Abstract
The erythrocyte receptor for Escherichia coli K99 fimbrial adhesin was isolated from equine erythrocytes and characterized as Neu5Gc-alpha(2----3)-Galp-beta(1----4)-GLcp-beta(1----1)-Ceramide. This glycolipid acted as the receptor for K99 by four different experimental approaches: inhibition of equine erythrocyte hemagglutination by preincubation of K99-positive bacteria or purified K99 fimbriae with the isolated glycolipid; inhibition of attachment of K99-positive bacteria to porcine intestinal epithelial cells in the presence of the isolated glycolipid; induction of binding of K99-positive bacteria or purified K99 fimbriae to normally unreactive guinea pig erythrocytes by coating these cells with the isolated glycolipid; and isolation of the receptor by affinity chromatography with K99 coupled to CNBr-activated Sepharose 4B, indicating a strong interaction between K99 and the isolated glycolipid.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariga T., Sekine M., Yu R. K., Miyatake T. Disialogangliosides in bovine adrenal medulla. J Biol Chem. 1982 Mar 10;257(5):2230–2235. [PubMed] [Google Scholar]
- Dabrowski J., Hanfland P., Egge H. Structural analysis of glycosphinoglipids by high-resolution 1H nuclear magnetic resonance spectroscopy. Biochemistry. 1980 Nov 25;19(24):5652–5658. doi: 10.1021/bi00565a030. [DOI] [PubMed] [Google Scholar]
- Eden C. S., Eriksson B., Hanson L. A. Adhesion of Escherichia coli to human uroepithelial cells in vitro. Infect Immun. 1977 Dec;18(3):767–774. doi: 10.1128/iai.18.3.767-774.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firon N., Ofek I., Sharon N. Interaction of mannose-containing oligosaccharides with the fimbrial lectin of Escherichia coli. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1426–1432. doi: 10.1016/0006-291x(82)90947-0. [DOI] [PubMed] [Google Scholar]
- Fukuda M. N., Hakomori S. Structures of branched blood group A-active glycosphingolipids in human erythrocytes and polymorphism of A- and H-glycolipids in A1 and A2 subgroups. J Biol Chem. 1982 Jan 10;257(1):446–455. [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
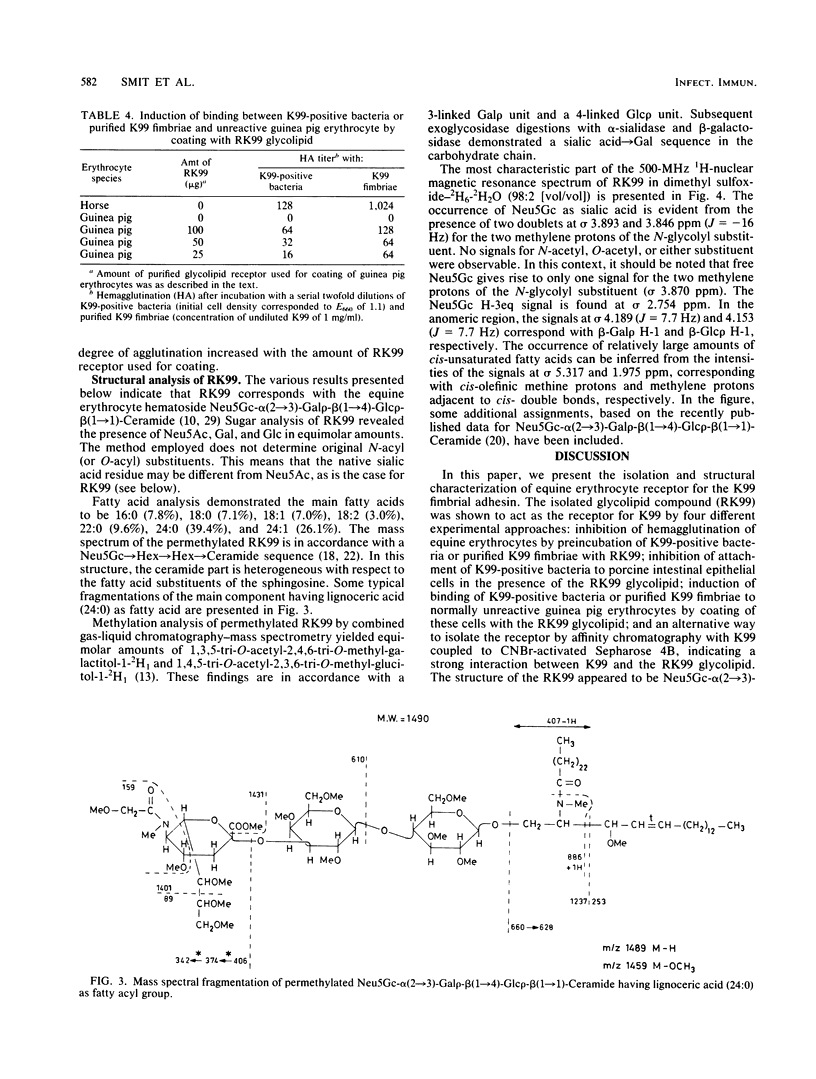
- Gasa S., Makita A., Kinoshita Y. Further study of the chemical structure of the equine erythrocyte hematoside containing O-acetyl ester. J Biol Chem. 1983 Jan 25;258(2):876–881. [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H., Agterberg C. M. Detection of the K99 antigen by means of agglutination and immunoelectrophoresis in Escherichia coli isolates from calves and its correlation with entertoxigenicity. Infect Immun. 1976 May;13(5):1369–1377. doi: 10.1128/iai.13.5.1369-1377.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Kamerling J. P., Dorland L., van Halbeek H., Vliegenthart J. F., Messer M., Schauer R. Structural studies of 4-O-acetyl-alpha-N-acetylneuraminyl-(2 goes to 3)-lactose, the main oligosaccharide in echidna milk. Carbohydr Res. 1982 Mar 1;100:331–340. doi: 10.1016/s0008-6215(00)81046-0. [DOI] [PubMed] [Google Scholar]
- Kamerling J. P., Gerwig G. J., Vliegenthart J. F., Clamp J. R. Characterization by gas-liquid chromatography-mass spectrometry and proton-magnetic-resonance spectroscopy of pertrimethylsilyl methyl glycosides obtained in the methanolysis of glycoproteins and glycopeptides. Biochem J. 1975 Dec;151(3):491–495. doi: 10.1042/bj1510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K. A., Pascher I., Pimlott W., Samuelsson B. E. Use of mass spectrometry for the carbohydrate composition and sequence analysis of glycosphingolipids. Biomed Mass Spectrom. 1974 Feb;1(1):49–56. doi: 10.1002/bms.1200010111. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A., Samuelsson B. E., Steen G. O. The sphingolipid composition of bovine kidney cortex, medulla and papilla. Biochim Biophys Acta. 1973 Sep 25;316(3):317–335. doi: 10.1016/0005-2760(73)90072-6. [DOI] [PubMed] [Google Scholar]
- Koerner T. A., Jr, Prestegard J. H., Demou P. C., Yu R. K. High-resolution proton NMR studies of gangliosides. 1. Use of homonuclear two-dimensional spin-echo J-correlated spectroscopy for determination of residue composition and anomeric configurations. Biochemistry. 1983 May 24;22(11):2676–2687. doi: 10.1021/bi00280a014. [DOI] [PubMed] [Google Scholar]
- Leffler H., Svanborg-Edén C. Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect Immun. 1981 Dec;34(3):920–929. doi: 10.1128/iai.34.3.920-929.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi M., Yamakawa T. Isolation and characterization of glycosphingolipids in pig adipose tissue. J Biochem. 1977 Jun;81(6):1675–1690. doi: 10.1093/oxfordjournals.jbchem.a131627. [DOI] [PubMed] [Google Scholar]
- Parkkinen J., Finne J., Achtman M., Väisänen V., Korhonen T. K. Escherichia coli strains binding neuraminyl alpha 2-3 galactosides. Biochem Biophys Res Commun. 1983 Mar 16;111(2):456–461. doi: 10.1016/0006-291x(83)90328-5. [DOI] [PubMed] [Google Scholar]
- Runnels P. L., Moon H. W., Schneider R. A. Development of resistance with host age to adhesion of K99+ Escherichia coli to isolated intestinal epithelial cells. Infect Immun. 1980 Apr;28(1):298–300. doi: 10.1128/iai.28.1.298-300.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellwood R., Gibbons R. A., Jones G. W., Rutter J. M. Adhesion of enteropathogenic Escherichia coli to pig intestinal brush borders: the existence of two pig phenotypes. J Med Microbiol. 1975 Aug;8(3):405–411. doi: 10.1099/00222615-8-3-405. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Klemm P., Gaastra W. Purification, characterization, and partial covalent structure of Escherichia coli adhesive antigen K99. Infect Immun. 1981 Sep;33(3):877–883. doi: 10.1128/iai.33.3.877-883.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., Roorda I. Production, purification, and characterization of the fimbrial adhesive antigen F41 isolated from calf enteropathogenic Escherichia coli strain B41M. Infect Immun. 1982 May;36(2):751–758. doi: 10.1128/iai.36.2.751-758.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Embden J. D., de Graaf F. K., Schouls L. M., Teppema J. S. Cloning and expression of a deoxyribonucleic acid fragment that encodes for the adhesive antigen K99. Infect Immun. 1980 Sep;29(3):1125–1133. doi: 10.1128/iai.29.3.1125-1133.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]