Abstract
Background
Dogs are the most common pet animals worldwide. They may harbour a wide range of parasites with zoonotic potential, thus causing a health risk to humans. In Nigeria, epidemiological knowledge on these parasites is limited.
Methods
In a community-based study, we examined 396 dogs in urban and rural areas of Ilorin (Kwara State, Central Nigeria) for ectoparasites and intestinal helminths. In addition, a questionnaire regarding knowledge and practices was applied to pet owners.
Results
Nine ectoparasite species belonging to four taxa and six intestinal helminth species were identified: fleas (Ctenocephalides canis, Pulex irritans, Tunga penetrans), mites (Demodex canis, Otodectes sp., Sarcoptes scabiei var. canis), ticks (Rhipicephalus sanguineus, Ixodes sp.), and lice (Trichodectes canis); and Toxocara canis, Ancylostoma sp., Trichuris vulpis, Dipylidium caninum, Taenidae and Strongyloides sp. Overall prevalence of ectoparasites was 60.4% and of intestinal helminths 68.4%. The occurrence of C. canis, R. sanguineus, T. canis, Ancylostoma sp. and T. vulpis was most common (prevalence 14.4% to 41.7%). Prevalence patterns in helminths were age-dependent, with T. canis showing a decreasing prevalence with age of host, and a reverse trend in other parasite species. Knowledge regarding zoonoses was very limited and the diseases not considered a major health problem. Treatment with antiparasitic drugs was more frequent in urban areas.
Conclusion
Parasites of importance for human health were highly prevalent in Nigerian dogs. Interventions should include health education provided to dog owners and the establishment of a program focusing on zoonotic diseases.
Background
Dogs are the most successful canids, adapted to human habitation worldwide. They have contributed to physical, social and emotional well-being of their owners, particularly children [1,2]. However, in spite of the beneficial effects, close bonds of dogs and humans (in combination with inappropriate human practices and behaviour) remain a major threat to public health, with dogs harbouring a bewildering number of infective stages of parasites transmissible to man and other domestic animals [2-4]. For example, well-known and important zoonotic diseases are cutaneous and visceral larva migrans, hydatid disease and tungiasis [5-8].
In low-income settings, treatments to eliminate these parasites are – if done at all - often applied in advanced stages of disease, causing distress on pets and their owners [9,10].
In many African countries, including Nigeria, appropriate policies regarding pet ownership and their effects on individual and community health are nonexistent. Prevalence of parasite infection in dogs with importance for human health is usually high, resulting in risk of zoonotic transmission from dogs to humans. The risk is further increased by non-favourable ecological and human behavioural factors [11-13].
Previous epidemiological studies on dog parasites in Nigeria were focused on the prevalence with little or no information on quantitative measure of infection and/or were not community-based [14-17]. Thus, we examined a representative population of dogs in urban and rural areas in a Nigerian city for the presence of possibly zoonotic parasites.
Methods
Study Area
The study was conducted in the city of Ilorin (Central Nigeria), and the neighbouring rural communities (longitude 4° 30' – 4° 45'N and latitude 8° 28' – 8° 38'E; Figure 1). Ilorin is an urban centre and the capital of Kwara State. The city covers an area of about 38 square miles, with an estimated population of 1.4 million people. It is located in Nigeria's central savannah region with intense rainfalls from April to October and daily temperatures between 23°C and 37°C.
Figure 1.
Map of the study area highlighting Nigeria (left), Kwara State and the study area (right).
The urban area of Ilorin is surrounded by rural villages with mainly agricultural-based economy. Living conditions are particularly poor in these rural communities, and a substantial proportion of the villagers keeping dogs have no access to veterinary services. Therefore, most dogs have never been treated for any form of parasitic diseases prior to the study. In addition, most dogs are not vaccinated.
Study design
A random house-to-house screening of dogs was conducted between October 2006 and May 2007. With the informed consent of dog owners, interviews were conducted using pre-tested structured questionnaires to obtain information on the dogs' age, sex, regimen, defaecation sites, previous anthelminthic treatment and disease-related knowledge of owners. Thereafter, pre-labeled specimen containers were distributed for the collection of stool samples. A screening of ectoparasites on dogs was performed before fecal specimen collection.
In households with more than one dog, only one dog (chosen by the dog owner) was included.
Sample collections
Dogs were thoroughly examined for ectoparasites by combing the entire body surfaces on a clear sheet of white paper. To facilitate the extraction of ectoparasites, the dogs were rubbed with a piece of cotton-wool soaked in ether. The ectoparasites recovered were preserved in 70% alcohol for identification.
For the diagnosis of intestinal helminths, freshly passed faecal samples from dogs were collected from dog owners and examined macroscopically for proglottides. Thereafter, a sub-sample of faeces was taken into a pre-labelled clean sterile universal plastic bottle containing 10% formaldehyde solution. All samples were carried to the parasitology laboratory at the University of Ilorin and processed for microscopic examination.
Laboratory procedures
Fleas, ticks and lice were cleared in 10% potassium hydroxide (KOH) solution overnight, dehydrated in ascending strength of alcohol and mounted in Canada balsam. Mites were mounted directly in Berlese fluid. Examination was done at 40× magnification under a dissecting microscope.
A duplicate 50 mg Kato-Katz thick smear was prepared from each faecal sample, using the Kato-Katz technique, as modified by Forrester and Scott [18]. In short, a small portion (1–3 g) was sieved through double-ply gauze to remove rough materials. The filtrate was centrifuged at 3000 rpm for 3 min, the supernatant decanted, and the tube allowed to stand for 10 min. Fifty mg of the sediment delivered by Kato-Katz template was taken onto a degreased glass slide, and covered with a cellophane strip soaked overnight in 50% solution of glycerol-malachite green. Slides were examined for helminths eggs under a light microscope immediately after preparation. Parasite eggs were identified based on the morphological characteristics. Density of infection, as expressed by eggs per gram (EPG) of faeces, was calculated by multiplying each slide count by 20 [19].
Data Entry and Statistical Analysis
Data were entered using an excel spreadsheet and checked for entry errors, by comparing data entries with the original data forms. Then, data were transferred to Stata® software package (version 9.0; Stata Corporation, College Station, USA) for analysis. The Fisher's exact test was applied to determine the significance of differences of relative frequencies and the one-way ANOVA test to determine significance of differences of mean egg counts.
Results
A total of 396 dogs, consisting of 180 (45.5%) males and 216 (54.5%) females was examined; 192 (48%) dogs lived in urban, and 204 (52%) in rural areas.
All dog owners agreed to participate and completed the questionnaires. Table 1 summarizes the differences in dog regimen and the perception of dog owners to diseases transmissible by their animals, stratified by urban and rural areas. In the rural area, significantly more individuals kept dogs for hunting and observed their dogs catching prey than in the city (p < 0.0001), whereas 29.2% and 18.1% of dog owners in the urban and rural areas kept dogs as watch dogs, respectively (Table 1). Treatment with antiparasitic drugs was a more frequent practice for dogs from urban than rural areas.
Table 1.
Characteristics of dogs and knowledge and attitudes of dog owners regarding potential zoonotic disease in urban and rural communities.
| Variables | Urban n = 192 | Rural n = 204 | Urban Vs. rural | ||
| N | % | N | % | p value | |
| Sex of dogs | |||||
| Male | 91 | 47.4% | 89 | 43.6% | 0.5 |
| Female | 101 | 52.6% | 115 | 56.4% | 0.5 |
| Age of dogs (months) | |||||
| 0–6 | 61 | 31.8% | 73 | 35.8% | 0.4 |
| 7–11 | 49 | 25.5% | 59 | 28.9% | 0.4 |
| ≥ 12 | 82 | 42.7% | 72 | 35.3% | 0.1 |
| Reasons for keeping dogs | |||||
| Hunting | 71 | 37.0 | 115 | 56.4 | < 0.0001 |
| Watch dog | 56 | 29.2 | 37 | 18.1 | 0.013 |
| Companion | 43 | 22.4 | 32 | 15.7 | 0.1 |
| No specific reason | 22 | 11.5 | 20 | 9.8 | 0.6 |
| Where do dogs usually roam? | |||||
| Confined to dog house on compound | 18 | 9.4 | 7 | 3.4 | 0.022 |
| Inside the house | 3 | 1.6 | 11 | 5.4 | 0.055 |
| Within the compound | 57 | 29.7 | 33 | 16.2 | 0.002 |
| Anywhere within and outside the compound | 114 | 59.4 | 153 | 75.0 | 0.001 |
| How do dogs leave house premises | |||||
| Always accompanied | 35 | 18.2 | 10 | 4.9 | < 0.0001 |
| Occasionally accompanied | 67 | 34.9 | 80 | 39.2 | 0.4 |
| Never Accompanied | 90 | 46.9 | 114 | 55.9 | 0.09 |
| Usual place of defecation | |||||
| Within the house premises | 66 | 34.4 | 54 | 26.5 | 0.1 |
| Within/out of house premises | 126 | 65.6 | 150 | 73.5 | 0.1 |
| Preferred type of floor where dogs defecate | |||||
| Only impervious (cemented/tiles) | 29 | 15.1 | 16 | 7.8 | 0.027 |
| Only pervious (grass, soil, etc) | 56 | 29.2 | 86 | 42.2 | 0.009 |
| Both pervious/impervious | 107 | 55.7 | 102 | 50.0 | 0.3 |
| Observation on dogs catching prey | 118 | 61.5 | 164 | 80.4 | < 0.0001 |
| Last anthelminthic treatment of dogs | |||||
| < 12 months ago | 42 | 21.9 | 20 | 9.8 | 0.001 |
| ≥ 12 months ago | 56 | 29.2 | 38 | 18.6 | 0.018 |
| Never | 94 | 49.0 | 146 | 71.6 | < 0.0001 |
| Dog owners' knowledge of possible diseases/conditions transmitted or caused by dogs* | |||||
| Rabies | 124 | 64.6 | 136 | 66.7 | 0.7 |
| Wound from dog bite | 75 | 39.1 | 85 | 41.7 | 0.6 |
| Scabies | 35 | 18.2 | 28 | 13.7 | 0.3 |
| Worms | 11 | 5.7 | 15 | 7.4 | 0.5 |
| Dysentery | 6 | 3.1 | 10 | 4.9 | 0.4 |
| Other bacterial/viral diseases | 5 | 2.6 | 12 | 5.9 | 0.14 |
| Do children play with dogs? | |||||
| Yes | 191 | 99.5 | 204 | 100 | 0.5 |
| No | 1 | 0.5 | 0 | 0 | 0.5 |
| Dog owners' perception of diseases transmitted by dogs | |||||
| Serious | 35 | 18.2 | 23 | 11.3 | 0.064 |
| Not serious | 91 | 47.4 | 72 | 35.3 | 0.019 |
| Do not cause any disease | 66 | 34.4 | 109 | 53.4 | < 0.0001 |
*more than one option possible
Interestingly, more than half of dog owners in the rural communities, and about a third in the urban area did not perceive diseases transmitted by dogs as a health problem (p < 0.0001). The bonds of humans with their animals were close, and children played with virtually all dogs included in the study (Table 1). When asked about possible diseases transmitted by their dogs, less than 10% of owners mentioned helminths ("worms") as a health problem, but about two third were aware of the risk of rabies transmission (Table 1).
Ectoparasites
At least one of nine ectoparasite species identified, belonging to four taxa, was encountered in 239 (60.4%) of the 396 dogs. Dogs from rural areas (77.9%) were more commonly infested than those from urban areas (41.7%; p < 0.0001). Eighty (20.3%) dogs harboured two or more species. Dogs from rural areas were more frequently parasitized with two or more ectoparasites than the urban dogs (Table 2).
Table 2.
Prevalence of ectoparasites in dogs, stratified by urban or rural communities.
| Overall (n = 396) | Urban (n = 192) | Rural (n = 204) | Urban vs. Rural | ||||
| N infected | % (95%CI) | N infected | % (95%CI) | N infected | %(95%CI) | p value | |
| Fleas | |||||||
| Ctenocephalides canis | 127 | 32.1(27.5 – 6.9) | 40 | 20.8(14.9 – 26.7) | 87 | 42.6(35.8 – 49.7) | < 0.0001 |
| Pulex irritans | 26 | 6.6(4.3 – 9.5) | 7 | 3.6(1.5 – 7.4) | 19 | 9.3(5.7 – 14.2) | 0.026 |
| Tunga penetrans | 2 | 0.5 (0.0 – 1.8) | 0 | 0.0 | 2 | 1.0(0.0 – 3.5) | 0.5 |
| Mites | |||||||
| Demodex canis | 4 | 1.0 (0.0 – 2.6) | 0 | 0.0 | 4 | 2.0(0.0 – 4.9) | 0.12 |
| Otodectes sp. | 39 | 9.8(7.1 – 13.2) | 6 | 3.1(1.2 – 6.7) | 33 | 16.2(11.4 – 22.0) | < 0.0001 |
| Sarcoptes scabiei var. canis | 8 | 2.0(0.1 – 3.4) | 1 | 0.5(0.0 – 2.9) | 7 | 3.4(1.3 – 6.9) | 0.069 |
| Ticks | |||||||
| Rhipicephalus sanguineus | 76 | 19.2(15.4 – 23.4) | 25 | 13.0(8.6 – 18.6) | 51 | 25.0(19.2 – 31.5) | 0.003 |
| Ixodes sp. | 18 | 4.5(2.7 – 7.1) | 7 | 3.6(1.5 – 7.4) | 11 | 5.4(2.7 – 9.4) | 0.5 |
| Lice | |||||||
| Trichodectes canis | 42 | 10.6(7.8 – 14.1) | 15 | 7.8(4.4 – 12.6) | 27 | 13.2(8.9 – 18.7) | 0.1 |
| Number of ectoparasite species per host | |||||||
| One ectoparasite species | 159 | 40.2(35.3 – 45.2) | 60 | 31.3(24.8 – 38.3) | 99 | 48.5(41.5 – 55.6) | < 0.0001 |
| Two ectoparasite species | 72 | 18.2(14.5 – 22.3) | 19 | 9.9(6.1 – 15.0) | 53 | 26.0(20.1 – 32.6) | < 0.0001 |
| Three ectoparasite species | 7 | 1.8(0.7 – 3.6) | 1 | 0.5(0.0 – 2.9) | 6 | 2.9(1.1 – 6.3) | 0.12 |
| Four or more ectoparasite species | 1 | 0.3(0.0 – 1.4) | 0 | 0.0 | 1 | 0.5(0.0 – 2.7) | 1.0 |
In total, 155 (39.1%) were infested with fleas, 94 (23.7%) with ticks, 51 (12.9%) with mites, and 42 (10.6%) with lice. The prevalence detailed for each ectoparasite species is depicted in Table 2, stratified by urban and rural areas.
The common dog flea, Ctenocephalides canis, was the most prevalent species and present in almost one third of dogs, followed by the brown dog tick Rhipicephalus sanguineus, Trichodectes canis, Otodectes sp., Pulex irritans and Ixodes sp. (Table 2). Infestations due to the sand flea Tunga penetrans, the mange mite Sarcoptes scabiei var. canis and Demodex canis were less common.
The prevalence of C. canis and of Otodectes sp. was significantly higher in rural dogs than in urban dogs. A similar trend was observed for P. irritans and R. sanguineus (Table 2).
Intestinal helminths
In total, 271 (68.4%) of the examined dogs were infected with at least one intestinal helminth species. Six species, namely Toxocara canis, Ancylostoma sp.Trichuris vulpis, Dipylidium caninum, Taenidae and Strongyloides sp. were identified in dogs of both urban and rural areas (Table 3).
Table 3.
Prevalence of intestinal helminths parasite in dogs, stratified by communities.
| Overall (n = 396) |
Urban (n = 192) |
Rural (n = 204) |
Urban vs. Rural | ||||
| N infected | % (95% Cl) | N infected | % (95% Cl) | N infected | % (95% Cl) | p value | |
| Parasite species | |||||||
| Toxocara canis | 165 | 41.7 (36.8 – 46.7) | 72 | 37.5 (30.6 – 44.8) | 93 | 45.6 (39.6 – 53.7) | 0.13 |
| Ancylostoma sp. | 67 | 16.9 (13.4 – 21.0) | 27 | 14.1 (9.5 – 19.8) | 40 | 19.6 (14.4 – 25.7) | 0.18 |
| Trichuris vulpis | 57 | 14.4 (9.7 – 16.6) | 28 | 14.6 (9.9 – 20.4) | 29 | 14.2 (9.7 – 19.8) | 1.0 |
| Dipylidium caninum | 36 | 9.1 (6.5 – 12.4) | 11 | 5.7 (2.9 – 10.0) | 25 | 12.3 (8.1 – 17.6) | 0.035 |
| Taenidae | 33 | 8.3 (5.8 – 11.5) | 3 | 1.6 (0.3 – 4.5) | 30 | 14.7 (10.2 – 20.3) | < 0.0001 |
| Strongyloides sp. | 15 | 3.8 (2.1 – 6.2) | 3 | 1.6 (0.3 – 4.5) | 12 | 5.8 (3.1 – 10.1) | 0.033 |
| Number of intestinal helminth species per host | |||||||
| One helminth species | 196 | 49.4 (44.2 – 54.3) | 92 | 47.9 (40.7 – 55.2) | 104 | 51.0 (43.9 – 58.3) | 0.6 |
| Two helminth species | 52 | 13.1 (10.0 – 16.9) | 17 | 8.9 (5.2 – 13.8) | 35 | 17.5 (12.3 – 23.0) | 0.017 |
| Three helminth species | 18 | 4.6 (2.7 – 7.1) | 4 | 2.1 (0.6 – 5.2) | 14 | 6.9 (3.8 – 11.2) | 0.029 |
| Four or more helminth species | 5 | 1.3 (0.0 – 2.9) | 0 | - | 5 | 2.5 (0.1 – 5.6) | 0.062 |
The most common parasites were T. canis, followed by Ancylostoma sp. and T. vulpis (Table 3). Prevalence of the dog tapeworm, D. caninum, Taenidae and Strongyloides sp. were <10%.
Except for D. caninum, Taenidae and Strongyloides sp., the prevalence of intestinal helminths was not statistically different in urban or rural areas. However, multiple infections with 2 and 3 parasites species per host were significantly higher in rural than in urban areas (Table 3).
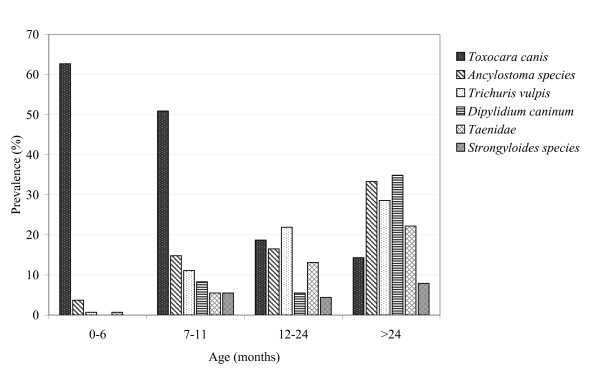
The pattern of prevalence and distribution of helminth parasites, stratified by age of dogs, is depicted in Figure 2. In general, prevalence of parasite infection increased with age of the dog. An exception was observed in T. canis infection, which was by far the most common infection in puppies, and showed decreasing prevalence with age. The density of infection, expressed by mean egg counts per gramme (epg) paralleled the prevalence data (Table 4).
Figure 2.
Prevalence of intestinal helminths species diagnosed in dogs, stratified by age of dogs.
Table 4.
Density of intestinal parasites infection in dogs, stratified by rural and urban communities.
| Parasite | Overall n = 396 |
Urban n = 192 |
Rural n = 204 |
Urban vs. Rural |
| Mean (SD) | Mean (SD) | Mean (SD) | p value | |
| Toxocara canis | 375.6 (569.5) | 264.1 (441.3) | 480.47 (651.9) | 0.001 |
| Ancylostoma sp. | 84.2 (221.5) | 70.4 (207.6) | 97.29 (233.5) | 0.54 |
| Trichuris vulpis | 147.8 (440.6) | 126.23 (404.2) | 168.12 (477.4) | 0.79 |
| Dipylidium caninum | 46.8 (189.7) | 10.12 (85.9) | 81.29 (246.2) | 0.028 |
| Taenidae | 126.8 (435.0) | 77.38 (336.6) | 173.29 (507.1) | 0.001 |
| Strongyloides sp. | 11.3 (68.1) | 7.25 (57.8) | 15.18 (76.5) | 0.92 |
Discussion
The present study provides the first systematic assessment on quantitative estimates of parasites in dogs in Nigeria's Kwara State. The results show that ectoparasitic and intestinal helminth species were abundant, and that prevalence and density of infection was very high. The knowledge and perception of dog owners regarding zoonotic diseases transmitted by pets was insufficient.
The parasites reported in this study have been previously documented in dogs throughout the world, with a pronounced difference in prevalence and density between regions [16,17,20-27]. In our study, the overall prevalence of intestinal helminths (68%) was similar to that reported from different ecological and epidemiological settings in Nigeria [17,26] and to the prevalence of 71% reported from Spain [28]. In South Africa (76%), Mexico (85%) and Morocco (100%), prevalences were even higher [22,23,29].
This potential for human zoonotic disease has rarely been addressed in control programs in Nigeria and other low income countries. Considering the high prevalence of ectoparasites and intestinal helminth infections found in dogs, and the close bonds in which dogs live together with people, the risk of transmission of these parasites to humans seems to be obvious. For example, Toxocara infection in humans may cause visceral larva migrans, in severe cases leading to blindness [30], and dog hookworm infections put humans at risk for cutaneous larva migrans which is endemic in many resource-poor communities [31]. Rhipicephalus ticks have been described to parasitize humans [32], and may transmit rickettsial disease and visceral leishmaniasis [33]. Fleas may transmit human plague, rickettsioses and trypanosomes [34], and serve as intermediate hosts for the dog tapeworm, D. caninum.
Our data show that the prevalence pattern was age-dependent; T. canis decreased with age of dog, whereas A. caninum, T. vulpis, Taenidae, D. caninum and Strongyloides sp. increased with age, even though to a less extent. These patterns have been observed previously [16,17,20,23,27]. In Nigeria, Sowemimo and Asaolu [27] found by far the highest prevalence of toxocariasis in puppies, whereas the age dependency of hookworm infection was less pronounced. The high prevalence of ascarid infections in puppies is in accordance with the transmission pattern of the parasite, which is mainly by transplancental and transmammary routes; acquired age-dependent immunity may be caused by repeated exposure [35,36]. Increased infection rates in older dogs are caused by parasite species which are not transmitted to dogs at early age, and thus do not elicit a specific immune response.
The prevalence detected in our study differs from those of Sowemimo and Asaolu [27] who recorded 24% in a Nigerian city in a neighbouring state with similar characteristics as Ilorin. However, these data were not population-based, but included dogs presented to veterinary clinics. These authors also argued that the reduction of prevalence as compared to a study done in the 1970s [31] may be caused by increased awareness of pet holders regarding deworming practices. In contrast, our data can be regarded as representative for the dog population, as pet owners who bring their animals to veterinary clinics may deworm their animals more regularly. As a consequence, studies based on veterinary clinics underestimate prevalence of parasitic infections and infestations. Our data, though, show that the majority of dogs received antiparasitic treatment never or more than a year ago, and only few people were aware of the zoonotic potential of dog parasites; 60% of dogs examined had never visited a clinic for any form of treatment.
The reduced prevalence of D. caninum over time was claimed to be caused by the reduced prevalence of the intermediate host C. canis. This may hold true for pets brought to veterinary clinics, but our study shows that C. canis is very common in dogs in the community and thus probably continue being important for the transmission of D. caninum.
The intensities of T. canis, Taenidae and D. caninum were statistically higher in rural dogs than those in the urban area. Similarly, Habluetzel et al. [38] observed that twice as many dogs from rural areas had nematodes infections, as compared to urban dogs. These differences in the level of infection from different locations have been described also in other studies [39,40] and may be partly due to variation in local environmental conditions affecting spatial aggregation and infective stages of parasites. Besides, differences in health care and animal management practices may account for these differing characteristics. Urban dog owners may feel encouraged by their proximity to veterinary clinics, which are nonexistent in rural areas.
The number of intestinal parasite species per host revealed that single infection was more common; polyparasitism with more than two parasites species was less frequently observed. A similar pattern was observed in ectoparasite infestation. These results are in agreement with Fontanarrosa et al. [24] who explained that interactions among parasite species depend on parasite burden rather than the mere presence of other species.
The high prevalence of ectoparasites (60%) was consistent with another study, where fleas and ticks were the most commonly found taxa [41]. Ugochukwu and Nnadozie [42] recorded in Bendel State (Nigeria) a low prevalence of ectoparasites in dogs, including Demodex canis, R. sanguineus and C. canis. Bryson et al. [43] identified several species of ixodid ticks, fleas and lice from dogs in South Africa. However, C. felis and Echidnophaga gallinacea which were frequently reported in dogs in other study areas [39,42-44] were not encountered in our study.
The variation in distribution and prevalence of ectoparasites can be ascribed to differences in the availability of infective stages, host habitat/climatic factors and the sampling period. Peak prevalences of ectoparasites usually occur during the warm dry months [40,45,46]. Gracia et al. [40] revealed that accumulation of organic wastes and the presence of other pet animals influence the survival and abundance of ectoparasites, especially fleas. This also explains why P. irritans and T. penetrans, relatively low host-specific ectoparasites, occurred only in rural areas, where dogs were frequently in contact with other natural host animals, such as pigs, rats and small ruminants [47-49].
Unfortunately, due to the absence of funding, we were unable to identify the prevalence of other zoonotic diseases and to specify the species in Taenidae encountered, such as Echinococcous granulosus causing hydatid disease. The diagnostic technique of parasites done in this study, based on the morphological characteristics of ova under light microscope, has the disadvantage that it fails to distinguish E. granulosus from other Taenidae. Thus, E. granulosus, a major zoonotic parasite of livestock and dogs in Nigeria [11,14,15], has possibly been present but not reported in our survey. The fact that dogs enjoy unrestrained association with humans, scavenge for food in an environment contaminated with faecal material of potential intermediate hosts and feed on offal of slaughtered livestock in abattoirs (Ugbomoiko, personal communication) makes transmission of hydatid disease predictable in the setting studied.
In general, the trend in prevalence, density and species composition of parasites observed in this study may reflect the degree of environmental contamination and inequalities in the health care service between urban and rural areas. In particular, T. canis, A. caninum and D. caninum are zoonotic parasites constituting public health problems in the study areas.
Conclusion
Our study shows that parasites of importance for human health were highly prevalent in Nigerian dogs and that intervention measures are necessary to reduce the risk of transmission of parasites from dogs to humans. Interventions should focus on health education provided to dog owners and the establishment of a program based on zoonotic diseases.
Authors' contributions
USU: study design, conducted the study, statistical analysis, contributed to the manuscript; LA: statistical analysis, contributed to the manuscript; JH: study design, statistical analysis, contributed to the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
LA received a PhD scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil). The study was supported in part by a PROÁFRICA grant from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil). JH is research fellow from CNPq.
Contributor Information
Uade Samuel Ugbomoiko, Email: samugbomoiko@yahoo.com.
Liana Ariza, Email: arizaliana@hotmail.com.
Jorg Heukelbach, Email: heukelbach@web.de.
References
- Dohoo IR, McDonell WN, Rhodes CS, Elazhary YL. Veterinary research and human health. Can Vet J. 1998;39:549–556. [PMC free article] [PubMed] [Google Scholar]
- Robertson ID, Irwin PJ, Lymbery AJ, Thompson RCA. The role of companion animals in the emergence of parasitic disease. Int J Parasitol. 2000;30:1369–1377. doi: 10.1016/S0020-7519(00)00134-X. [DOI] [PubMed] [Google Scholar]
- McCarthy J, Moore T. Emerging helminth zoonoses. Int J Parasitol. 2000;30:1351–1360. doi: 10.1016/S0020-7519(00)00122-3. [DOI] [PubMed] [Google Scholar]
- Molyneux DH. 'Neglected' Diseases but unrecognized successes-challenges and opportunities for infectious disease control. Lancet. 2004;364:380–383. doi: 10.1016/S0140-6736(04)16728-7. [DOI] [PubMed] [Google Scholar]
- Dakkak A. Guidelines for diagnosis, surveillance and control of echinococcosis. Veterinary Public Health, World Health Organization, Geneva Switzerland; 1992. Echinococcus-hydatidiosis in North Africa: geographical distribution of species and strains and prevalence in man and animals. [Google Scholar]
- Heukelbach J, Mencke N, Feldmeier H. Cutaneous larva migrans and tungiasis: the challenge to control zoonotic ectoparasitoses associated with poverty. Trop Med Int Health. 2002;7:907–910. doi: 10.1046/j.1365-3156.2002.00961.x. [DOI] [PubMed] [Google Scholar]
- Heukelbach J, Wilcke T, Meier A, Moura RCS, Feldmeier H. A longitudinal study of cutaneous larva migrans in an impoverished Brazilian township. Travel Med Infect Dis. 2003;1:213–218. doi: 10.1016/j.tmaid.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Akao N, Ohta N. Toxocariasis in Japan. Parasitol Int. 2007;56:87–93. doi: 10.1016/j.parint.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Morrison G. Zoonotic infection from pets. Understanding the risk and treatment. Postgrad Med. 2001;110:24–26. doi: 10.3810/pgm.2001.07.970. [DOI] [PubMed] [Google Scholar]
- Irwin PJ. Companion animal parasitology: clinical perspective. Int J Parasitol. 2002;32:591–593. doi: 10.1016/S0020-7519(01)00361-7. [DOI] [PubMed] [Google Scholar]
- Dada BJO, Adeboye DS, Mohammed AN. The epidemiology of Echinococcus infection in Kaduna State, Nigeria. Vet Rec. 1979;104:312–313. doi: 10.1136/vr.104.14.312. [DOI] [PubMed] [Google Scholar]
- Malgor R, Oku Y, Gallardo R, Yarzabal I. High prevalence of Ancylostoma species infection in dogs associated with endemic focus of human cutaneous larva migrans in Tacuaremba Uruguay. Parasite. 1996;3:131–134. doi: 10.1051/parasite/1996032131. [DOI] [PubMed] [Google Scholar]
- Patz JA, Gractzyk TK, Gella N, Vittor AY. Effects of environmental changes on emerging parasitic diseases. Int J Parasitol. 2002;30:1395–1405. doi: 10.1016/S0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Dada BJO. Taeniasis, cysticercosis and echinococcosis/hydatidiosis in Nigeria: iv. Prevalence of Echinococcus granulosus infection in stray dogs. J Helminthol. 1980;54:299–301. doi: 10.1017/s0022149x00006805. [DOI] [PubMed] [Google Scholar]
- Ayanwale FO, Dipeolu OO, Esuruoso GO. The incidence of Echinococcus infection in dogs, sheep and goats slaughtered in Ibadan, Nigeria. Int J Zoonoses. 1982;9:65–68. [PubMed] [Google Scholar]
- Chiejina SN, Ekwe TO. Canine toxocariasis and the associated environmental contamination of urban areas in Eastern Nigeria. Vet Parasitol. 1986;22:157–161. doi: 10.1016/0304-4017(86)90019-1. [DOI] [PubMed] [Google Scholar]
- Anene BM, Nnaji TO, Chime AB. Intestinal Parasitic infections of dogs in the Nsukka area of Enugu, Nigeria. Prev Vet Med. 1996;27:89–94. doi: 10.1016/0167-5877(95)00527-7. [DOI] [Google Scholar]
- Forrester JE, Scott ME. Measurement of Ascaris infection intensity and dynamic of expulsion following treatment with Mebendazole. Parasitology. 1990;100:303–308. doi: 10.1017/s003118200006131x. [DOI] [PubMed] [Google Scholar]
- Pitchford RJ, Visser PS. A simple rapid technique for quantitative estimation of helminthes eggs in human and animals excreta with special reference to Schistosoma species. Trans R Soc Trop Med Hyg. 1975;69:318–322. doi: 10.1016/0035-9203(75)90126-1. [DOI] [PubMed] [Google Scholar]
- Ugochukwu EI, Ejimadu KN. Studies on the prevalence of gastrointestinal helminths of dogs in Calabar Nigeria. Int J Zoonoses. 1985;12:214–218. [PubMed] [Google Scholar]
- Oliveira-Sequeira TCG, Amarante AFT, Ferrari TB, Nunes LC. Prevalence of intestinal parasites in dogs from São Paulo State, Brazil. Vet Parasitol. 2002;103:19–27. doi: 10.1016/S0304-4017(01)00575-1. [DOI] [PubMed] [Google Scholar]
- Minnaar WN, Krecek RC, Fourie LJ. Helminths in dogs from a peri-urban resource-limited community in Free State Province, South Africa. Vet Parasitol. 2002;107:343–349. doi: 10.1016/S0304-4017(02)00155-3. [DOI] [PubMed] [Google Scholar]
- Eguia-Aguilar P, Cruz-Reyes A, Martinez-Maya JJ. Ecological analysis and description of the intestinal helminthes present in dogs in Mexico City. Vet Parasitol. 2005;127:139–146. doi: 10.1016/j.vetpar.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Fontanarrosa MF, Vezzani D, Basabe J, Eiras D. An epidemiological study of gastrointestinal parasites of dogs from Southern Greater Buenos Aires (Argentina): Age, gender, breed, mixed infections, and seasonal and spatial patterns. Vet Parasitol. 2006;136:283–295. doi: 10.1016/j.vetpar.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Yacob HT, Ayele T, Fikru R, Basu AK. Gastrointestinal nematodes in dogs from Debre Zeit, Ethiopia. Vet Parasitol. 2007;148:144–148. doi: 10.1016/j.vetpar.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Omudu EA, Amutu EU. Parasitology and urban livestock farming in Nigeria: Prevalence of ova in faecal and soil samples and animals ectoparasites in Makurdi. J S Afr Vet Assoc. 2007;78:40–45. doi: 10.4102/jsava.v78i1.285. [DOI] [PubMed] [Google Scholar]
- Sowemimo OA, Asaolu SO. Epidemiology of intestinal helminth parasites of dogs in Ibadan, Nigeria. J Helminthol. 2008;82:89–93. doi: 10.1017/S0022149X07875924. [DOI] [PubMed] [Google Scholar]
- Martinez-Moreno FJ, Hernandez S, Lopez-Cobos E, Becerra C, Acosta I, Martinez-Moreno A. Estimation of canine intestinal parasites in Cordoba (Spain) and their risk to public health. Vet Parasitol. 2007;143:7–13. doi: 10.1016/j.vetpar.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Pandey VS, Dakkak A, Elmamoune M. Parasites of stray dogs in Rabat region, Morocco. Ann Trop Med Parasitol. 1987;81:53–55. doi: 10.1080/00034983.1987.11812090. [DOI] [PubMed] [Google Scholar]
- Taylor MR. The epidemiology of ocular toxocariasis. J Helminthol. 2001;75:109–118. [PubMed] [Google Scholar]
- Heukelbach J, Walton SF, Feldmeier H. Ectoparasitic infestations. Curr Infect Dis Rep. 2005;7:373–380. doi: 10.1007/s11908-005-0012-2. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F, Figueredo LA, Brandao-Filho SP. Rhipicephalus sanguineus (Acari: ixodidae), the brown dog tick, parasitizing humans in Brazil. Rev Soc Bras Med Trop. 2006;39:64–67. doi: 10.1590/s0037-86822006000100012. [DOI] [PubMed] [Google Scholar]
- Coutinho MT, Bueno LL, Sterzik A, Fujiwara RT, Botelho JR, De Maria M, Genaro O, Linardi PM. Participation of Rhipicephalus sanguineus (Acari: Ixodidae) in the epidemiology of canine visceral leishmaniasis. Vet Parasitol. 2005;128:149–155. doi: 10.1016/j.vetpar.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Zanatta-Coutinho MT, Linardi PM. Can fleas from dogs infected with canine visceral leishmaniasis transfer the infection to other mammals? Vet Parasitol. 2007;147:320–325. doi: 10.1016/j.vetpar.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Sprent JFA. Post-parturient infection of the bitches with Toxocara canis. J Parasitol. 1961;47:284. doi: 10.2307/3275307. [DOI] [Google Scholar]
- Sprent JFA. Observation on the development of Toxocara canis (Werner, 1782) in the dogs. Parasitology. 1957;48:184–193. doi: 10.1017/s0031182000021168. [DOI] [PubMed] [Google Scholar]
- Olufemi BE, Bobade PA. Prevalence of gastrointestinal helminth parasites of dogs in Ibadan, Nigeria. Nig Vet J. 1979;23:21–25. [Google Scholar]
- Habluetzel A, Traldi G, Ruggieri S, Attili AR, Scuppa P, Marchetti R, Menghini G, Esposito F. An estimation of Toxocara canis prevalence in dogs, environmental egg contamination and risk of human infection in the Marche region of Italy. Vet Parasitol. 2003;113:243–252. doi: 10.1016/S0304-4017(03)00082-7. [DOI] [PubMed] [Google Scholar]
- Durden LA, Judy TN, Martin JE, Spedding LS. Fleas parasitizing domestic dogs in Georgia, USA: Species composition and seasonal abundance. Vet Parasitol. 2005;130:157–162. doi: 10.1016/j.vetpar.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Gracia MJ, Calvete C, Estrada R, Castillo JA, Peribañez MA, Lucientes J. Fleas parasitizing domestic dogs in Spain. Vet Parasitol. 2008;151:301–309. doi: 10.1016/j.vetpar.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Estares L, Chavez A, Casas E. Prevalencia de ectoparasitos de Canis familiaris en los dos distritos de San Juan de Lurigancho, San Martin de Porres Comas e Independencia de Lima Metropolitana. Revista de Investigaciones Veterinarias del Perú. 1999;10:1–9. [Google Scholar]
- Ugochukwu EI, Nnadozie CC. Ectoparasitic infestation in dogs in Bendel State, Nigeria. Int J Zoonoses. 1985;12:308–312. [PubMed] [Google Scholar]
- Bryson NR, Horak IG, Hohn EW, Louw JP. Ectoparasites of dogs belonging to people in resource poor communities in North West Province, South Africa. J S Afr Vet Assoc. 2000;71:175–179. doi: 10.4102/jsava.v71i3.709. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Castro DC, Gonzalez S. Ectoparasitic species of Canis familiaris (Linne) in Buenos Aires Province, Argentina. Vet Parasitol. 2004;120:123–129. doi: 10.1016/j.vetpar.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Dipeolu OO. A survey of the ectoparasitic infestations of dogs in Nigeria. J Small Anim Pract. 1975;16:123–129. doi: 10.1111/j.1748-5827.1975.tb05725.x. [DOI] [Google Scholar]
- Heukelbach J, Wilcke T, Harm G, Feldmeier H. Seasonal variation of tungiasis in an endemic community. Am J Trop Med Hyg. 2005;72:145–149. [PubMed] [Google Scholar]
- Obasaju MF, Otesile EB. Ctenocephalides canis infestation of sheep and goats. Trop Anim Health Prod. 1980;12:116–118. doi: 10.1007/BF02242620. [DOI] [PubMed] [Google Scholar]
- Heukelbach J, Costal AM, Wilcke T, Mencke N, Feldmeier H. The animal reservoir of Tunga penetrans in severely affected community of North east Brazil. Med Vet Entomol. 2004;18:329–335. doi: 10.1111/j.0269-283X.2004.00532.x. [DOI] [PubMed] [Google Scholar]
- Ugbomoiko US, Ariza L, Heukelbach J. Pigs are the most important animal reservoir for Tunga Penetrans (Jigger flea) in rural Nigeria. Trop Doct. 2008;30:226–227. doi: 10.1258/td.2007.070352. [DOI] [PubMed] [Google Scholar]