred for extensive work-up by two nephrology departments. To be eligible, patients had to be ≥18 yr of age and neither be on dialysis nor have received a kidney transplant. Pregnant women were excluded. These patients were seen annually with an extensive set of standardized measures to assess CKD progression and complications. All patients signed informed consent before inclusion in the cohort. We analyzed baseline data from the 1038 patients included between January 2000 and December 2006.
Measures
We recorded data during a 5-h in-person visit at each hospital physiology department. They included demographics, renal diagnosis, medical history, height and weight, resting BP, and medications. We collected blood and urine samples to determine levels of serum plasma creatinine, Hb, PTH, phosphate, potassium, tCO2, and albumin, as well as proteinuria.
We assessed the mGFR by 51Cr-EDTA renal clearance as described previously.22 Briefly, we injected 1.8 to 3.5 MBq of 51Cr-EDTA (GE Healthcare, Velizy, France) intravenously as a single bolus. We then determined average renal 51Cr-EDTA clearance during five to six consecutive 30-min clearance periods. We measured plasma creatinine for all patients with a modified kinetic Jaffe colorimetric method and a Konelab 20 analyzer. We then estimated GFR with the MDRD Study equation with plasma creatinine values calibrated two different ways, first to the Cleveland Clinic Laboratory (using the original four-variables MDRD formula: eGFRcl = 186 × [Scr (mg/dl)]−1.154 × [age (years)]−0.203 × 0.742 [if female] × 1.210 [if black])30 and, second, according to isotope dilution mass spectrometry (IDMS; using the IDMS-modified MDRD formula: eGFRms = 175 × [Scr (mg/dl)]−1.154 × [age (years)]−0.203 × 0.742 [if female] × 1.210 [if black]).23 Calibration of our creatinine assay to the Beckmann CX3 colorimetric assay in use at the Cleveland Clinic Foundation has been described previously.22 Standardization to IDMS-traceable creatinine assay was performed with reference to international Standard Reference Materials (SRM) NIST-SRM-967 (National Institute of Standards and Technology: http://www.nist.gov/srm) and IRMM-BCR-573, BCR-574, BCR-575 (Institute for Reference Materials and Measurements: http://irmm.jrc.ec.europa.eu/html/homepage.htm). We performed serial measurements of these commutable reference materials. We performed regression analysis between the five certified values (66.5, 346.2, 68.7, 105.0, and 404.1 μmol/L for SRM-967 level 1, SRM-967 level 2, BCR-573, BCR-574, and BCR-575, respectively) and applied measured concentrations to confirm linearity of our assay and to determine the calibration equation able to convert our clinical creatinine measurements into IDMS-traceable creatinine values: IDMS-SCr (mg/dl) = 1.0450 Jaffe-SCr − 0.0713 (r2 = 0.9954, P < 0.0001). The black correction for race was equally applied to black patients for eGFRcl and eGFRms.
We measured PTH concentration by a second-generation two-site immunoradiometric assay (Allegro-Intact PTH and then the Allegro-calibrated Intact PTH Advantage assay; Nichols Institute Diagnostics, San Clemente, CA).44 We measured plasma phosphate concentration by colorimetry (phosphomolybdate assay), potassium by flame photometry, venous tCO2 with a specific electrode (Beckman SX9), and albuminemia by immunonephelometry. We measured proteinuria by colorimetry (pyrogallol red with molybdate) and expressed as g/g creatinine.
We based thresholds used to define metabolic complications on current international guidelines whenever available. Patients treated for a given metabolic complication were considered to have this complication regardless of their test values. We used K/DOQI criteria to define anemia, which is a Hb concentration <110 g/L.31 To compare our findings with others, we also provided prevalence estimates based on WHO gender-specific thresholds: Hb concentration <130 g/L for men and <120 g/L for women.32 Patients treated with erythropoiesis-stimulating agents were also classified as having anemia. Hyperparathyroidism was defined as an intact PTH concentration >60 pg/ml (laboratory reference values 10 to 60 pg/ml) or treatment with active vitamin D, hyperphosphatemia as a plasma phosphate value >4.3 mg/dl (or 1.38 mmol/L)1 or phosphate binder medication, metabolic acidosis as a tCO2 value <22 mmol/L1 or treatment with oral bicarbonate, and hyperkalemia as serum potassium >5 mmol/L or treatment with ion exchange resin.45 Data were available for all patients for all complications, except proteinuria data were missing for 9%.
Statistical Analysis
We studied the prevalence of metabolic complications by strata of 10 ml/min per 1.73 m2 of mGFR and eGFR as follows: 89 to 60 (reference category), 59 to 50, 49 to 40, 39 to 30, 29 to 20, and <20 ml/min per 1.73 m2. We applied logistic regression to estimate adjusted OR and 95% CI of each complication according to age, gender, race, BMI (in kg/m2), diabetes, high BP (130/80 mmHg [the therapeutic goal for patients with CKD] or higher46) ACEi or ARB treatment, and proteinuria. We adjusted all OR for mGFR treated as a quantitative variable and then for each eGFR. We analyzed ROC and determined mGFR and eGFR thresholds associated with the detection of each complication with 90% sensitivity. Internal validation of the ROC analysis used the jackknife method to estimate unbiased GFR levels and 95% CI.47 We used P < 0.05 to determine statistical significance. We performed statistical analyses with SAS 9.1 (SAS Institute, Cary, NC).
DISCLOSURES
J.R. has received honoraria and/or research funds from Affymax, Amgen, Ortho-Biotech, Roche and Shire; M.F. has received honoraria and/or research funds from Affymax, Hospira and Roche; P.H. has received honoraria and/or research funds from Amgen, Genzyme and Hybrigenics. B.F. and J.R. have been employed by Amgen full-time since August 2007 (B.F.) and December 2006 (J.R.) but were the study coordinator and an investigator, both academic affiliated to university, from the start of the study until after work on this manuscript began.
Acknowledgments
Participants in the NephroTest Study Group: Pierre Ronco, Pablo Urena, Jean-Philippe Rougier, Emmanuelle Plaisier, Marinos Fysekidis (Tenon); Francois Vrtovsnik, Martin Flamant, Marie Essig (Bichat); Renaud de La Faille, Christian d’Auzac, Alexandre Karras, Marie Briet, Gérard Maruani, Carmen Lefaucheur, Laurence Nicolet-Barousse (HEGP).
The NephroTest study received funding from a joint program of the French Ministry of Research and INSERM (CRB cohortes et collections 2001) and from the Agence de Biomedecine. This study is part of the CKD Epidemiology and Clinical Research Network granted by INSERM (coordinator B.S.; grant A08022LS). The NephroTest initiative received logistic support from Ortho-Biotech.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 2.Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, Hallan HA, Lydersen S, Holmen J: International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol 17: 2275–2284, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Bailey JL: Metabolic acidosis: An unrecognized cause of morbidity in the patient with chronic kidney disease. Kidney Int Suppl S15–S23, 2005 [DOI] [PubMed]
- 5.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Wattanakit K, Folsom AR, Selvin E, Coresh J, Hirsch AT, Weatherley BD: Kidney function and risk of peripheral arterial disease: Results from the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol 18: 629–636, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, Chambless LE, Coresh J: Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol 18: 1307–1315, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Fried LF, Biggs ML, Shlipak MG, Seliger S, Kestenbaum B, Stehman-Breen C, Sarnak M, Siscovick D, Harris T, Cauley J, Newman AB, Robbins J: Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol 18: 282–296, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Hsu C-y, McCulloch CE, Curhan GC: Epidemiology of anemia associated with chronic renal insufficiency among Adults in the United States: Results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 13: 504–510, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Astor BC, Muntner P, Levin A, Eustace JA, Coresh J: Association of kidney function with anemia: The Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 162: 1401–1408, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Clase CM, Kiberd BA, Garg AX: Relationship between glomerular filtration rate and the prevalence of metabolic abnormalities: results from the Third National Health and Nutrition Examination Survey (NHANES III). Nephron Clin Pract 105: c178–c184, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Cirillo M, Laurenzi M, Mancini M, Zanchetti A, Lombardi C, De Santo NG: Low glomerular filtration in the population: Prevalence, associated disorders, and awareness. Kidney Int 70: 800–806, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Stevens PE, O'Donoghue DJ, de Lusignan S, Van Vlymen J, Klebe B, Middleton R, Hague N, New J, Farmer CK: Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int 72: 92–99, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Kraut JA, Kurtz I: Metabolic acidosis of CKD: Diagnosis, clinical characteristics, and treatment. Am J Kidney Dis 45: 978–993, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Eustace JA, Astor B, Muntner PM, Ikizler TA, Coresh J: Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney Int 65: 1031–1040, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Arnaud CD: Hyperparathyroidism and renal failure. Kidney Int 4: 89–95, 1973 [DOI] [PubMed] [Google Scholar]
- 19.Pitts TO, Piraino BH, Mitro R, Chen TC, Segre GV, Greenberg A, Puschett JB: Hyperparathyroidism and 1,25-dihydroxyvitamin D deficiency in mild, moderate, and severe renal failure. J Clin Endocrinol Metab 67: 876–881, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Hsu CY, Chertow GM: Elevations of serum phosphorus and potassium in mild to moderate chronic renal insufficiency. Nephrol Dial Transplant 17: 1419–1425, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Gennari FJ, Segal AS: Hyperkalemia: An adaptive response in chronic renal insufficiency. Kidney Int 62: 1–9, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P: Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol 16: 763–773, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Vassalotti JA, Uribarri J, Chen SC, Li S, Wang C, Collins AJ, Calvo MS, Whaley-Connell AT, McCullough PA, Norris KC, Kidney Early Evaluation Program Investigators: Trends in mineral metabolism: Kidney Early Evaluation Program (KEEP) and the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis 51: S56–S68, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Gupta A, Kallenbach LR, Zasuwa G, Divine GW: Race is a major determinant of secondary hyperparathyroidism in uremic patients. J Am Soc Nephrol 11: 330–334, 2000 [DOI] [PubMed] [Google Scholar]
- 26.De Boer IH, Gorodetskaya I, Young B, Hsu CY, Chertow GM: The severity of secondary hyperparathyroidism in chronic renal insufficiency is GFR-dependent, race-dependent, and associated with cardiovascular disease. J Am Soc Nephrol 13: 2762–2769, 2002 [DOI] [PubMed] [Google Scholar]
- 27.El-Achkar TM, Ohmit SE, McCullough PA, Crook ED, Brown WW, Grimm R, Bakris GL, Keane WF, Flack JM, Kidney Early Evaluation Program: Higher prevalence of anemia with diabetes mellitus in moderate kidney insufficiency: The Kidney Early Evaluation Program. Kidney Int 67: 1483–1488, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Bosman DR, Winkler AS, Marsden JT, Macdougall IC, Watkins PJ: Anemia with erythropoietin deficiency occurs early in diabetic nephropathy. Diabetes Care 24: 495–499, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Ravanan R, Spiro JR, Mathieson PW, Smith RM: Impact of diabetes on haemoglobin levels in renal disease. Diabetologia 50: 26–31, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract]. J Am Soc Nephrol 11: 155A, 2000 [Google Scholar]
- 31.KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis 50: 471–530, 2007 [DOI] [PubMed] [Google Scholar]
- 32.WHO: Nutritional Anemia, Report of a World Health Organisation Scientific Group, Geneva, World Health Organization, 1968
- 33.McFarlane SI, Chen SC, Whaley-Connell AT, Sowers JR, Vassalotti JA, Salifu MO, Li S, Wang C, Bakris G, McCullough PA, Collins AJ, Norris KC, Kidney Early Evaluation Program Investigators: Prevalence and associations of anemia of CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis 51: S46–55, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Thomas MC, MacIsaac RJ, Tsalamandris C, Molyneaux L, Goubina I, Fulcher G, Yue D, Jerums G: The burden of anaemia in type 2 diabetes and the role of nephropathy: A cross-sectional audit. Nephrol Dial Transplant 19: 1792–1797, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Kamper AL, Nielsen OJ: Effect of enalapril on haemoglobin and serum erythropoietin in patients with chronic nephropathy. Scand J Clin Lab Invest 50: 611–618, 1990 [DOI] [PubMed] [Google Scholar]
- 36.Aloia JF, Feuerman M, Yeh JK: Reference range for serum parathyroid hormone. Endocr Pract 12: 137–144, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutierrez OM, Isakova T, Andress DL, Levin A, Wolf M: Prevalence and severity of disordered mineral metabolism in blacks with chronic kidney disease. Kidney Int 73: 956–962, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K: Obesity is associated with secondary hyperparathyroidism in men with moderate and severe chronic kidney disease. Clin J Am Soc Nephrol 2: 1024–1029, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Morfis L, Smerdely P, Howes LG: Relationship between serum parathyroid hormone levels in the elderly and 24 h ambulatory blood pressures. J Hypertens 15: 1271–1276, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Gennari FJ, Hood VL, Greene T, Wang X, Levey AS: Effect of dietary protein intake on serum total CO2 concentration in chronic kidney disease: Modification of Diet in Renal Disease study findings. Clin J Am Soc Nephrol 1: 52–57, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Braden GL, O'Shea MH, Mulhern JG: Tubulointerstitial diseases. Am J Kidney Dis 46: 560–572, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Couchoud C, Stengel B, Jacquelinet C: REIN annual report 2005. Renal Epidemiology and Information Network & Agence de la biomedecine [in French]. Nephrol Ther 3[Suppl 1]: S1–82, 2007 [PubMed] [Google Scholar]
- 43.Archibald G, Bartlett W, Brown A, Christie B, Elliott A, Griffith K, Pound S, Rappaport I, Robertson D, Semple Y, Slane P, Whitworth C, Williams B: UK Consensus Conference on Early Chronic Kidney Disease: 6 and 7 February 2007. Nephrol Dial Transplant 22: 2455–2457, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Souberbielle JC, Boutten A, Carlier MC, Chevenne D, Coumaros G, Lawson-Body E, Massart C, Monge M, Myara J, Parent X, Plouvier E, Houillier P: Inter-method variability in PTH measurement: Implication for the care of CKD patients. Kidney Int 70: 345–350, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Kratz A, Ferraro M, Sluss PM, Lewandrowski KB: Case records of the Massachusetts General Hospital: Weekly clinicopathological exercises: Laboratory reference values. N Engl J Med 351: 1548–1563, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Efron B, Gong G: A leisurely look at the Bootstrap, the Jackknife, and cross-validation. Am Stat 37: 36–48, 1983 [Google Scholar]