Abstract
Background
Neuronal nicotinic acetylcholine receptors (nAChRs) are a key target in medication development efforts for a range of neuropsychiatric disorders, including nicotine dependence. Varenicline, a partial agonist at the α4β2 nAChRs, is a new efficacious medication for nicotine dependence. Its effects on the affective and cognitive dimensions of nicotine withdrawal have yet to be well characterized.
Methods
Sixty-seven treatment-seeking smokers were administered varenicline (× 21 days) and placebo (× 21 days) in a double-blind within-subject cross-over design. Following the medication run-up phase (days 1–10), there was a 3-day mandatory smoking abstinence phase (days 11–13) during which subjective symptoms and cognitive performance were assessed. Participants were re-exposed to a scheduled smoking lapse (day 14) and followed for days to lapse (days 15–21) in each medication period.
Results
In the varenicline period, compared to placebo, withdrawal symptoms (p=.04), smoking urges (p<.001), and negative affect (p=.01) were significantly reduced, and levels of positive affect (p=.046), sustained attention (p=.018) and working memory (p=.001) were significantly greater during mandatory abstinence. Varenicline also significantly reduced the subjective rewarding effects of the scheduled smoking lapse (e.g., satisfaction, relief, liking) (p=.003). Medication effects on days to lapse following the scheduled smoking lapse were dependent on treatment order (p=.001); among participants who received placebo in the first period, varenicline increased days of abstinence in the follow-up period.
Conclusions
These data identify novel affective and cognitive effects of varenicline, and may have implications for medication development for other neuropsychiatric conditions.
Keywords: nicotine, tobacco, dependence, varenicline, affect, cognition
Introduction
One in five adults in the U.S. is a current smoker, and among those who receive treatment, fewer than one-third quit successfully in the long-term (1). To accelerate medication development for nicotine dependence, comprehensive characterization of the affective, cognitive, and behavioral effects of efficacious medications is needed (2). Such work may also identify clinical effects that could inform medication development for other neuropsychiatric conditions.
Neuronal nicotinic acetylcholine receptors (nAChRs) are a key target of drug discovery efforts for nicotine dependence, as well as for neuropsychiatric conditions (3). Varenicline, a partial agonist at the α4β2 nAChRs, and a full agonist at the α7 nAChRs, is a new, efficacious treatment for nicotine dependence (4–6). Varenicline produces a moderate level of nAChR stimulation and lower sustained levels of dopamine release, thereby replacing some of nicotine’s effects (7). Further, competitive nAChR binding should, theoretically, reduce nicotine reward (7, 8).
Varenicline’s clinical effects have been examined only within efficacy trials. These trials document treatment-related reductions in smoking urges and withdrawal-related negative affect (4, 5, 9), as well as reduced smoking reward during a lapse (10). Because participants in clinical trials self-select to abstinence or relapse, and the time to relapse varies among participants, assessments of medication effects on abstinence symptoms and smoking reward can be biased.
We conducted a placebo-controlled cross-over study to characterize the effects of varenicline on subjective abstinence symptoms (withdrawal, smoking urges, and affect) during the first 72 hours of abstinence, as this coincides with peak symptom severity (11–14). Based on evidence for the role of α4β2 nAChRs in cognition and learning (15, 16), and the important role of cognitive deficits in smoking relapse (17, 18), we also examined varenicline’s effects on working memory and sustained attention. In addition, we examined whether varenicline altered subjective and behavioral responses to a scheduled smoking lapse following brief abstinence. We hypothesized that varenicline, relative to placebo, would attenuate abstinence-induced changes in withdrawal symptoms, urges, and negative affect; enhance cognitive performance; reduce the rewarding value of the smoking lapse cigarette; and increase days to lapse during a subsequent follow-up phase on medication. Such a comprehensive assessment of an efficacious medication could provide a profile against which novel compounds can be compared and selected for further development, and may also identify novel effects of relevance to other neuropsychiatric conditions.
Methods and Materials
Study Participants
Smokers responding to local advertisements for a smoking cessation program were screened for eligibility in September 2006 – August 2007. Eligible smokers were ≥ 18 years of age and had smoked ≥ 10 cigarettes per day for the previous 12 months. To increase the generalizability of results to the clinical setting, we enrolled treatment-seeking smokers (those planning to quit in the next 3 months) (19, 20). Exclusion criteria included: history of seizures, pregnancy, lactation or planning pregnancy; unstable angina, history of heart attack or stroke in previous 6 months, insulin dependent diabetes, current diagnosis or history of DSMIV Axis I psychiatric disorders or substance abuse (other than nicotine), and current use of smoking cessation treatment, psychotropic medications (e.g., anti-depressants, antipsychotics), or contraindicated medications (i.e., blood thinners, insulin).
Procedures
All procedures were approved by the University of Pennsylvania Institutional Review Board. Participants provided written informed consent and completed a physical examination that included a urine drug screen (Screeners®, Drug Detection Devices, Ltd., Alpharetta, GA) and a pregnancy test (for females; QuPI® One-Step pregnancy test, Boerne, TX). Participants testing positive for illicit drugs (e.g., amphetimines, cocaine, methamphetamines, opiates, methadone, barbituates, benzodiazapines, tricyclic antidepressants, PCP) or pregnancy were excluded. The MINI International Neuropsychiatric Interview (MINI) was administered by trained research technicians to screen for psychiatric and substance abuse disorders (21). Ten percent of MINI interviews were recorded and reviewed by a licensed clinician. Once eligibility was confirmed, participants completed a baseline assessment of demographics, smoking history, mood and a battery of computerized neurocognitive tasks (see “Outcomes”, below).
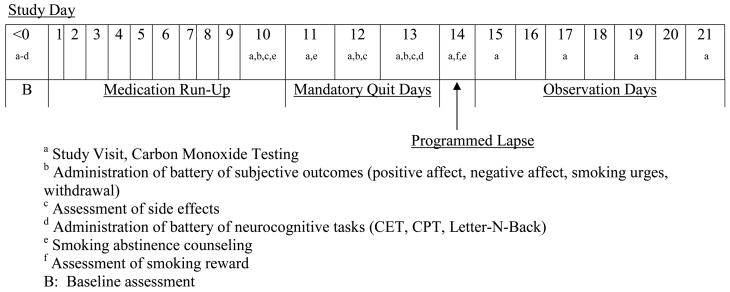
In this placebo-controlled double-blind crossover study, participants completed two 21-day medication periods (varenicline and placebo in counterbalanced order) and a 5–7 day washout period (four participants had longer washout periods due to scheduling issues) (see Figure 1). The placebo was a sucrose capsule that resembled the active medication (active medication was encapsulated also). All drug packaging was completed by the University of Pennsylvania Investigational Drug Service. Varenicline was administered according to standard treatment guidelines (22): days 1–3 (0.5mg once a day), days 4–7 (0.5mg twice a day), beginning on day 8 (1mg twice a day). Participants smoked as usual during the 10-day medication run-up period. On day 10, they received a 15-minute counseling session to help them prepare for the mandatory abstinence phase, which was presented as a “practice” quit attempt. On day 10 (smoking as usual) and during the mandatory abstinence phase of each medication period (days 11–13), participants completed assessments of withdrawal, smoking urges, mood, and carbon monoxide (CO ≤ 10 ppm needed to confirm abstinence on days 11–13). On the third day of mandatory abstinence (day 13), participants completed the same battery of computerized neurocognitive tasks that had been administered at baseline. Participants who did not remain abstinent (n=3) were excluded.
Figure 1.
Study Schema.
On the morning of day 14, participants smoked one of their own cigarettes and completed subjective measures of reward. They were instructed to smoke 4 additional cigarettes in their natural environment and returned to the Center at 6pm for CO assessment (23, 24). A 15-minute booster counseling session was conducted to prepare for the post-lapse quit attempt (days 15–21). Participants meeting abstinence criteria received a small ($15 daily) incentive (24) and were coached to consider these post-lapse quit attempts as “practice” quit attempts for their “final” quit attempt that would occur following completion of the two 21-day study periods. Following the study, they received 13-week smoking cessation treatment with varenicline and counseling.
Subjective Outcomes
We assessed changes in subjective nicotine abstinence symptoms from day 10 (smoking as usual) to days 11–13 (scores were averaged across the three days of mandatory abstinence). A 19-item withdrawal symptom checklist (25) was used to create a summary score for common withdrawal symptoms. Urges to smoke were rated using the 10-item Questionnaire on Smoking Urges-Brief Form (26). The Positive and Negative Affect Schedule (PANAS) (27), a 20-item Likert-format self-report measure, was used to assess positive and negative affect. Cronbach’s alphas for all scales ranged from .79 to .96.
Neurocognitive Outcomes
For each medication period, we assessed cognitive function at baseline (smoking as usual, before starting medication) and day 13 (day 3 of mandatory abstinence), using validated computerized tasks: the Letter-N-back task of working memory (28), the Penn Continuous Performance Task (P-CPT) of sustained attention (29), and the Conditional Exclusion Test (CET) of abstraction in executive function (30, 31). These tasks were selected to target cognitive domains that may be important to nicotine withdrawal. For each cognitive task, the median reaction time for correct responses was used as the primary outcome; number of true positives was also examined. Detailed descriptions of these tasks are included in the supplemental information.
Smoking Lapse Measures
To assess the rewarding value of a smoking lapse following brief abstinence, the Cigarette Evaluation Scale (CES) (32) and the Sensory Questionnaire (SQ) (33) were administered immediately following the lapse cigarette on day 14 of each medication period. Subscale measures for the CES include satisfaction, psychological relief, and toxicity, and for the SQ include liking and strength; in addition, an overall score was created using all items from both instruments. Cronbach’s alpha coefficients were .63 and .64 for the toxicity subscale, and ranged from .68 to .90 for the other measures. The number of days to the first cigarette during the observation phase (days 15–21) was assessed by self report and biochemically verified by CO (≤ 10 ppm) during visits (days 15, 17, 19 and 21).
Medication Adherence and Side Effects
Medication adherence was calculated as the percentage of pills consumed (out of the total of 39 dispensed during the period) based on pill count. Side effect severity was assessed on the final day of each run-up period by a self-report instrument developed for this study consisting of 15 items representing likely side effects of varenicline (e.g., dry mouth, nausea, sleep disorder); participants rated the severity with which they had experienced each side effect in the previous week using a 4-point response scale (0 = not present, 1 = mild, 2 = moderate, 3 = severe).
Statistical Analysis
Descriptive statistics were generated for all variables. Mixed effects models were used to examine medication (varenicline vs. placebo) effects on outcome measures. All models controlled for sex, cigarettes per day at baseline, and order of medication phases. The models of subjective outcomes also included day 10 (smoking as usual) levels of the measure as a time-varying covariate; thus, these models tested the effect of medication on changes in outcomes from pre-abstinence (day 10) to abstinence (mean of days 11–13). The models of neurocognitive outcomes included baseline scores as a time-invariant covariate. For the N-back model, a variable representing working memory load (i.e., 0- vs. 1- vs. 2- vs. 3-back) was included as an additional predictor. The model of side effect severity scores included baseline scores as a time-invariant covariate. Finally, to provide an indication of effect size, Cohen’s d was calculated for the raw difference between varenicline and placebo (not controlling for any other variables) on each outcome measure with effect sizes greater than 0.8 considered large. (In calculating Cohen’s d, the mean of the standard deviations at placebo and varenicline was used, rather than the standard deviation of the difference scores.)
Results
Sixty-seven participants were included in the analyses (three had incomplete data for the Penn Continuous Performance task; see Supplementary Figure 1 for subject accrual information). Of these, 38 (56.7%) were female; 40 (59.7%) were white and 25 (37.3%) were African American; and 46 (68.7%) had some college education. Mean age was 44.6 years (sd = 11.6), and the mean number of cigarettes smoked per day at baseline was 21.5 (sd = 10.0). There were no significant differences in these variables by medication order, nor were there any differences in smoking rate during the two run up periods (all Ps>.20).
Subjective Outcomes
There were significant effects of medication on all subjective abstinence symptoms during the mandatory abstinence phase (days 11–13, Table 1). During the varenicline period, compared to placebo, participants reported lower levels of withdrawal symptoms (p=.04), smoking urges (p<.001), and negative affect (p=.013). Levels of positive affect were also greater during the varenicline period compared to placebo (p=.046). There were no significant effects of medication order for any of these measures, and effects of cigarettes per day and sex were not significant. Scores on day 10 (smoking as usual) were a significant predictor in all models (p<.001). The effects of medication on positive affect, negative affect, smoking urges and withdrawal symptoms were small to moderate in size (Cohen’s d for difference between change scores (varenicline vs. placebo) = 0.26 to 0.42) (34).
Table 1.
Effects of Varenicline vs. Placebo on Changes in Subjective Outcomes from Day 10 (Smoking as Usual) to the Average of Days 11–13 (Abstinence).
| Measure | Change from Day 10 to Average of Days 11–13 | Drug effecta | ||||
|---|---|---|---|---|---|---|
| Varenicline | Placebo | F | p | |||
| Mean | SD | Mean | SD | |||
| Positive affect | −2.08 | 5.94 | −3.74 | 6.60 | 4.20 | .046 |
| Negative affect | −0.57 | 4.33 | 0.68 | 5.16 | 6.62 | .013 |
| Smoking urges | 0.94 | 13.37 | 6.54 | 13.04 | 14.53 | < .001 |
| Withdrawal symptoms | −0.54 | 5.98 | 1.50 | 6.46 | 4.37 | .041 |
Medication effect in mixed effects model. Dependent variable is average scores from days 11–13. Models controlled for treatment order, sex, cigarettes per day at baseline, and day 10 (smoking as usual) scores.
Neurocognitive Outcomes
As shown in Table 2, for the Letter-N-back task, there was a significant medication effect in the model of correct reaction time (F = 11.08, p = .001) but not in the model of true positives (F = .001, p = .98); in both models, there were significant effects of baseline performance and working memory load (p < .001), and the medication by memory load interaction was not significant (p > .4). In the model of CPT true positives, there was a significant effect of medication (F = 7.28, p = .009); in the model of CPT correct reaction time, there were effects of medication (F = 5.86, p = .018) and baseline performance (F = 117.1, p < .001). Effects of sex, cigarettes per day, and medication order were not significant in these models (p > .2). Only baseline performance was significant in the models of CET true positives (p = .02) and correct reaction time (p < .001). The effects of medication on CPT true positives and N-Back correct reaction time were small in size (Cohen’s d for difference between scores at varenicline vs. placebo = 0.33 and 0.21 respectively) (34).
Table 2.
Effects of Varenicline vs. Placebo on Neurocognitive Outcomes at Baseline and Day 13.
| Measure | Mean (SD) at: | Drug effecta | |||
|---|---|---|---|---|---|
| Baseline | Varenicline | Placebo | F | p | |
| CPT true positivesb | 57.20 (5.26) | 59.22 (1.39) | 58.73 (1.56) | 7.28 | .009 |
| N-Back true positivesb | 49.97 (7.04) | 52.34 (4.42) | 52.36 (4.03) | 0.001 | .98 |
| CET true positivesb | 41.87 (11.79) | 41.97 (12.80) | 40.78 (11.00) | 0.38 | .54 |
| CPT correct RTc | 485.7 (50.04) | 489.8 (50.53) | 499.1 (53.40) | 5.86 | .018 |
| N-Back correct RTc | 559.9 (139.9) | 546.1 (129.2) | 575.0 (140.7) | 11.08 | .001 |
| CET correct RTc | 2609.0 (1025.3) | 2541.2 (1173.5) | 2380.2 (793.6) | 1.21 | .28 |
Effect of medication (varenicline vs. placebo) in mixed effects model controlling for treatment order, sex, baseline score, cigarettes per day at baseline, and (in N-back models) working memory load (0- vs. 1- vs. 2- vs. 3-back).
Number of correct responses.
Median reaction time (milliseconds) for correct responses.
Smoking Reward and Days to Lapse
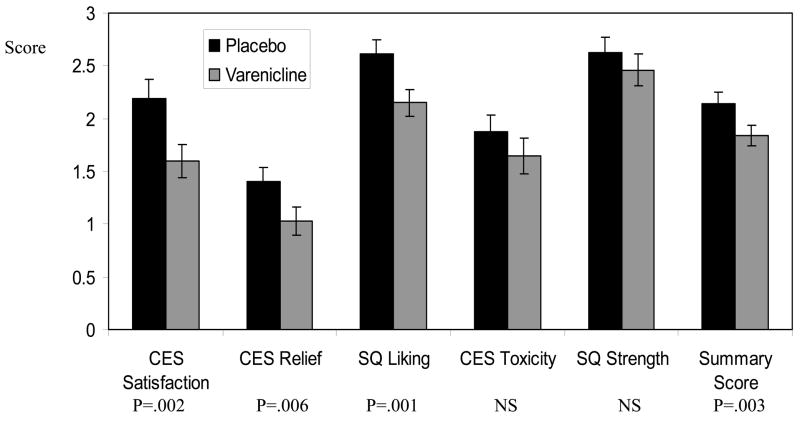
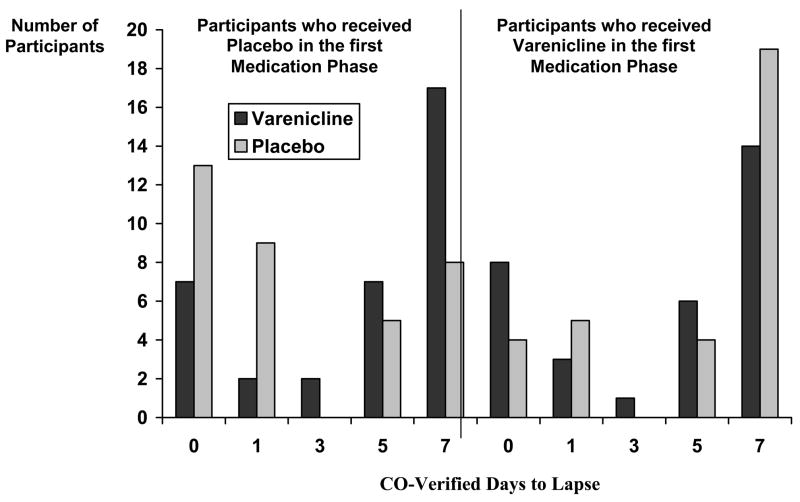
As shown in Figure 2, compared to placebo, varenicline attenuated the subjective rewarding value of the scheduled lapse cigarette (day 14) (F = 9.25, p = .003; Cohen’s d = 0.36). Significant effects were observed for satisfaction (F = 10.05, p = .002; d = 0.43), relief (F = 8.12, p = .006; d = 0.34), and liking (F = 11.09, p = .001; d = 0.41). For days to lapse in the observation phase (days 15–21), there was a significant treatment by order interaction (F = 11.99, p = .001). Among participants receiving placebo first, mean days to lapse were 4.63 (SD=2.86) and 2.57 (SD=2.94) for the varenicline and placebo study periods, respectively (Z=3.16, p=.002 by Wilcoxon matched pairs signed-ranks test; d = 0.71); among those receiving varenicline first, mean days to lapse were 4.19 (SD=3.04) and 4.94 (SD=2.87) for varenicline and placebo, respectively (Z=1.34, p=.18 by Wilcoxon test; d = −0.25) (see Figure 3).
Figure 2.
Varenicline Effects on Lapse Cigarette Ratings.
Figure 3.
CO-Verified Days to Lapse by Medication Phase and Treatment Order.
Medication Adherence and Side Effects
Mean adherence levels were 99.27% for the placebo period and 98.70% for the varenicline period (Z = 1.89, p = .06 by Wilcoxon test). Average side effect severity scores were 0.44 (sd = 0.46) on day 10 of the varenicline period and 0.24 (sd = 0.29) on day 10 of the placebo period. In the model of side effect severity scores, the effect of medication was significant (F = 18.93, p < .001), as was the effect of baseline score (F = 14.81, p < .001). Side effects reported as either moderate or severe by over 5% of participants on day 10 of either medication period are shown in Table 3. The percentage of participants reporting at least one side effect as either moderate or severe was 55.2% (37/67) on day 10 of the varenicline period and 28.4% (19/67) on day 10 of the placebo period. The mixed effects models of all other outcomes reported so far were repeated, with experience of at least one moderate or severe side effect on day 10 (0 = no, 1 = yes) included as a covariate, All significant effects of treatment on outcomes remained significant, with the exception of the treatment effect in the model of positive affect, which became nonsignificant when the side effect measure was included (F=1.62, p=.21).
Table 3.
Side effects reported as moderate or severe by over 5% of participants on Day 10 of either medication period.
| Side effect | Participants rating side effect as moderate or severe at Day 10 of: | P | |||
|---|---|---|---|---|---|
| Varenicline | Placebo | ||||
| N | % | N | % | ||
| Dry mouth | 15 | 22.4 | 6 | 9.0 | .035 |
| Abnormal dreams | 14 | 20.9 | 2 | 3.0 | .004 |
| Increased flatulence | 14 | 20.9 | 9 | 13.4 | .18 |
| Fatigue | 10 | 14.9 | 5 | 7.5 | .18 |
| Nausea | 10 | 14.9 | 1 | 1.5 | .004 |
| Reflux | 10 | 14.9 | 1 | 1.5 | .004 |
| Sleep disorder | 8 | 11.9 | 2 | 3.0 | .07 |
| Insomnia | 7 | 10.4 | 6 | 9.0 | 1.00 |
| Abdominal pain | 6 | 9.0 | 2 | 3.0 | .22 |
| Constipation | 6 | 9.0 | 0 | 0.0 | .031 |
| Stomach pain | 5 | 7.5 | 0 | 0.0 | .06 |
| Headache | 4 | 6.0 | 3 | 4.5 | 1.00 |
| Taste problems | 4 | 6.0 | 2 | 3.0 | .69 |
Note. P-values are for McNemar tests comparing varenicline to placebo.
Discussion
Neuronal nicotinic acetylcholine receptors (nAChRs) are a key target of medication development efforts for nicotine dependence and other neuropsychiatric conditions (3). In chronic smokers, abstinence from nicotine increases the availability of unbound α4β2 nAChRs, which in turn contributes to abstinence symptoms (35). Aside from urges to smoke, the most common symptoms involve emotional dysregulation and cognitive deficits (13, 14). Data from the present study indicate that varenicline, a partial agonist at the α4β2 nAChRs, enhances mood and cognitive function during early abstinence from nicotine. As expected, varenicline also reduced withdrawal symptoms and urges to smoke, reduced the rewarding value of a smoking lapse following brief abstinence, and increased the number of abstinent days following the smoking lapse; however, the abstinence effect was observed only among participants who received placebo prior to varenicline. The effects of varenicline on subjective abstinence symptoms were of a magnitude similar to those observed in clinical efficacy trials (4, 36).
The beneficial effects of varenicline on affect during abstinence are consistent with preclinical evidence for the role of α4β2 nAChRs in nicotine’s effect on hedonic state. Nicotine reduces, and nicotine withdrawal increases, brain reward thresholds in rodents (37, 38). These effects are mediated, in part, by α4β2 nAChRs (39), consistent with varenicline’s mechanism of action. Further, genetic modification of α4 and β2 nAChR subunits alters anxiety-related behaviors in mouse models, supporting the importance of these receptor complexes in affective regulation (40–42). Further, it is possible that varenicline’s agonist effects at α7 nAChRs contribute to the observed mood enhancing effects, given the role of α7 nAChRs in nicotine induced dopamine release (43).
Varenicline’s effects on mood during early abstinence are likely to play an important role in its clinical efficacy. Depression symptoms are common among smokers and motivate smoking to reduce negative affect (44). Further, increases in negative affect predict clinical relapse (45–47). Varenicline’s effects on positive affect may also be clinically important, as low levels of positive affect predict poorer outcomes following smoking cessation, beyond the effects of negative affect measures (48).
Varenicline treatment also significantly enhanced sustained attention and working memory after three days of abstinence, although effects were small. The involvement of β2-containing nAChRs in varenicline’s cognitive enhancing properties is supported by evidence that nicotine and withdrawal effects on learning and memory are attenuated in β2 nAChR subunit knock-out mice (49, 50). Subjective and performance measures of abstinence-induced cognitive deficits predict smoking cessation in clinical trials (17, 18). Thus, small changes in cognitive performance during nicotine withdrawal may contribute to varenicline’s efficacy, and may be worth exploring further as a target for future efforts for medication development for nicotine dependence (51). Prior evidence for the cognitive enhancing effects of selective nAChR agonists suggests further that varenicline might have additional potential benefits in this area beyond those specific to nicotine withdrawal (52).
Strengths of the present study include the large sample size for a human laboratory investigation and a comprehensive battery of subjective and performance measures of abstinence symptoms. The within-subject cross-over design and enrollment of treatment-seeking smokers provides a more sensitive test of medication effects (19). However, order effects were observed for the smoking abstinence outcome; specifically, effects of varenicline on days to lapse were observed only for participants receiving placebo in the first medication phase followed by varenicline and not for those receiving varenicline followed by placebo. One interpretation of this observation is that participants who experienced varenicline-induced success with abstinence in the first period may have had greater self-efficacy to maintain abstinence in the second period with placebo. Alternatively, varenicline may produce longer-lasting modulation of nAChRs.
In summary, this study provides the first evidence from a controlled laboratory investigation for varenicline’s hypothesized effects on withdrawal, craving, affect, and smoking reward. Furthermore, the data suggest two novel and potentially important clinical effects of varenicline, namely enhancement of positive affect and cognitive function. These data, together with evidence for anti-depressant effects of the α4β2 nAChR partial agonist cytisine (53), and evidence for the potential benefits of the nicotinic receptor antagonist mecamylamine (54) in combination therapy for major depression, suggest that varenicline might be explored as a treatment for affective disorders. It should be noted that there have been recent reports of adverse psychiatric events with varenicline therapy; however, it is not possible to determine whether these events are partly attributable to nicotine withdrawal (55). Nonetheless, given the low abuse potential of varenicline (56), further research on its use in the treatment of other neuropsychiatric conditions is warranted.
Supplementary Material
Acknowledgments
This research was supported by AstraZeneca and NCI/NIDA grant P5084718 (C.L.). The authors thank Dr. Maxine Stitzer for her helpful advice on the study design.
Footnotes
Financial Disclosures
Dr. Lerman has served as a paid consultant and has received funding from pharmaceutical companies which manufacture smoking cessation medications, including Pfizer (maker of Chantix®, varenicline) and GlaxoSmithKline. Dr. Frey is employed by Astra Zeneca. Dr. Siegel is a consultant to NuPathe and has received research support from AstraZeneca. Dr. Patterson, Dr. Jepson, Dr. Strasser, Dr. Loughead, Dr. Gur, and Dr. Perkins report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schnoll RA, Lerman C. Current and emerging pharmacotherapies for treating tobacco dependence. Expert Opin Emerg Drugs. 2006;11:429–44. doi: 10.1517/14728214.11.3.429. [DOI] [PubMed] [Google Scholar]
- 2.Lerman C, Lesage MG, Perkins KA, O’Malley SS, Siegel SJ, Benowitz NL, et al. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov. 2007;6:746–62. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- 3.Arneric SP, Holladay M, Williams M. Neuronal nicotinic receptors: a perspective on two decades of drug discovery research. Biochem Pharmacol. 2007;74:1092–101. doi: 10.1016/j.bcp.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. Jama. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 5.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. Jama. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 6.Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–5. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- 7.Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–7. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 8.Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007;28:316–25. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 9.West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology (Berl) 2008;197:371–7. doi: 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- 10.Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166:1571–7. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- 11.Shiffman S, Patten C, Gwaltney C, Paty J, Gnys M, Kassel J, et al. Natural history of nicotine withdrawal. Addiction. 2006;101:1822–32. doi: 10.1111/j.1360-0443.2006.01635.x. [DOI] [PubMed] [Google Scholar]
- 12.Teneggi V, Tiffany ST, Squassante L, Milleri S, Ziviani L, Bye A. Smokers deprived of cigarettes for 72 h: effect of nicotine patches on craving and withdrawal. Psychopharmacology (Berl) 2002;164:177–87. doi: 10.1007/s00213-002-1176-1. [DOI] [PubMed] [Google Scholar]
- 13.Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–27. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- 14.Ward MM, Swan GE, Jack LM. Self-reported abstinence effects in the first month after smoking cessation. Addict Behav. 2001;26:311–27. doi: 10.1016/s0306-4603(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 15.Chan WK, Wong PT, Sheu FS. Frontal cortical alpha7 and alpha4beta2 nicotinic acetylcholine receptors in working and reference memory. Neuropharmacology. 2007;52:1641–9. doi: 10.1016/j.neuropharm.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27:10870–7. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, et al. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rukstalis M, Jepson C, Patterson F, Lerman C. Increases in hyperactive-impulsive symptoms predict relapse among smokers in nicotine replacement therapy. J Subst Abuse Treat. 2005;28:297–304. doi: 10.1016/j.jsat.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacology (Berl) 2006;184:628–36. doi: 10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- 20.Perkins KA, Lerman C, Stitzer M, Fonte CA, Briski JL, Scott JA, et al. Development of procedures for early screening of smoking cessation medications in humans. Clin Pharmacol Ther. 2008;84:216–21. doi: 10.1038/clpt.2008.30. [DOI] [PubMed] [Google Scholar]
- 21.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. 34–57. [PubMed] [Google Scholar]
- 22.Pfizer. Chantix Prescribing Information. [Investigator Brochure] 2007 December, 2007 [cited 2007 November 16, 2007]; Available from: http://www.pfizer.com/files/products/uspi_chantix.pdf.
- 23.Chornock WM, Stitzer ML, Gross J, Leischow S. Experimental model of smoking re-exposure: effects on relapse. Psychopharmacology (Berl) 1992;108:495–500. doi: 10.1007/BF02247427. [DOI] [PubMed] [Google Scholar]
- 24.Juliano LM, Donny EC, Houtsmuller EJ, Stitzer ML. Experimental evidence for a causal relationship between smoking lapse and relapse. J Abnorm Psychol. 2006;115:166–73. doi: 10.1037/0021-843X.115.1.166. [DOI] [PubMed] [Google Scholar]
- 25.Hughes JR, Hatsukami DK, Pickens RW, Krahn D, Malin S, Luknic A. Effect of nicotine on the tobacco withdrawal syndrome. Psychopharmacology (Berl) 1984;83:82–7. doi: 10.1007/BF00427428. [DOI] [PubMed] [Google Scholar]
- 26.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 27.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 28.Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, et al. Working memory for complex figures: an fMRI comparison of letter and fractal n-back tasks. Neuropsychology. 2002;16:370–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Kurtz MM, Ragland JD, Bilker W, Gur RC, Gur RE. Comparison of the continuous performance test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophr Res. 2001;48:307–16. doi: 10.1016/s0920-9964(00)00060-8. [DOI] [PubMed] [Google Scholar]
- 30.Kurtz MM, Ragland JD, Moberg PJ, Gur RC. The Penn Conditional Exclusion Test: a new measure of executive-function with alternate forms of repeat administration. Arch Clin Neuropsychol. 2004;19:191–201. doi: 10.1016/S0887-6177(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 31.Kurtz MM, Wexler BE, Bell MD. The Penn Conditional Exclusion Test (PCET): relationship to the Wisconsin Card Sorting Test and work function in patients with schizophrenia. Schizophr Res. 2004;68:95–102. doi: 10.1016/S0920-9964(03)00179-8. [DOI] [PubMed] [Google Scholar]
- 32.Westman E, Levin E, Rose J. Smoking while wearing the nicotine patch: is smoking satisfying or harmful? Clinical Research. 1992;40:871A. [Google Scholar]
- 33.Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol Biochem Behav. 2000;67:71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J. A power primer. Psychological Bulletin. 1992:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 35.Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, et al. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci. 2006;26:8707–14. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166:1561–8. doi: 10.1001/archinte.166.15.1561. [DOI] [PubMed] [Google Scholar]
- 37.Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl) 2003;168:347–58. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- 38.Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–11. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- 39.Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. J Neurosci. 2005;25:6208–12. doi: 10.1523/JNEUROSCI.4785-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labarca C, Schwarz J, Deshpande P, Schwarz S, Nowak MW, Fonck C, et al. Point mutant mice with hypersensitive alpha 4 nicotinic receptors show dopaminergic deficits and increased anxiety. Proc Natl Acad Sci U S A. 2001;98:2786–91. doi: 10.1073/pnas.041582598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owens JC, Balogh SA, McClure-Begley TD, Butt CM, Labarca C, Lester HA, et al. Alpha 4 beta 2* nicotinic acetylcholine receptors modulate the effects of ethanol and nicotine on the acoustic startle response. Alcohol Clin Exp Res. 2003;27:1867–75. doi: 10.1097/01.ALC.0000102700.72447.0F. [DOI] [PubMed] [Google Scholar]
- 42.Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, et al. Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci. 2000;20:6431–41. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schilstrom B, Svensson HM, Svensson TH, Nomikos GG. Nicotine and food induced dopamine release in the nucleus accumbens of the rat: putative role of alpha7 nicotinic receptors in the ventral tegmental area. Neuroscience. 1998;85:1005–9. doi: 10.1016/s0306-4522(98)00114-6. [DOI] [PubMed] [Google Scholar]
- 44.Lerman C, Audrain J, Orleans CT, Boyd R, Gold K, Main D, et al. Investigation of mechanisms linking depressed mood to nicotine dependence. Addict Behav. 1996;21:9–19. doi: 10.1016/0306-4603(95)00032-1. [DOI] [PubMed] [Google Scholar]
- 45.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 46.Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu A, et al. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depend. 2002;67:219–23. doi: 10.1016/s0376-8716(02)00067-4. [DOI] [PubMed] [Google Scholar]
- 47.Swan GE, Ward MM, Jack LM. Abstinence effects as predictors of 28-day relapse in smokers. Addict Behav. 1996;21:481–90. doi: 10.1016/0306-4603(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 48.Leventhal AM, Ramsey SE, Brown RA, Lachance HR, Kahler CW. Dimensions of depressive symptoms and smoking cessation. Nicotine Tob Res. 2008;10:507–17. doi: 10.1080/14622200801901971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Portugal GS, Kenney JW, Gould TJ. Beta2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiol Lern Mem. 2008;89:106–13. doi: 10.1016/j.nlm.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, et al. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 51.Lerman C, LeSage MG, Perkins KA, O’Malley SS, Siegel SJ, Benowitz NL, et al. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov. 2007;6:746–62. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- 52.Cincotta SL, Yorek MS, Moschak TM, Lewis SR, Rodefer JS. Selective nicotinic acetylcholine receptor agonists: potential therapies for neuropsychiatric disorders with cognitive dysfunction. Curr Opin Investig Drugs. 2008;9:47–56. [PubMed] [Google Scholar]
- 53.Mineur YS, Somenzi O, Picciotto MR. Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacology. 2007;52:1256–62. doi: 10.1016/j.neuropharm.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.George TP, Sacco KA, Vessicchio JC, Weinberger AH, Shytle RD. Nicotinic antagonist augmentation of selective serotonin reuptake inhibitor-refractory major depressive disorder: a preliminary study. J Clin Psychopharmacol. 2008;28:340–4. doi: 10.1097/JCP.0b013e318172b49e. [DOI] [PubMed] [Google Scholar]
- 55.Kuehn BM. FDA warns of adverse events linked to smoking cessation drug and antiepileptics. Jama. 2008;299:1121–2. doi: 10.1001/jama.299.10.1121. [DOI] [PubMed] [Google Scholar]
- 56.McColl SL, Burstein AH, Reeves KR, Billing CB, Jr, Stolar M, Sellers EM. Human abuse liability of the smoking cessation drug varenicline in smokers and nonsmokers. Clin Pharmacol Ther. 2008;83:607–14. doi: 10.1038/sj.clpt.6100510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.