Abstract
Besides its classical effects on salt homeostasis in renal epithelial cells, aldosterone promotes inflammation and fibrosis and modulates cell proliferation. The proinflammatory transcription factor NF-κB has been implicated in cell proliferation, apoptosis, and regulation of transepithelial sodium transport. The effect of aldosterone on the NF-κB pathway in principal cells of the cortical collecting duct, a major physiologic target of aldosterone, is unknown. Here, in both cultured cells and freshly isolated rat cortical collecting duct, aldosterone activated the canonical NF-κB signaling pathway, leading to increased expression of several NF-κB–targeted genes (IκBα, plasminogen activator inhibitor 1, monocyte chemoattractant protein 1, IL-1β, and IL-6). Small interfering RNA–mediated knockdown of the serum and glucocorticoid-inducible kinase SGK1, a gene induced early in the response to aldosterone, but not pharmacologic inhibition of extracellular signal–regulated kinase and p38 kinase, attenuated aldosterone-induced NF-κB activation. Pharmacologic antagonism or knockdown of the mineralocorticoid receptor prevented aldosterone-induced NF-κB activity. In addition, activation of the glucocorticoid receptor inhibited the transactivation of NF-κB by aldosterone. In agreement with these in vitro findings, spironolactone prevented NF-κB–induced transcriptional activation observed in cortical collecting ducts of salt-restricted rats. In summary, aldosterone activates the canonical NF-κB pathway in principal cells of the cortical collecting duct by activating the mineralocorticoid receptor and by inducing SGK1.
Classically, aldosterone acting through the mineralocorticoid receptor (MR) regulates sodium reabsorption in renal distal tubular cells. Cumulating evidence indicates that aldosterone can elicit additional effects in epithelial and nonepithelial cells. Aldosterone promotes tissue inflammation and fibrosis and plays a role in cell proliferation and apoptosis.1–4 Activation of the EGF receptor and mitogen-activated protein kinases (MAPK), induction of oxidative stress, and MR-dependent transcription of proinflammatory genes are some of the mechanisms proposed to account for the injurious effects induced by aldosterone.5–8 As outlined by recent reports, mineralocorticoid also mediates inflammation and fibrosis through NF-κB activation in liver, heart, and glomerular mesangial cells9–13 via a pathway involving the aldosterone early-induced gene, serum and glucocorticoid-induced kinase 1 (SGK1).11,12
NF-κB is a transcription factor composed of dimers of Rel family proteins (p65/RelA, p50/p105, p52/p100, RelB, and c-Rel). Under resting conditions, NF-κB is sequestered in the cytoplasm in association with an inhibitory protein of the IκB family. Cell stimulation leads to a signaling cascade that culminates in IκBα phosphorylation by the IκB kinase (IKK) complex and its proteasome-mediated degradation. Free NF-κB dimers, mainly p65/RelA:p50 heterodimers, then translocate to the nucleus, where they promote transcription of target genes carrying κB DNA-binding motifs.14 Besides its pivotal role in regulating inflammation and apoptosis,15 NF-κB modulates transepithelial sodium transport. The synthesis of the amiloride-sensitive sodium channel is regulated at a transcriptional level by NF-κB in a lung alveolar cell model,16 and its expression at the plasma membrane has been shown to be increased by IKKβ overexpression.17 Conversely, we have shown that increased transepithelial sodium transport induces the dissociation of the p65/RelA:IκBα:protein kinase A (PKA) complex in collecting duct (CD) principal cells.18
In light of these reports, we examined whether aldosterone modulates the NF-κB pathway in physiologically targeted CD principal cells. In this study, we show that aldosterone stimulates the NF-κB signaling pathway in two immortalized mouse CD principal cell lines and in native rat cortical CD (CCD). This effect was dependent on both MR and SGK1. The pathologic relevance of this effect of aldosterone was demonstrated by increased expression of NF-κB–controlled genes in CCD from salt-restricted rats, which classically exhibit increased aldosterone levels.
RESULTS
Aldosterone Stimulates NF-κB Transcriptional Activity via an IKKβ- and IκBα-Dependent Pathway in CD Principal Cells
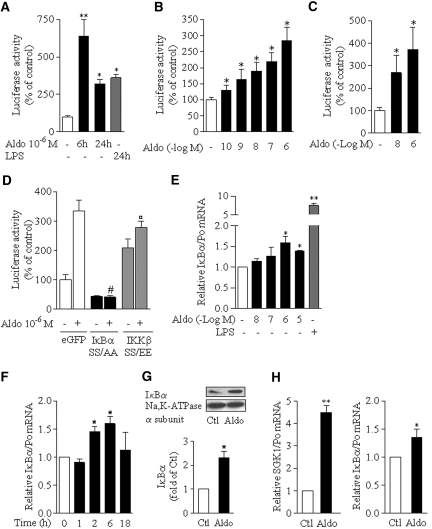
We first investigated whether aldosterone regulates NF-κB transcriptional activity by reporter gene experiments in mpkCCDcl4 cells transfected with an NF-κB–controlled luciferase reporter gene (pLuc-NF-κB). Luciferase activity induced by 10−6 M aldosterone was time dependent and maximal after 6 h of stimulation. After a 24-h stimulation, the activation level was comparable to that produced by 100 ng/ml bacterial LPS, a potent agonist of NF-κB (Figure 1A). Aldosterone-induced luciferase activity increased in a concentration-dependent manner within concentrations ranging from 10−10 to 10−6 M, spanning the physiologic and pathologic range of aldosterone circulating levels (Figure 1B). This effect was not specific to mpkCCDcl4 cells, because a dosage-dependent increase of luciferase activity was also observed in aldosterone-treated mCCDcl1 cells (Figure 1C). To study the pathway leading to NF-κB activation, we assessed the effects of constitutively active IKKβ SS/EE and super-repressor IκBα SS/AA mutants. Aldosterone-induced NF-κB transcriptional activity was abolished in cells co-transfected with pLuc-NFκB and an expression vector containing IκBα SS/AA (Figure 1D), and luciferase activity in cells transfected with IKKβ SS/AA, increased by two-fold over basal conditions, was not significantly further stimulated by aldosterone (Figure 1D). These results suggest that aldosterone activates the so-called “canonical” NF-κB pathway.
Figure 1.
Aldosterone stimulates NF-κB transcriptional activity via an IKKβ- and IκBα-dependent pathway in CD principal cell lines. (A through C) mpkCCDcl4 (A and B) or mCCDcl1 (C) cells were transfected with NF-κB–dependent luciferase reporter gene (pLuc-NF-κB). Transfected cells seeded on polycarbonate filters were incubated in the absence or presence of aldosterone (Aldo) or 100 ng/ml of LPS from Escherichia coli before measurement of luciferase activity. (A) Time-dependent effect of 10−6 M aldosterone on NF-κB–dependent luciferase activity. (B and C) Dosage-dependent effect of 6-h aldosterone treatment on NF-κB–dependent luciferase activity. (D) Effect of constitutive inhibition or activation of NF-κB. mpkCCDcl4 cells transiently co-transfected with pLuc-NF-κB and eGFP, super-repressor IκBα SS/AA or constitutionally active IKKβ SS/EE mutants were grown on polycarbonate filters before measurement of luciferase activity. Results are expressed as a percentage of control (untreated cells in A, B, and C and untreated eGFP transfected cells in D). Bars are means ± SEM from three to six independent experiments. *P < 0.05 and **P < 0.001 versus control values; #P < 0.001 versus aldosterone-stimulated eGFP vector; °no significant difference versus nonstimulated IKKβ SS/EE vector. (E through G) Aldosterone upregulated IκBα expression. mCCDcl1 grown to confluence on polycarbonate filters (E and F) or on plastic support (G) were treated with aldosterone (Aldo) or 100 ng/ml LPS or left untreated (Ctl). IκBα mRNA was detected by RT-PCR (E and F), and IκBα protein was detected by immunoblot (G). (E) Dosage-dependent induction of IκBα mRNA expression by 6-h treatment with aldosterone. (F) Time-dependent induction of IκBα mRNA expression in cells treated with 10−6 M aldosterone. Results are expressed as fold of control values set to 1. Bars are means ± SEM from four independent experiments. (G) Representative immunoblot showing the increased abundance of IκBα protein in cells stimulated by 10−6 M aldosterone for 6 h. Na-K-ATPase α-subunit was taken as a loading control. The bar graph represents the densitometric quantification of IκBα immunoreactivity, expressed as fold of control level. Data are means ± SEM from four independent experiments. *P < 0.05 versus Ctl. (H) Isolated rat CCD were incubated for 2 h with or without 10−8 M aldosterone. SGK1, taken as a positive control of aldosterone effect and IκBα mRNA was detected by RT-PCR. Bars are means ± SEM from 12 experiments. *P < 0.05 versus Ctl.
Typically, activation of the canonical NF-κB pathway induces IκBα mRNA synthesis via binding of NF-κB subunits to the IκBα promoter, generating a negative feedback loop downregulating NF-κB activation. Results show that IκBα mRNA levels were increased by aldosterone in a dosage-dependent manner (Figure 1E). This increase was transient, maximal after 6 h of treatment with 10−6 M aldosterone (Figure 1F), and was associated with an increase of IκBα protein abundance (Figure 1G). Aldosterone also induced IκBα mRNA expression, along with a large increase of SGK1 expression, in native CCD cells as demonstrated by real-time PCR (RT-PCR) experiments performed on isolated rat CCD treated with 10−8 M aldosterone for 2 h (Figure 1H). Thus, the effect of aldosterone on NF-κB–dependent transcription is not species specific or restricted to cultured CD cells and constitutes a physiologic feature of CD principal cells.
Aldosterone Induces p65/RelA Phosphorylation and Nuclear Translocation of p65/RelA and p50 in CD Principal Cells
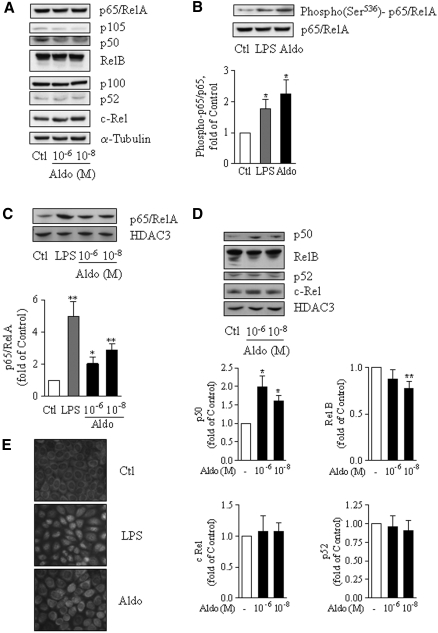
The composition of NF-κB complexes defines specific function and mechanisms of activation. Immunoblotting of total cell extracts revealed that mCCDcl1 cells (Figure 2A) and mpkCCDcl4 cells (data not shown) constitutively express all five NF-κB proteins—p65/RelA, RelB, c-Rel, p105/p50, and p100/p52—whose expression levels were not altered by aldosterone treatment. In contrast, phosphorylation of serine 536, an index of transcriptional activation of p65/RelA,14 increased both in LPS-treated cells, taken as a positive control, and in aldosterone-treated cells by approximately two-fold (Figure 2B). p65/RelA phosphorylation was associated with its nuclear translocation as suggested by immunofluorescence studies (Figure 2E) and confirmed by immunoblots performed on cell nuclear extracts. Treatment of mCCDcl1 cells by 10−6 or 10−8 M aldosterone induced a two- to threefold-increase of p65/RelA levels in nuclei (Figure 2C) and enhanced the nuclear abundance of p50, whereas c-Rel and p52 protein levels remained unchanged (Figure 2D). Nuclear abundance of RelB decreased in response to aldosterone.
Figure 2.
Aldosterone induces p65/RelA transactivation and nuclear translocation of p65/RelA and p50 NF-κB subunits. (A through D) mCCDcl1 cells grown on plastic support were incubated for 6 h in the absence (Ctl) or presence of either 10−8 M (A, C, and D) or 10−6 M (A through D) aldosterone (Aldo), or 100 ng/ml LPS (B and C). NF-κB subunits were detected by immunoblot in total cell lysates (A and B) or nuclear extracts (C and D). (A) Representative immunoblots showing the constitutional expression of p65/RelA, RelB, c-Rel, p100/p50, and p100/p52 proteins in mCCDcl1 cells. NF-κB subunit abundance was not altered by aldosterone treatment. α-Tubulin was taken a loading control (n = 2). (B) Representative immunoblots demonstrating increased p65/RelA phosphorylation [Phospho(Ser536)-p65/RelA] after stimulation by aldosterone or LPS. The bar graph represents the densitometric quantification of phospho-p65/RelA to p65/RelA immunoreactivity, expressed as fold of control value (n = 3). (C and D) Representatives immunoblots demonstrating that the nuclear abundance of p65/RelA and p50 increased in response to aldosterone. The nuclear abundance of RelB decreased, whereas nuclear c-Rel and p52 were unchanged. Anti–histone deacetylase 3 (HDAC3) was used as a loading control. Diagrams represent densitometric analysis of immunoblots expressed as fold of control value (n = 6). Values are means ± SEM. *P < 0.05 and **P < 0.01 versus Ctl. (E) Immunolocalization of p65/RelA by indirect immunofluorescence in mCCDcl1 cells grown on glass coverslips and treated for 6 h by 10−6 M aldosterone or 100 ng/ml LPS. Representative images from six independent experiments are shown.
Effect of Aldosterone on Proinflammatory NF-κB–Dependent Gene Expression in CD Principal Cells
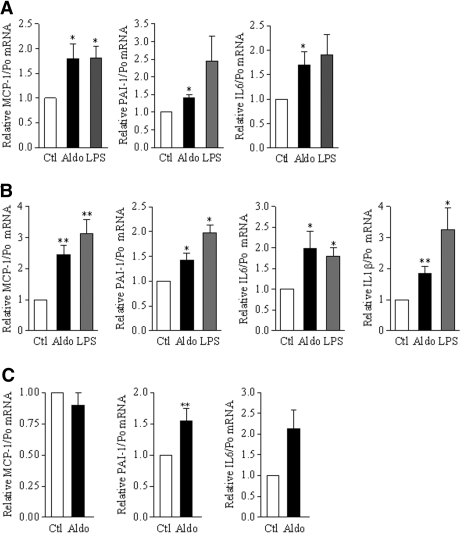
To examine whether aldosterone-induced NF-κB activity in cultured CD cells was associated with upregulation of pro-inflammatory genes, we assessed by RT-PCR the expression of several NF-κB–dependent cytokines or chemokines. Aldosterone stimulation (10−6 M) for 6 h slightly but significantly increased the expression levels of IL-6, monocyte chemoattractant protein 1 (MCP-1), IL-1β, and plasminogen activator inhibitor 1 (PAI-1) mRNA to levels comparable to those induced by LPS in mCCDcl1 and mpkCCDcl4 cells (Figure 3, A and B). Treatment of isolated rat CCD with 10−8 M aldosterone also increased PAI-1 and IL-6 mRNA expression (Figure 3C), whereas MCP-1 expression remained unchanged. Thus, the aldosterone-dependent proinflammatory response is not restricted to cultured CD cells, although the response pattern in cultured cells is slightly different from that of native tissue.
Figure 3.
Aldosterone stimulates the expression of NF-κB–dependent genes. (A and B) mCCDcl1 (A) or mpkCCDcl4 (B) cells grown to confluence on polycarbonate filters were treated or not (Ctl) with 10−6 M aldosterone (Aldo) or 100 ng/ml LPS for 6 h. mRNA of various NF-κB–dependent genes (MCP-1, PAI-1, IL-6, and IL-1β) were detected by RT-PCR. Aldosterone, like LPS, induced an increased expression of MCP-1, PAI-1, IL-6 and IL-1β mRNA in both cell lines. (C) Isolated rat CCD were incubated for 2 h with or without 10−8 M aldosterone. MCP-1, PAI-1, and IL-6 mRNA were detected by RT-PCR. Results are expressed as fold of control value set to 1. Bars are means ± SEM from 12 independent experiments. *P < 0.05 and **P < 0.01 versus Ctl.
Aldosterone-Induced Expression of Proinflammatory Genes Is Achieved via the NF-κB Signaling Pathway
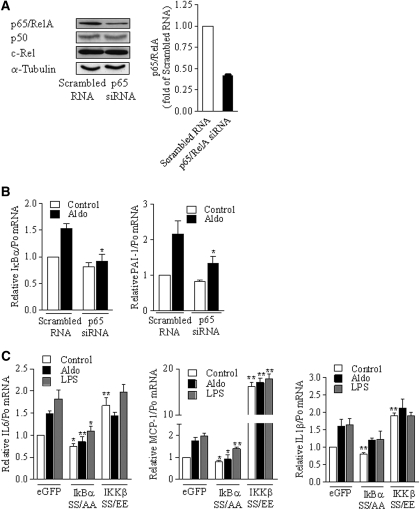
To demonstrate that the aldosterone-induced increase of proinflammatory gene expression depends on NF-κB activation, we analyzed the effect of p65/RelA silencing in mCCDcl1 cells transfected with small interfering RNA (siRNA) targeting p65/RelA mRNA (Figure 4A). Knockdown of p65/RelA prevented the effect of aldosterone on IκBα or PAI-1 mRNA expression (Figure 4B). In agreement with this finding, transfection of mpkCCDcl4 cells with IκBα SS/AA super-repressor mutant prevented the effect of aldosterone on MCP-1 and IL-6 mRNA abundance, whereas the effects of transfection with IKKβ SS/EE constitutively active mutant and aldosterone were not additive (Figure 4C). IL-1β mRNA expression displayed the same profile, although the inhibitory effect of IκBα SS/AA failed to reach statistical significance, suggesting that induction of IL-1β expression in response to both aldosterone and LPS is not solely dependent on the classical NF-κB pathways.
Figure 4.
The effect of aldosterone relies on the activation of the canonical NF-κB pathway. (A to B) mCCDcl1 cells were transfected with either siRNA targeting p65/RelA or scrambled RNA, seeded on plastic support (A) or permeable filters (B), and incubated in the absence or presence of 10−6 M aldosterone for 6 h, before protein (A) or RNA (B) extraction. (A) Specific p65/RelA knockdown by siRNA. p65/RelA, p50, c-Rel, and Na,K-ATPase α-subunit, taken as a loading control, were detected by immunoblot. Representative blots from three independent experiments are shown. Bars show the densitometric quantification of p65/RelA immunoreactivity expressed as fold of OD measured in scrambled-RNA transfected cells. (B) Silencing of p65/RelA by siRNA blunted the increased expression of NF-κB–targeted genes in response to aldosterone. IκBα and PAI-1 mRNA were detected by RT-PCR. (C) mpkCCDcl4 cells transfected with eGFP, super-repressor IκBα SS/AA, or constitutionally active IKKβ SS/EE mutants were processed as described in B. LPS- or aldosterone-induced expression of MCP-1, IL-6, or IL-1β mRNA were blunted by IκBα SS/AA or IKKβ SS/EE mutants. Results are expressed as fold of value in nonstimulated scrambled RNA or eGFP transfected cells. Bars are means ± SEM from six independent experiments. *P < 0.05 or **P < 0.01 versus scrambled RNA or eGFP.
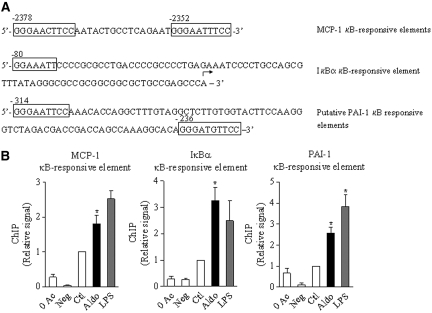
To demonstrate further that aldosterone increases NF-κB activity and regulates proinflammatory gene expression, we performed chromatin immunoprecipitation (ChIP) analysis on mCCDcl1 cell chromatin fragments immunoprecipitated with p65/RelA antibodies (see the Concise Methods section). Functional κB sites have been described in mouse MCP-1 and IκBα promoters.19,20 Computer-assisted analysis revealed two putative κB sites in mouse PAI-1 promoter, displaying the consensus sequence 5′-GGGRNNYYCC-3′,21 located 314 and 236 bp upstream of the ATG start codon (Figure 5A). The functionality of these κB sites was substantiated by ChIP analysis performed on cells exposed to either LPS or 10−6 M aldosterone for 4 h, which revealed a two- to four-fold increase of p65/RelA binding to κB sites located in MCP-1, PAI-1, and IκBα promoters (Figure 5B).
Figure 5.
Aldosterone stimulates p65/RelA DNA binding on IκBα, MCP-1, and PAI-1 promoters. (A) The localization of previously described mouse κB-responsive elements on MCP-1 and IκBα promoters and putative κB binding sites on mouse PAI-1 promoter is shown. (B) ChIP was performed with p65/RelA antibodies on chromatin isolated from mCCDcl1 cells grown on plastic support, untreated (Ctl) or treated with 10−6 M aldosterone (Aldo) or 100 ng/ml LPS for 4 h. Negative controls consisted of chromatin fragments precipitated in the absence of antibody (0 Ac) or protein-A incubated with RIPA buffer and antibodies (Neg). p65/RelA bound to κB-binding sites of MCP-1, PAI-1, and IκBα promoters was quantified by RT-PCR using primers flanking the specific κB-responsive elements. Bars are means ± SEM from four independent experiments. Results are expressed as fold of control value set to 1. *P < 0.05 versus Ctl.
SGK1 Contributes to Aldosterone-Induced NF-κB Activation
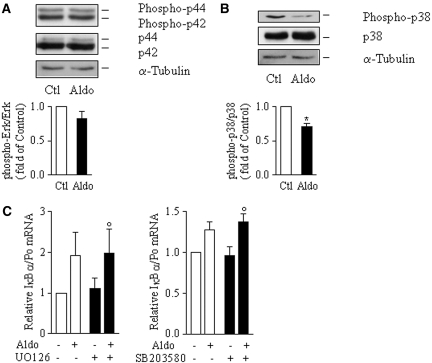
Depending on the stimulus, numerous transduction pathways participate in the cascade of phosphorylation events leading to NF-κB activation. Aldosterone has been shown to interfere with extracellular signal–regulated kinase (ERK) and p38 kinase pathways; therefore, we examined the effect of aldosterone on ERK and p38 activity using specific antibodies against their dual phosphorylated (hence activated) form. Immunoblot analysis of total mCCDcl1 cell extracts revealed that phosphorylation levels of ERK1/2 were not altered by 10−6 M aldosterone after 6 h of stimulation, whereas p38 kinase phosphorylation strongly decreased (Figure 6A). Treatment of mCCDcl1 cells with either 10−5 M SB203580, a p38 kinase inhibitor, or 10−6 M UO-126, a MEK1 and thereby ERK1/2 inhibitor, did not alter the increase of IκBα mRNA induced by aldosterone (Figure 6B).
Figure 6.
ERK and p38 kinase are not involved in aldosterone-induced activation of NF-κB. (A and B) mCCDcl1 cells grown to confluence on plastic support were left untreated (Ctl) or treated with 10−6 M aldosterone (Aldo) for 6 h. Phosphorylated and total ERK (A) or p38 kinases (B) were detected by immunoblot. α-Tubulin was taken as a loading control. Representative blots of three independent experiments are shown. Bars represent densitometric analysis of immunoblots expressed as fold of control value. Data are means ± SEM from three independent experiments. *P < 0.05 versus Ctl. (C) mCCDcl1 cells grown on polycarbonate filters were incubated for 6 h in the presence or absence (Ctl) of 10−6 M aldosterone and/or 10−6 M UO-126, a specific MEK1/ERK pathway inhibitor (left), or 10−5 M SB203580, a specific p38 kinase inhibitor (right), before detection of IκBα mRNA by RT-PCR. Results are expressed as fold of control value set to 1. Bars are means ± SEM from four independent experiments. °No significant difference versus Aldo.
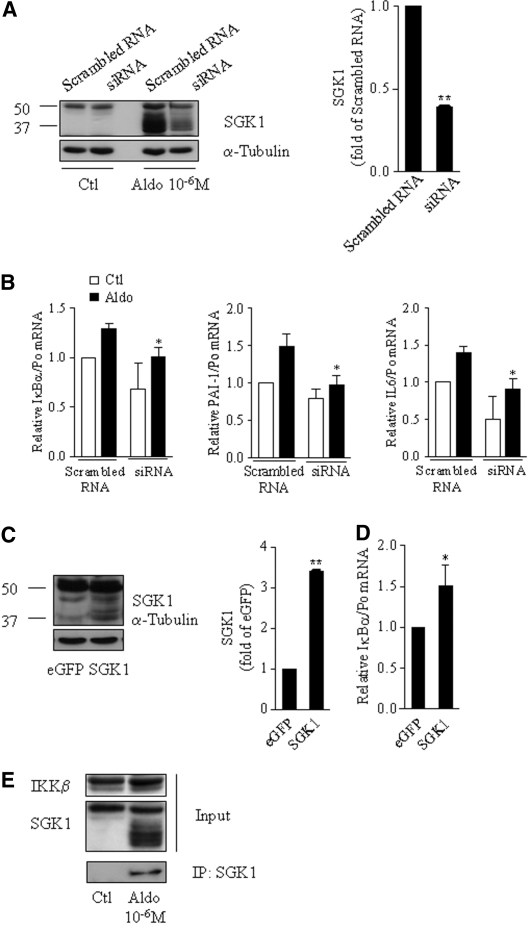
To test whether aldosterone-induced NF-κB activation in CD cells is mediated by the serine-threonine kinase SGK1, as recently shown in mesangium and heart,11,12 we transfected mCCDcl1 cells with siRNA targeting SGK1. SGK1 knockdown decreased SGK1 protein abundance by 60% in aldosterone-treated cells (Figure 7A) and blunted the aldosterone-induced increase of IκBα, IL-6, or PAI-1 mRNA expression (Figure 7B). In contrast, cell transfection with an SGK1 expression vector, which resulted in a three- to four-fold increase of SGK1 protein abundance (Figure 7C), increased IκBα mRNA expression (Figure 7D). Finally, IKKβ was detected by immunoblot after immunoprecipitation of protein extracts from aldosterone-treated mCCDcl1 cells with anti-SGK1 antibodies, indicating that aldosterone induced a physical association between SGK1 and IKKβ in CD cells (Figure 7E), as already described in cultured breast cells.22 Taken together, these results suggest that SGK1 at least partly mediates aldosterone-induced NF-κB activation in CD principal cells.
Figure 7.
Aldosterone-induced NF-κB activation requires induction of SGK1. (A through D) mCCDcl1 were transfected with siRNA targeting SGK1 or scrambled RNA (A and B) or with SGK1 or eGFP (C and D). Cells seeded on plastic support (A and C) or on polycarbonate filters (B and D) were incubated in the absence (Ctl) or presence of 10−6 M aldosterone (Aldo) for 6 h, before protein (A and C) or RNA (B and D) extraction. (A) Representative immunoblot showing the efficient knockdown of SGK1 by siRNA. Bars represent densitometric analysis of immunoblots from aldosterone-treated cells transfected with siRNA, expressed as fold of stimulated scrambled RNA transfected cell OD (n = 2). (B) Silencing of SGK1 prevented the effect of aldosterone on NF-κB–dependent genes. IκBα, PAI-1, and IL-6 mRNA were detected by RT-PCR. Results are expressed as fold of control values set to 1. Bars are means ± SEM from six independent experiments. *P < 0.05 versus aldosterone-stimulated scrambled RNA. (C) Representative immunoblot showing that SGK1 protein abundance increased three-fold in cells transfected with SGK1 (n = 2). Bars represent densitometric analysis of immunoblots, expressed as fold of eGFP OD. (D) SGK1 overexpression increased IκBα mRNA abundance, detected by RT-PCR. Results are expressed as fold of control values set to 1. Bars are means ± SEM from four independent experiments. *P < 0.05 versus scrambled RNA. (E) mCCDcl1 cells grown on plastic support were treated or not (Ctl) with 10−6 M aldosterone (Aldo) for 6 h. Total protein extracts were immunoprecipitated with an anti-SGK1 antibody (see the Concise Methods section). Total cell lysates (Inputs) and immunoprecipitated proteins (IP: SGK1) were separated by 10% SDS-PAGE, and IKKβ and SGK1 were detected by immunoblotting. A representative blot from four independent experiments is shown.
MR but not Glucocorticoid Receptor Is Required for Aldosterone-Induced NF-κB Activation
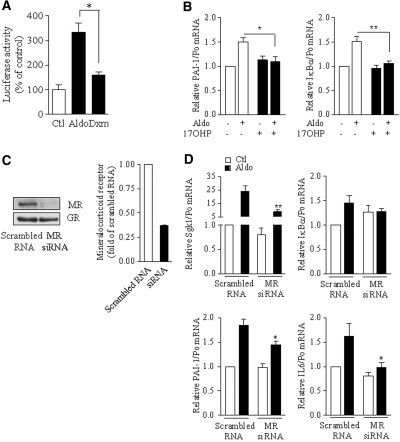
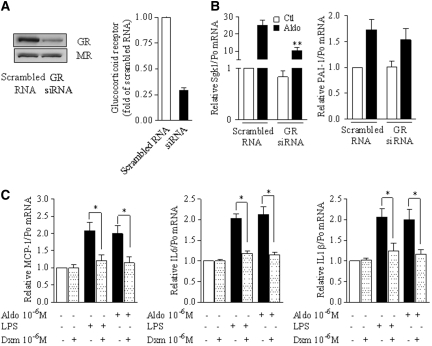
Because SGK1 was originally identified as a glucocorticoid-induced gene and aldosterone binds with a similar affinity to both MR and glucocorticoid receptor (GR), we investigated whether aldosterone-induced NF-κB activation depends on the MR or the GR. For this purpose, we first assessed the effect of the glucocorticoid agonist dexamethasone on NF-κB transcriptional activity by luciferase-reporter gene assay in mpkCCDcl4 cells. Luciferase activity weakly increased in response to 10−6 M dexamethasone, but this stimulation was 2.5-fold lower than that produced by 10−6 M aldosterone (Figure 8A), suggesting that the MR rather than the GR mediates aldosterone-induced NF-κB activation. In agreement with this hypothesis, the progesterone derivative 17-α-hydroxyprogesterone, that specifically antagonizes MR transactivation without significantly altering GR activity23 (unpublished data; see the Concise Methods section) also fully prevented the effect of 10−6 M aldosterone on NF-κB–dependent gene transcription (Figure 8B). Moreover, siRNA targeting the MR, which decreased MR abundance by 70% without altering GR expression (Figure 8C), prevented aldosterone-induced IκBα, IL-6, and PAI-1 mRNA expression (Figure 8D), whereas effective knockdown of the GR did not (Figure 9, A and B). Finally, in mpkCCDcl4 cells, dexamethasone prevented the increase of NF-κB–controlled gene transcripts observed in response to either LPS or aldosterone (Figure 9C); therefore, the MR mediates NF-κB activation by aldosterone, and this activation is inhibited by activated GR in CD principal cells.
Figure 8.
MR is required for NF-κB activation by aldosterone. (A) Effect of the GR agonist dexamethasone on NF-κB activity. mpkCCDcl4 cells transiently were transfected with NF-κB–dependent luciferase-reporter gene, seeded on polycarbonate filters and incubated with 10−6 M dexamethasone (Dxm) or aldosterone (Aldo) for 6 h before measurement of luciferase activity. Results are expressed as a percentage of control (untreated cells; Ctl). Bars represent mean ± SEM from six independent experiments. *P < 0.05. (B) The effect of aldosterone was prevented by an MR antagonist. mCCDcl1 cells grown on polycarbonate filters were incubated for 6 h in the presence or absence of 10−6 M Aldo and/or 10−5 M of the specific MR antagonist 17OHP. IκBα and PAI-1 mRNA was detected by RT-PCR. Results are expressed as fold of control values set to 1. Bars are mean ± SEM from six independent experiments. *P < 0.05, **P < 0.01. (C and D) Silencing of MR prevented the effects of aldosterone. mCCDcl1 cells transfected with siRNA targeting MR or scrambled RNA, seeded on plastic support (C) or polycarbonate filters (D), were incubated in the absence (Ctl) or presence of 10−6 M aldosterone (Aldo) for 6 h, before protein (B) or RNA extraction (C). (C) MR protein abundance, assessed by immunoblot, confirmed the specific knockdown of MR by siRNA. Representative blots from two independent experiments are shown. (D) SGK1, IκBα, PAI-1, or IL-6 mRNA was detected by RT-PCR. Results are expressed as fold of control value set to 1. Bars are means ± SEM from six independent experiments. *P < 0.05 versus aldosterone-stimulated scrambled RNA.
Figure 9.
Aldosterone-mediated NF-κB activation does not involve the GR. (A and B) Silencing of GR did not prevent the aldosterone-induced NF-κB activation. mCCDcl1 cells were transfected with siRNA targeting GR or scrambled RNA and processed as described in Figure 8, C and D. (A) GR protein abundance, assessed by immunoblot, confirmed the specific knockdown of GR by siRNA. Representative blots from two independent experiments are shown. (B) SGK1 and PAI-1 mRNA were detected by RT-PCR. (C) GR agonist inhibited the aldosterone-induced NF-κB activation. mpkCCDcl4 cells grown on polycarbonate filters were incubated for 6 h with 10−6 M dexamethasone and/or 10−6 M aldosterone or 100 ng/L LPS. MCP-1, IL-1β, and IL-6 mRNA were detected by RT-PCR. Results are expressed as fold of control values set to 1. Bars are means ± SEM from four independent experiments. *P < 0.05. Results are expressed as fold of control value set to 1. Bars are means ± SEM from six independent experiments. *P < 0.05 versus aldosterone-stimulated scrambled RNA.
Salt Restriction Increases NF-κB–Induced Gene Expression in an Aldosterone-Dependent Manner in Rat CCD
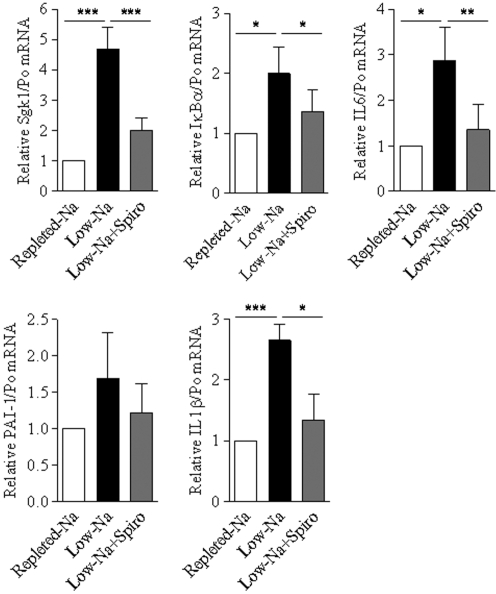
To assess the physiologic relevance of aldosterone-dependent activation of NF-κB, we performed RT-PCR experiments on isolated CCD from rats under normal- or low-Na diet, which classically exhibit increased aldosterone levels. As shown in Figure 10, NF-κB–induced transcripts (IκBα, IL-6, PAI-1, and IL-1β), along with SGK1 mRNA, were upregulated in Na-restricted rats, and this effect was largely prevented by the MR antagonist spironolactone.
Figure 10.
Low-Na diet–induced NF-κB activation is dependent on MR in rat CCD. Isolated CCD were microdissected from rats under normal- or low-Na diet treated or not with spironolactone (Spiro). SGK1, IκBα, IL-6, PAI-1, or IL-1β mRNA was detected by RT-PCR. Results are expressed as fold of values obtained in CCD from rats under normal-Na diet set to 1. Bars are means ± SEM from 10 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
This study shows that aldosterone activates the NF-κB pathway in its physiologically targeted epithelial CD principal cells. The effect of aldosterone is MR mediated and involves upregulation of SGK1 and subsequent activation of the canonical IKKβ-IκBα-p65/RelA pathway.
According to our results, aldosterone increased NF-κB–inducible reporter gene expression and mRNA abundance of NF-κB target genes in CD principal cells in two differentiated mouse cultured cell lines,24,25 in native rat microdissected CCD, and in salt-restricted rats, a model of chronic high circulating aldosterone levels. Thus, aldosterone-induced NF-κB activation is not restricted to nonepithelial cells, such as mesangial cells or fibroblasts, as described previously,11,12 but is also observed in epithelial CD principal cells.
Although frequently activated simultaneously, the classical and alternative NF-κB activation pathways have distinct regulatory functions.26 In CD cells, disruption of the NF-κB pathway by either p65/RelA siRNA or IκBα SS/AA overexpression demonstrated that aldosterone-induced NF-κB activation is dependent on both IκBα and p65/RelA, indicating that this event involves activation of the canonical IKKβ/IκBα-dependent NF-κB pathway. In agreement with this interpretation, both p50 and p65/RelA accumulated in the nucleus, and p65/RelA DNA-binding on promoters of NF-κB–dependent genes increased in response to aldosterone. In addition, aldosterone decreased the nuclear abundance of RelB, an NF-κB subunit that can act both as a transcriptional activator and as a repressor.27 Proteasome-dependent degradation of RelB may occur in response to aldosterone stimulation as described after T cell activation.28 Thus, in addition to the nuclear translocation of p50 and p65/RelA, an altered balance between NF-κB subunits may play a role in the control of NF-κB–dependent gene expression in resting and aldosterone-treated CD principal cells.
NF-κB activity is controlled by a diverse array of signaling pathways. On the basis of the observation that many genes regulated by MAPK are also controlled by NF-κB, some studies have demonstrated that activation of p38 kinase and ERK pathways enhance the transactivation potential of p65/RelA.29 Aldosterone activates MAPK through various mechanisms, including MR-dependent transactivation of the EGF receptor,5 or redox-sensitive pathways.6 Nevertheless, we found that several hours of aldosterone treatment did not alter the phosphorylation levels of ERK1/2 and decreased phosphorylation of p38 kinase, suggesting that the activity of these MAPK is unaltered or decreased, respectively. Moreover, pharmacologic inhibition of ERK or p38 kinase did not alter the aldosterone-dependent increase of IκBα expression; therefore, it is unlikely that ERK or p38 kinase participates in the transactivation of p65/RelA by aldosterone in CD cells. SGK1, an important player in sodium reabsorption mediated by MR and in chronic nephropathy progression,30–32 also increases p65/RelA transactivation activity. Recent studies reported that stimulation of NF-κB–dependent luciferase reporter gene activity by aldosterone is dependent on SGK1 in glomerular mesangial cells and in fibroblasts.11,12 Moreover, SGK1 has been shown to associate physically with and phosphorylate IKKβ,22 which, once activated, phosphorylates p65/RelA.33 In agreement with these findings, our results indicate that knockdown or overexpression of SGK1, respectively, inhibited or increased NF-κB–targeted gene expression in response to aldosterone. Aldosterone also induced a physical interaction between SGK1 and IKKβ in CD cells. SGK1 may therefore stimulate IKKβ and thereby activate NF-κB in CD cells.
“Cross-talk,” or interaction, between nuclear receptors and NF-κB has been described with glucocorticoid, estrogen, and peroxisome proliferator–activated receptors.34 The time course of NF-κB activation by aldosterone was consistent with a genomic effect, suggesting the involvement of nuclear receptors. Glucocorticoids, which strongly induce SGK1 expression, classically inhibit NF-κB in nonepithelial cells,35 but this repressive effect is more controversial in epithelial cells. GR knockdown experiments did not prevent the effect of aldosterone on NF-κB–induced transcripts. Moreover, in contrast with a previous report from de Haij et al.36 that NF-κB–controlled cytokine production is insensitive to dexamethasone in renal epithelial cells, we observed a strong reduction of LPS- or aldosterone-induced cytokine mRNA accumulation in CD cells. Because inhibition of the NF-κB pathway by glucocorticoids relies on GR-dependent transrepression or transactivation mechanisms,37 it is very unlikely that GR mediates aldosterone-dependent NF-κB activation. Conversely, inhibition of aldosterone-induced expression of NF-κB–controlled genes by either knockdown or MR pharmacologic inhibition demonstrates that MR mediates the activation of NF-κB by aldosterone. In addition, our results confirm previous observations suggesting that MR and GR may elicit distinct physiologic responses in CD principal cells.38–40
Although NF-κB–inducible reporter gene was activated in response to aldosterone concentrations as low as 10−10 M in cultured CD cells, enhanced proinflammatory gene expression was observed in response to supraphysiologic concentrations of aldosterone in cultured cells and freshly isolated CCD; however, aldosterone-induced NF-κB activation observed in CD cells might be relevant to physiopathologic conditions. Indeed, rats under low-Na diet, which classically exhibit high aldosterone circulating levels,41 exhibit increased expression levels of several NF-κB–induced genes, and hyperaldosteronism is frequently observed in both experimental nephropathies42 and in human chronic renal failure.43,44 Results obtained in CCD from rats under low-Na diet and evidence obtained in cultured nonclassical aldosterone-responsive proximal tubular cells45 or glomerular mesangial cells12 strongly suggest that aldosterone may induce or enhance tubulointerstitial and glomerular inflammation and fibrosis via NF-κB activation. Along with our results, these observations provide a rationale for the beneficial effects of administration of MR antagonists in both experimental45–47 and human chronic kidney disease.48
Independent from this suspected role in chronic renal disease progression, activation of NF-κB by aldosterone may play a role in controlled sodium and water reabsorption by the CD. As demonstrated in our previous study,18 increased sodium entry and TNF-α induced dissociation of a p65/RelA:IκBα:PKA complex, leading to transient activation of transepithelial sodium transport. Whereas dissociation of the p65/IκBα/PKA complex transiently (minutes) increases sodium transport in CD principal cells, activation of NF-κB led to long-term (hours) transcriptional effects that may decrease both sodium and water reabsorption. In this regard, NF-κB agonists inhibit sodium transport in colon, lung, and renal CCD.40,49–51 More recently, we demonstrated in CD cells that prolonged NF-κB activation inhibits transepithelial sodium transport via downregulation of SGK1 expression and subsequent decreased expression of aldosterone-induced sodium transporters.52 Interestingly, inhibition of sodium transport by IL-1β is more pronounced in mineralocorticoid- than in glucocorticoid-stimulated CD cells.40 This observation might be explained by an additional stimulation of NF-κB by activated MR and by its inhibition by activated GR. We have also demonstrated that NF-κB activation directly inhibits aquaporin 2 (AQP2) gene transcription via binding of NF-κB to the AQP2 promoter.53 Activation of NF-κB may then participate in the downregulated expression of AQP2 mRNA observed in response to aldosterone in CD cells.54
In conclusion, activation of NF-κB by aldosterone in renal tubule epithelial cells may constitute a negative feedback mechanism that decreases aldosterone-dependent sodium transport, modulates water reabsorption, and/or participates in chronic tubulointerstitial inflammation.
CONCISE METHODS
Cell Culture
mpkCCDcl4 and mCCDcl1 cells were seeded on permeable filters (Transwell; Corning Costar, Cambridge, MA; for luciferase assay and real-time PCR), on glass coverslips (for immunofluorescence microscopy), or on a plastic support (for immunoblotting) and grown to confluence in culture medium supplemented with 2% FCS24,25; and then in serum- and hormone-free medium for 48 h before performance of experiments. All experiments were performed on at least 3 separate days, using different splittings.
Transfection
Transfection was performed by electroporating cells as described previously.55 Briefly, 4 × 106 cells, along with 400 μl of culture medium supplemented with 10% serum and 1.25% DMSO and 2 nmol siRNA or 8 pmol of plasmid constructs, were transferred to electroporation cuvettes (Gene Pulser cuvette 0.4 cm; Bio-Rad, Hercules, CA), electroporated (300 mV, 960 pF, pulse duration 15 to 17 ns in mpkCCDcl4 cells and 22 to 24 ns in mCCDcl1 cells) using a Bio-Rad Gene Pulser. Cells were seeded on permeable filters at a density of 500,000 cells per well, allowed to recover for 24 h in culture medium containing 10% serum, and then maintained in serum and hormone-free medium for another 24 h for siRNA or 48 h for plasmids.
Constitutively active IKKβ SS/EE, obtained by mutating Ser176 and Ser180 residues to Glu,56 and super-repressor IκBα SS/AA, obtained by mutating Ser 32 and 36 to Ala,57 were generous gifs from Dr S. Gosh (Yale University School of Medicine, New Haven, CT). SGK1 expression vector was a gift from Dr. D. Pearce (University of California, San Francisco, CA). siRNA were as follows: For targeting p65, siRNA 5′-GCACCUGCAGUUUGAUGCUGAUGAA-3′, and scrambled RNA 5′-GCACGUUGAGUUGUAGUCUACCGAA-3′; for targeting SGK1, siRNA 5′-GGGCUGUCCUGUAUGAGAUGCUCUATT-3′ and scrambled RNA 5′-CGACACUCCCAUAUCCUCACAGGAATT-3′; for targeting MR, siRNA 5′-AGACUAUUAUUCCCUAUCATT-3′ and scrambled RNA 5′-GUCCUAAUCAUUCACAUAUTT-3′ for targeting p50, siRNA 5′-GGGAGGAGAUUUACCUUCUCUGUGATT-3′ and scrambled RNA 5′-CAGCCGGAUACUUCCAAACUUAAUUTT-3′, for targeting the GR, siRNA 5′-UGACUGCCUUACUAAGAAATT-3′ and scrambled RNA 5′-GACGUAGCCUAAUAAAUCUTT-3′.
Animal Experimental Protocol
After 1 wk of acclimation, male Sprague-Dawley rats (150 to 200 g body weight; Charles-River, Saint Germain de l’Arbresle, France) were housed for 1 wk in metabolic cages and fed either low- or normal-Na diet. Low-Na diet was prepared by mixing commercially available Na-free synthetic rat chow (0.0041% NaCl wt/wt; formula 53140000; Ziegler, Gardner, PA) with deionized water (12.5 ml/7.5 g rat chow) and agar (0.5% wt/wt) for gelation. Rats fed this low-Na diet were given 0.005 mmol Na/100 g body wt per d. Normal Na diet was prepared by addition of NaCl to Na-free gel diet to provide 1.0 mmol Na/100 g body wt per d. Rats were pair-fed with 7.5 g of synthetic rat chow/100 g body wt per d and had free access to water. Spironolactone was dissolved in olive oil (50 mg spironolactone/ml olive oil) and added to the food mixture before gelation. An equal amount of olive oil was given to untreated rats. One group of rats under low-Na diet received 0.17 mg/100 g body wt per d spironolactone for the first 3 d of treatment and were then administered 0.35 mg/100 g body wt per d spironolactone for the last 4 d of treatment.
The efficacy of low-Na diet was assessed by measurement of urinary Na concentration, which was <10 mmol/L in both untreated and spironolactone-treated rats, whereas urinary Na concentration was 144 ± 14 mmol/L in rats under normal-Na diet. Animal protocols were approved by the local ethics committee.
Isolated Rat Kidney Tubules
As described previously,58 rats were anesthetized with 5 mg/100 g pentobarbital intraperitoneal, and the left kidney was perfused via the abdominal aorta with incubation solution (120 mM NaCl, 5 mM KCl, 4 mM NaHCO3, 1 mM CaCl2, 1 mM MgSO4, 0.2 mM NaH2PO4, 0.15 mM Na2HPO4, 5 mM glucose, 10 mM lactate, 1 mM pyruvate, 4 mM essential and nonessential amino acids, 0.03 mM vitamins, 20 mM HEPES, and 0.1% BSA [pH 7.45]) containing 5% (vol/vol) of collagenase (Blendzyme 2; Roche, Basel, Switzerland); removed; sliced into small pyramids; and incubated for 20 min at 30°C in oxygenated (95% O2 and 5% CO2) incubation solution containing 1% (vol/vol) of collagenase. Single CCD were isolated by microdissection in ice-cold oxygenated incubation solution and stored at 4°C until used. Each experimental group was constituted by pools of 50 CCD from the same rat.
Immunofluorescence Microscopy
Confluent cells grown on gelatin-coated glass coverslips were rinsed twice with PBS, fixed with 4% (wt/vol) paraformaldehyde for 30 min at room temperature, and then permeabilized with 0.1% Triton X-100 for 3 min. After blocking for 30 min with 1% (wt/vol) BSA in PBS, cells were incubated with monoclonal anti-p65 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:200 in PBS containing 1% BSA and after three washes in PBS incubated with a secondary anti-mouse IgG Alexa 488–conjugated antibody diluted 1:200 in PBS containing 1% BSA (Molecular Probes, Eugene, OR). Coverslips were mounted on glass slides with Vectashield (Vector Laboratories, Burlingame, CA), and fluorescence images were captured at 488 nm with an Axiovert 200M Zeiss microscope at ×40 magnification using Openlab software (Improvision, Boston, MA).
Preparation of Total and Nuclear Protein Extracts and Immunoblot Analysis
Total cell lysates were performed as described previously.59 For nuclear extract preparation, cells were scraped off, spun at 700 × g, and homogenized in cell lysis buffer (10 mM HEPES-KOH [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 1 mM 4-[2-aminoethyl]benzenesulfonylfluoride, 10 μg/ml leupeptin, and 4 μg/ml aprotinin) on ice for 10 min. The cell extract was then spun at 700 × g, resuspended in the same lysis buffer, and respun. The pellet was resuspended in nuclear lysis buffer (20 mM HEPES-KOH [pH 7.9], 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 M EDTA, 1 mM 4-[2-aminoethyl]benzenesulfonylfluoride, 10 μg/ml leupeptin, and 4 μg/ml aprotinin) on ice for 40 min and spun at 18,000 × g. The supernatant, corresponding to nuclear extract, was then collected. Equal amounts of protein (100 μg for nuclear extracts and 75 μg for total cell lysate) were separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, MA) as described previously.60 The membranes were incubated overnight with the indicated antibodies, followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h. The antigen–antibody complexes were detected using Immobilon Immuno Chemiluminescent HRP Substrate (Millipore, Billerica, MA). Bands were quantified using a video densitometer and ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Immunoprecipitation
Immunoprecipitation was performed as described previously.18 Briefly, after cell lysis, 500 μg of protein was incubated overnight at 4°C with saturating amounts of rabbit polyclonal anti-p65 (Santa Cruz Biotechnology) or anti-SGK1 (Sigma, St. Louis, MO) antibodies. After incubation with protein A–Sepharose beads and extensive washing, precipitated proteins were separated by 10% SDS-PAGE, and IKKβ or p65/RelA was revealed by immunoblotting using either a rabbit polyclonal anti-IKKβ or a mouse mAb or anti-p65/RelA antibody diluted 1:2000.
Antibodies and Reagents
Antibodies against IκBα, IKKβ, p65/RelA, p50, p52, RelB, c-Rel, and GR were purchased from Santa Cruz Biotechnology and used at a 1:2000 dilution. Antibodies against phospho(S536)p65/RelA and HDAC3 were purchased from Cell Signaling (Danvers, MA) and used at 1:200 or 1:2000 dilutions. Anti-mouse SGK1 and α-tubulin antibodies were purchased from Sigma and diluted 1:2000 and 1:106, respectively. The MR was detected with mixed mAb 905, 906, 907, and 908 and diluted 1:2000 (a gift from Dr. C. Gomez-Sanchez, Division of Endocrinology, University of Mississippi Medical Center, Jackson, MS). Goat HRP-conjugated anti-rabbit or anti-mouse IgG were purchased from Cell Signaling and diluted 1:20000.
Dexamethasone, aldosterone, spironolactone, and 17 α hydroxyprogesterone (17OHP) were purchased from Sigma. Steroids were 10 mM stock solution in 100% DMSO. Stock solutions were diluted to their final concentrations in culture medium. In all experiments using one or more of these compounds, control cells were incubated in the presence of the same concentration of DMSO as treated cells. As shown by Rafestin-Oblin etal.,23 17OHP behaves as a competitive inhibitor of the MR and was shown to antagonize up to 80% of aldosterone-induced transactivation activity of the MR. 17OHP also attenuated the aldosterone-stimulated amiloride-sensitive short-circuit current in murine mpkCCDcl4 cells. In addition, 17OHP is a weak agonist/antagonist of the GR because the GR transactivation activity induced by 10−6 M 17OHP amounted to approximately 15% of that induced by 10−6 M dexamethasone, and 10−6 M 17OHP inhibited only 20% of the GR transactivation activity induced by 10−8 M dexamethasone (M.-E.R.-O., unpublished data). UO126 and SB203580 were purchased from Calbiochem (San Diego, CA), and 1 mM stock solutions were prepared in 100% DMSO.
Real-Time PCR Analysis
RNA extraction, reverse transcription, and RT-PCR analyses were performed as described previously.59 The following primers were used: For detection of mouse acidic ribosomal phosphoprotein P0, 5′-AATCTCCAGAGGCACCATTG-3′ and 5′-GTTCAGCATGTTCAGCAGTG-3′; for detection of mouse MCP-1, 5′GGCTCAGCCAGATGCAGTTAA-3′ and 5′-CCTACTCATTGGGATCATCTTGCT-3′; for detection of mouse IL-6, 5′-CCAGAAACCGCTATGAAGTTCCT-3′ and 5′-CACCAGCATCAGTCCCAAGA-3′; for detection of mouse PAI-1, 5′-CAGGCACTGCAAAAGGTCAGGATCGA-3′ and 5′-GGGCCATGCGGGCTGAGATGA-3′; for detection of mouse IκBα, 5′-GGCCTTCCTCAACTTCCAGAA-3′ and 5′-GTCTCGGAGCTCAGGATCA-3′; for detection of mouse IL-1β, 5′-CCTTCCAGGATGAGGACATGA-3′ and 5′-GGAACGTCACACACCAGCAG-3′; for detection of rat acidic ribosomal phosphoprotein P0, 5′-CCTTCTCCTTCGGGCTGATC-3′ and 5′-GGGCTGTAGATGCTGCCATT-3′; for detection of rat SGK1, 5′-GGGACAACGTCCACCTTCTG-3′ and 5′-CGGCTGCTTATGGAGAACCT-3′; for detection of rat MCP-1, 5′-TCATCGCTGTTCATACAATCAGAA-3′ and 5′-CGCTTCTGGGCCTGTTGT-3′; for detection of rat IL-6, 5′-TCATCGCTGTTCATACAATCAGAA-3′ and 5′-AGGGAGATCTTGGAAATGAGAAAA; for detection of rat PAI-1, 5′-TCCGGATGGGCACGAGTA-3′ and 5′-GAGGGTTTCGCCGTGGTA-3′; for detection of rat IL-1β, 5′-AGCCTTTGTCCTCTGCCAAGT-3′ and 5′-CCAGAATGTGCCACGGTTTT-3′; and for detection of rat IκBα, 5′-CTGCAGGCCACCAACTACAA-3′ and 5′-GTAGCCATGGATAGAGGCTAAGTG-3′. P0 was used as an internal standard, and data were analyzed as described previously.13
Luciferase Assay
Luciferase plasmid constructs used for transfection were NF-κB–dependent luciferase plasmids containing three NF-κB enhancer elements (pκB)3 IFN-Luc plasmid.61 Luciferase activity was measured using the Luciferase Assay System (Promega, Madison, WI) according to the manufacturer's instructions. The light produced was measured using a Lumat LB 9507 luminometer (EG&G Berthold, Bad Wildbad, Germany).
ChIP Assay
ChIP assay was performed as described previously.62 Briefly, after stimulation, cells were rapidly fixed in ice-cold cross-linking solution (1% formaldehyde, 9 mM NaCl, and 4.5 mM HEPES [pH 8]) before cell lysis (0.5% NP40, 1 mM EDTA, 1 mM PMSF, 50 mM NaF, 1 mM orthovanadate, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 10 mM Tris [pH 8]). Nuclei were then isolated by centrifugation and lysed (1% Triton X100, 0.5% SDS, 0.5% Sarkosyl, 0.5 M NaCl, 1 mM EDTA, 1 mM PMSF, 50 mM NaF, 1 mM orthovanadate, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 10 mM Tris [pH 8]), and pelleted chromatin was resuspended (100 mM NaCl, 1 mM EDTA, and 10 mM Tris [pH 8]) before fragmentation (<1 kb) by sonication. For immunoprecipitation, chromatin extracts (100 μg) were precleared for 1 h with protein A–Sepharose beads (Amersham-Pharmacia Biotech, Little Chalfont, UK) and salmon sperm DNA in RIPA buffer (1% Triton X100, 0.1% SDS, 0.1% sodium deoxycholate, 140 mM NaCl, 1 mM EDTA, and 10 mM Tris [pH 8]) and then incubated overnight at 4°C with a polyclonal anti-p65 antibody (Santa Cruz Biotechnology). Immune complexes were precipitated with protein A–Sepharose beads for 3 h at 4°C. Reversal of cross-link was achieved by overnight incubation at 65°C with proteinase K and 1% SDS. DNA was extracted with phenol chloroform and precipitated with ethanol. RT-PCR was performed as described already. Primers flanking the κB site of the IκBα promoter were 5′-GCTTCTCAGTGGAGGACGAG-3′ and 5′-CTGGCTGAAACATGGCTGT-3, those flanking the κB sites of PAI-1 promoter were 5′-CTCTGTGATGGCTGTCTCCA-3′ and 5′-GCAAACTCTGGTTCCCTTGA-3′, and those flanking the κB sites of MCP-1 promoter were 5′-ATCTGGAGCTCACATTCCA-3′ and 5′-TCCCTCTCACTTCACTCTGTCA-3′.
Statistical Analysis
Results are given as means ± SEM from n independent experiments. We performed comparisons between two groups by one-sample t test. P < 0.05 was considered significant.
DISCLOSURES
None.
Acknowledgments
This work was supported in part by Swiss National Foundation grant 31-109473, a Genzyme Innovation Program grant, and an Ernst and Lucie Schmidheiny Foundation grant to E.F.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Salt in the Wound,” on pages 5–6.
REFERENCES
- 1.Marney AM, Brown NJ: Aldosterone and end-organ damage. Clin Sci (Lond) 113: 267–278, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Ishizawa K, Izawa Y, Ito H, Miki C, Miyata K, Fujita Y, Kanematsu Y, Tsuchiya K, Tamaki T, Nishiyama A, Yoshizumi M: Aldosterone stimulates vascular smooth muscle cell proliferation via big mitogen-activated protein kinase 1 activation. Hypertension 46: 1046–1052, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Huang W, Xu C, Kahng KW, Noble NA, Border WA, Huang Y: Aldosterone and TGF-β1 synergistically increase PAI-1 and decrease matrix degradation in rat renal mesangial and fibroblast cells. Am J Physiol Renal Physiol 294: F1287–F1295, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Mathew JT, Patni H, Chaudhary AN, Liang W, Gupta A, Chander PN, Ding G, Singhal PC: Aldosterone induces mesangial cell apoptosis both in vivo and in vitro. Am J Physiol Renal Physiol 295: F73–F81, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossmann C, Gekle M: Non-classical actions of the mineralocorticoid receptor: Misuse of EGF receptors? Mol Cell Endocrinol 277: 6–12, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Callera GE, Touyz RM, Tostes RC, Yogi A, He Y, Malkinson S, Schiffrin EL: Aldosterone activates vascular p38MAP kinase and NADPH oxidase via c-Src. Hypertension 45: 773–779, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Terada Y, Kobayashi T, Kuwana H, Tanaka H, Inoshita S, Kuwahara M, Sasaki S: Aldosterone stimulates proliferation of mesangial cells by activating mitogen-activated protein kinase 1/2, cyclin D1, and cyclin A. J Am Soc Nephrol 16: 2296–2305, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Brown NJ, Kim KS, Chen YQ, Blevins LS, Nadeau JH, Meranze SG, Vaughan DE: Synergistic effect of adrenal steroids and angiotensin II on plasminogen activator inhibitor-1 production. J Clin Endocrinol Metab 85: 336–344, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Li X, Meng Y, Wu P, Zhang Z, Yang X: Angiotensin II and aldosterone stimulating NF-kappaB and AP-1 activation in hepatic fibrosis of rat. Regul Pept 138: 15–25, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Neves MF, Amiri F, Virdis A, Diep QN, Schiffrin EL: Role of aldosterone in angiotensin II-induced cardiac and aortic inflammation, fibrosis, and hypertrophy. Can J Physiol Pharmacol 83: 999–1006, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Vallon V, Wyatt AW, Klingel K, Huang DY, Hussain A, Berchtold S, Friedrich B, Grahammer F, Belaiba RS, Gorlach A, Wulff P, Daut J, Dalton ND, Ross J Jr, Flogel U, Schrader J, Osswald H, Kandolf R, Kuhl D, Lang F: SGK1-dependent cardiac CTGF formation and fibrosis following DOCA treatment. J Mol Med 84: 396–404, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Terada Y, Kuwana H, Kobayashi T, Okado T, Suzuki N, Yoshimoto T, Hirata Y, Sasaki S: Aldosterone-stimulated SGK1 activity mediates profibrotic signaling in the mesangium. J Am Soc Nephrol 19: 238–309, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasler U, Nielsen S, Feraille E, Martin PY: Posttranscriptional control of aquaporin-2 abundance by vasopressin in renal collecting duct principal cells. Am J Physiol Renal Physiol 290: F177–F187, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Ghosh S, Karin M: Missing pieces in the NF-kappaB puzzle. Cell 109[Suppl]: S81–S96, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Gerondakis S, Grossmann M, Nakamura Y, Pohl T, Grumont R: Genetic approaches in mice to understand Rel/NF-kappaB and IkappaB function: Transgenics and knockouts. Oncogene 18: 6888–68895, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Baines DL, Janes M, Newman DJ, Best OG: Oxygen-evoked changes in transcriptional activity of the 5′-flanking region of the human amiloride-sensitive sodium channel (alphaENaC) gene: Role of nuclear factor kappaB. Biochem J 364: 537–545, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebowitz J, Edinger RS, An B, Perry CJ, Onate S, Kleyman TR, Johnson JP: IKKβ modulation of ENaC activity. J Biol Chem 279: 41985–41990, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Vinciguerra M, Hasler U, Mordasini D, Roussel M, Capovilla M, Ogier-Denis E, Vandewalle A, Martin PY, Feraille E: Cytokines and sodium induce protein kinase A-dependent cell-surface Na,K-ATPase recruitment via dissociation of NF-κB/IκB/protein kinase A catalytic subunit complex in collecting duct principal cells. J Am Soc Nephrol 16: 2576–2585, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Chiao P, Miyamato S, Verma I: Autoregulation of IκBα activity. Proc Natl Acad Sci U S A 91: 28–32, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ping D, Jones PL, Boss JM: TNF regulates the in vivo occupancy of both distal and proximal regulatory regions of the MCP-1/JE gene. Immunity 4: 455–469, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Ghosh S, May MJ, Kopp EB: NF-κB and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16: 225–260, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Cui R, Cheng X, Du J: Antiapoptotic effect of serum and glucocorticoid-inducible protein kinase is mediated by novel mechanism activating IκB kinase. Cancer Res 65: 457–464, 2005 [PubMed] [Google Scholar]
- 23.Rafestin-Oblin ME, Fagart J, Souque A, Seguin C, Bens M, Vandewalle A: 11β-Hydroxyprogesterone acts as a mineralocorticoid agonist in stimulating Na+ absorption in mammalian principal cortical collecting duct cells. Mol Pharmacol 62: 1306–1313, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A: Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol 10: 923–934, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Gaeggeler HP, Gonzalez-Rodriguez E, Jaeger NF, Loffing-Cueni D, Norregaard R, Loffing J, Horisberger JD, Rossier BC: Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol 16: 878–891, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Hayden MS, Ghosh S: Signaling to NF-κB. Genes Dev 18: 2195–2224, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Ruben SM, Klement JF, Coleman TA, Maher M, Chen CH, Rosen CA: I-Rel: A novel rel-related protein that inhibits NF-κB transcriptional activity. Genes Dev 6: 745–760, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Marienfeld R, Berberich-Siebelt F, Berberich I, Denk A, Serfling E, Neumann M: Signal-specific and phosphorylation-dependent RelB degradation: A potential mechanism of NF-κB control. Oncogene 20: 8142–8147, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W, Haegeman G: p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-κ B p65 transactivation mediated by tumor necrosis factor. J Biol Chem 273: 3285–3290, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Flores SY, Loffing-Cueni D, Kamynina E, Daidie D, Gerbex C, Chabanel S, Dudler J, Loffing J, Staub O: Aldosterone-induced serum and glucocorticoid-induced kinase 1 expression is accompanied by Nedd4–2 phosphorylation and increased Na+ transport in cortical collecting duct cells. J Am Soc Nephrol 16: 2279–2287, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Artunc F, Amann K, Nasir O, Friedrich B, Sandulache D, Jahovic N, Risler T, Vallon V, Wulff P, Kuhl D, Lang F: Blunted DOCA/high salt induced albuminuria and renal tubulointerstitial damage in gene-targeted mice lacking SGK1. J Mol Med 84: 737–746, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Feng Y, Wang Q, Wang Y, Yard B, Lang F: SGK1-mediated fibronectin formation in diabetic nephropathy. Cell Physiol Biochem 16: 237–244, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Perkins ND: Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene 25: 6717–6730, 2006 [DOI] [PubMed] [Google Scholar]
- 34.De Bosscher K, Vanden Berghe W, Haegeman G: Cross-talk between nuclear receptors and nuclear factor kappaB. Oncogene 25: 6868–6886, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS Jr: Role of transcriptional activation of IκBα in mediation of immunosuppression by glucocorticoids. Science 270: 283–286, 1995 [DOI] [PubMed] [Google Scholar]
- 36.de Haij S, Woltman AM, Bakker AC, Daha MR, van Kooten C: Production of inflammatory mediators by renal epithelial cells is insensitive to glucocorticoids. Br J Pharmacol 137: 197–204, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newton R, Holden NS: Separating transrepression and transactivation: A distressing divorce for the glucocorticoid receptor? Mol Pharmacol 72: 799–809, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Boulkroun S, Fay M, Zennaro M-C, Escoubet B, Jaisser F, Blot-Chabaud M, Farman N, Courtois-Coutry N: Characterization of rat NDRG2 (N-myc downstream regulated gene 2), a novel early mineralocorticoid-specific induced gene. J Biol Chem 277: 31506–31515, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Laplace JR, Husted RF, Stokes JB: Cellular responses to steroids in the enhancement of Na+ transport by rat collecting duct cells in culture: Differences between glucocorticoid and mineralocorticoid hormones. J Clin Invest 90: 1370–1378, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Husted RF, Zhang C, Stokes JB: Concerted actions of IL-1β inhibit Na+ absorption and stimulate anion secretion by IMCD cells. Am J Physiol 275: F946–F954, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Pacha J, Frindt G, Antonian L, Silver RB, Palmer LG: Regulation of Na channels of the rat cortical collecting tubule by aldosterone. J Gen Physiol 102: 25–42, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greene E, Kren S, Hostetter T: Role of aldosterone in the remnant kidney model in the rat. J Clin Invest 98: 1063–1068, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hene RJ, Boer P, Koomans HA, Dorhout Mees EJ: Plasma aldosterone concentrations in chronic renal disease. Kidney Int 21: 98–101, 1982 [DOI] [PubMed] [Google Scholar]
- 44.Reams GP, Bauer JH: Effect of enalapril in subjects with hypertension associated with moderate to severe renal dysfunction. Arch Intern Med 146: 2145–2148, 1986 [PubMed] [Google Scholar]
- 45.Han S-Y, Kim C-H, Kim H-S, Jee Y-H, Song H-K, Lee M-H, Han K-H, Kim H-K, Kang Y-S, Han J-Y, Kim Y-S, Cha D-R: Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J Am Soc Nephrol 17: 1362–1372, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Fujisawa G, Okada K, Muto S, Fujita N, Itabashi N, Kusano E, Ishibashi S: Spironolactone prevents early renal injury in streptozotocin-induced diabetic rats. Kidney Int 66: 1493–1502, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Aldigier JC, Kanjanbuch T, Ma LJ, Brown NJ, Fogo AB: Regression of existing glomerulosclerosis by inhibition of aldosterone. J Am Soc Nephrol 16: 3306–3314, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Bianchi S, Bigazzi R, Campese VM: Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int 70: 2116–2123, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Amasheh S, Barmeyer C, Koch CS, Tavalali S, Mankertz J, Epple HJ, Gehring MM, Florian P, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD: Cytokine-dependent transcriptional down-regulation of epithelial sodium channel in ulcerative colitis. Gastroenterology 126: 1711–1720, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Fukuda N, Jayr C, Lazrak A, Wang Y, Lucas R, Matalon S, Matthay MA: Mechanisms of TNF-α stimulation of amiloride-sensitive sodium transport across alveolar epithelium. Am J Physiol Lung Cell Mol Physiol 280: L1258–L1265, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Sakairi Y, Ando Y, Tabei K, Kusano E, Asano Y: Interleukin-1 inhibits sodium and water transport in rabbit cortical collecting duct. Am J Physiol Renal Physiol 266: F674–F680, 1994 [DOI] [PubMed] [Google Scholar]
- 52.de Seigneux S, Leroy V, Ghzili H, Rousselot M, Nielsen S, Rossier BC, Martin P-Y, Feraille E: NF-κB inhibits sodium transport via down-regulation of SGK1 in renal collecting duct principal cells. J Biol Chem 283: 25671–25681, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Hasler U, Leroy V, Jeon US, Bouley R, Dimitrov M, Kim JA, Brown D, Kwon HM, Martin PY, Feraille E: NF-κB modulates aquaporin-2 transcription in renal collecting duct principal cells. J Biol Chem 283: 28095–28105, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hasler U, Mordasini D, Bianchi M, Vandewalle A, Feraille E, Martin PY: Dual influence of aldosterone on AQP2 expression in cultured renal collecting duct principal cells. J Biol Chem 278: 21639–21648, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Mordasini D, Bustamante M, Rousselot M, Martin PY, Hasler U, Feraille E: Stimulation of Na+ transport by AVP is independent of PKA phosphorylation of the Na-K-ATPase in collecting duct principal cells. Am J Physiol Renal Physiol 289: F1031–F1039, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A: IKK-1 and IKK-2: Cytokine-activated IκB kinases essential for NF-κB activation. Science 278: 860–866, 1997 [DOI] [PubMed] [Google Scholar]
- 57.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI: Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-κB activation in hemopoietic progenitor cells. J Immunol 160: 1224–1232, 1998 [PubMed] [Google Scholar]
- 58.Feraille E, Carranza ML, Rousselot M, Favre H: Insulin enhances sodium sensitivity of Na-K-ATPase in isolated rat proximal convoluted tubule. Am J Physiol Renal Physiol 267: F55–F62, 1994 [DOI] [PubMed] [Google Scholar]
- 59.Hasler U, Vinciguerra M, Vandewalle A, Martin PY, Feraille E: Dual effects of hypertonicity on aquaporin-2 expression in cultured renal collecting duct principal cells. J Am Soc Nephrol 16: 1571–1582, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Hasler U, Mordasini D, Bens M, Bianchi M, Cluzeaud F, Rousselot M, Vandewalle A, Feraille E, Martin PY: Long term regulation of aquaporin-2 expression in vasopressin-responsive renal collecting duct principal cells. J Biol Chem 277: 10379–10386, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Fujita T, Nolan GP, Liou HC, Scott ML, Baltimore D: The candidate proto-oncogene bcl-3 encodes a transcriptional coactivator that activates through NF-κB p50 homodimers. Genes Dev 7: 1354–1363, 1993 [DOI] [PubMed] [Google Scholar]
- 62.Ryser S, Fujita T, Tortola S, Piuz I, Schlegel W: The rate of c-fos transcription in vivo is continuously regulated at the level of elongation by dynamic stimulus-coupled recruitment of positive transcription elongation factor b. J Biol Chem 282: 5075–5084, 2007 [DOI] [PubMed] [Google Scholar]