Summary
A number of distinct factors acting at different stages of the adeno-associated virus vector (AAV)-mediated gene transfer process were found to influence murine hepatocyte transduction. Foremost amongst these was the viral capsid protein. Self complementary (sc) AAV pseudotyped with capsid from serotype 8 or rh.10 mediated four-fold greater hepatocyte transduction for a given vector dose when compared to vector packaged with AAV7 capsid. An almost linear relationship between vector dose and transgene expression was noted for all serotypes with vector doses as low as 1×107vg/mouse (4×108vg/kg) mediating therapeutic levels of human FIX (hFIX) expression. Gender significantly influenced scAAV mediated transgene expression with two fold higher levels of expression observed in male compared to female mice. Pretreatment of mice with the proteasome inhibitor bortezomib, increased scAAV mediated hFIX expression from 4±0.6μg/ml to 9±2μg/ml in female mice although the effect of this agent was less profound in males. Exposure of mice to adenovirus 10-20 weeks after gene transfer with AAV vectors augmented AAV transgene expression two-fold by increasing the level of proviral mRNA. Hence, optimization of individual steps in the AAV gene transfer process can further enhance the potency of AAV-mediated transgene expression, thus increasing the probability of successful gene therapy.
Keywords: AAV, Gender, Adenovirus, Proteosome inhibitors
Introduction
Vectors based on adeno-associated virus (AAV), a non-pathogenic, replication-defective human parvovirus, have several attributes that make them highly suitable for gene transfer into post mitotic tissues. Foremost amongst these is the fact that a single administration of recombinant AAV (rAAV) into muscle, liver, retina or the brain results in long-term transgene expression without toxicity in a variety of animal models.(1) These preclinical studies have lead to a series of Phase-I/II gene therapy trials with AAV serotype 2, the first isolate to be fully characterized. A diverse range of disorders have been targeted including hemophilia B, α-1-antitrypsin deficiency, Duchene muscular dystrophy, Leber’s congenital amaurosis, leukodystrophies and Parkinson’s disease.(2-6) These early human studies have identified several obstacles which need to be addressed before the potential of AAV vectors can be fully realized. Firstly, pre-existing immunity to viral capsid is a significant impediment to stable transgene expression.(3) Since wild type (wt) AAV2 is endemic to humans, the majority of individuals participating in these clinical trials likely had pre-existing immunity to this serotype as a result of prior natural infection. Anti-AAV2 antibodies have the potential to block or greatly reduce gene transfer with rAAV2 vectors. In addition, cytotoxic T-cells generated at the time of infection with wt-AAV can expand and eliminate cells that are transduced, as suggested by the results of a recent hemophilia B gene therapy trial.(3) A strategy to overcome these immunological obstacles involves the switching of capsid protein to those serotypes of AAV which are less prevalent in the humans.(7-9) A large number of AAV serotypes have recently been described and need to be systematically evaluated in pre-clinical models to facilitate their transition to the clinic.(10;11)
Another limitation to the clinical use of AAV vectors is the need for high multiplicity of infection to achieve therapeutic gene transfer. For instance, in excess of 1014 AAV2 vector particles were required for therapeutic transgene expression from the liver in subjects with severe hemophilia B.(3) Efficient transduction of target cells is blocked at several levels including ineffective endocytosis, endosomal degradation, inefficient uncoating and the need to convert the single stranded AAV genome to a transcriptionally active double stranded form.(12-14) The latter process may be influenced by host factors such as gender.(15) A better understanding of these biological limitations has resulted in novel strategies to improve transduction with single-stranded AAV. For instance, blockade of endosomal degradation of AAV with proteaosome inhibitors significantly augments transduction in mice.(16) The clinical exploitation of this strategy will be further facilitated by demonstrating the same effect with clinically relevant proteasome inhibitors. The efficiency with which vectors uncoat and release their genome can be enhanced by switching AAV capsid protein to that of an alternative serotypes such as AAV8.(7;17) This is partly responsible for improving transduction of murine hepatocytes with AAV8 when compared with vectors pseudotyped with serotype 2 capsid.
Possibly the most important impediment to efficient gene transfer with AAV vectors is the need to convert the single-stranded AAV genome to a transcriptionally active double-stranded form.(12-14) This complex process is inefficient and appears to be influenced by additional host factors such as gender.(15) Consequently the majority of the single-stranded AAV genome that enters the nucleus is degraded. A solution to this problem has been derived by modifying the AAV genome such that complementary proviral single-stranded molecules are packaged within a single virion.(8;18;19) After uncoating these complementary strands anneal to form stable transcriptionally active dimers. These self complementary AAV vectors (scAAV) mediate stable transduction of the liver that is almost a log higher that that achieved with an equivalent dose of comparable single-stranded rAAV.(8;18;19) This raises the possibility of mediating therapeutic gene transfer with significantly lower doses of scAAV vector, which has favorable safety implications and may ease the burden on vector production. Hence, there is significant interest in this new generation of AAV vectors but it is unclear if the upstream obstacles that restricted gene transfer with single-stranded AAV vectors such as proteasome degradation will also impair the efficiency of scAAV vectors.
Another unanswered question relates to the consequences of delayed exposure to helper virus during the viraemic phase of a natural infection with adeno or herpes virus, as may occur in patients whose hepatocytes have been stably transduced with rAAV vector. These helper viruses play an important role in virtually all aspects of the life cycle of wt-AAV. For instance the adenoviral E1, E2a and E4 (orf6) genes and the VA1 RNA interact with the AAV ITRs and the rep and cap genes to facilitate rescue and replication of the wild type provirus. Co-administration of rAAV vectors, which lack the wild-type rep and cap genes, with adenovirus significantly enhances AAV transduction due to efficient synthesis of double-stranded transcriptionally active viral genome.(20) Since infection with these helper viruses is common in humans, their effects on expression mediated by stably retained transgene following rAAV gene transfer needs to be defined.(21)
In this study, transduction of the murine liver with scAAV vector pseudotyped with capsid proteins derived from four different recently isolated nonhuman serotypes of AAV was compared. The minimum vector dose required for therapeutic transgene expression in mice was defined. In addition, we determined whether scAAV mediated gene transfer into murine hepatocytes is influenced by gender and administration of a clinically available proteasome inhibitor. The consequences of late infection with adenovirus on AAV mediated gene transfer in murine liver were also investigated.
Results
Transduction of murine liver with alternative AAV serotypes
These experiments were performed with our previously described scAAV vector cassette encoding human FIX under the control of a liver specific promoter (LP1-hFIXco) flanked by AAV2 ITRs.(8) The use of scAAV dispenses with the need for second strand synthesis, thus removing a confounding variable. scAAV-LP1-hFIXco vectors pseudotyped with AAV-7, 8, 9 and rh.10 capsid were generated using previously described chimeric packaging plasmids encoding AAV2-rep, positioned upstream of the cap genes from serotype 7, 8, 9 or rh.10.(10;11;22) All pseudotyped scAAV vectors were purified using iodixanol density centrifugation. SDS-PAGE electrophoresis of the resulting vector stock demonstrated a similar high level of purity (<20% contaminating proteins). Equivalent numbers of vector genomes (range: 4×108 to 2×1011vg/kg) as determined by qPCR were then injected into the tail vein of 6-8 week old male C57Bl/6 mice (n=5–8 animals/cohort) to determine the efficiency with which these pseudotyped AAV vectors transduced the liver. The LP1 promoter has previously been shown to restrict transcription to hepatocytes, therefore, circulating hFIX protein detected following administration of scAAV-LP1-hFIXco was assumed to result from expression in the liver.(8)
At all doses assessed and for each pseudotyped vector, the profile of transgene expression generated from alternative scAAV vectors was similar (Figure 1). Plasma hFIX levels were detectable within 24 hours and increased rapidly to peak values by 7 days after vector administration. Thereafter expression stabilized for the duration of the study (57 days). scAAV pseudotyped with serotype 8 and rh.10 capsid proteins gave the highest levels of expression at all doses and time points. Fifty seven days after tail vein administration of 2×1011vg/kg, the mean hFIX levels were 36±0.3μg/mL and 34.0±0.2μg/mL in the scAAV-2/8 and -2/rh.10 cohort of mice, respectively. These levels correspond to almost 700% of normal hFIX levels in humans. When the vector dose was reduced to 4×1010vg/kg expression of hFIX in the scAAV-2/8 and -2/rh.10 cohorts was still almost 250% of physiologic values at 13±4μg/mL and 12±2μg/mL, respectively. Although the mean hFIX level in animals transduced with 4×1010vg/kg of scAAV2/9-LP1-hFIXco was lower at 7±3μg/mL, this difference was not statistically significant when compared to expression in the serotype 8 and rh.10 cohorts. In contrast, mice transduced with 4×1010vg/kg of scAAV-LP1-hFIXco pseudotyped with capsid from AAV7 had an almost 4 fold lower level of hFIX expression (3±1μg/mL) when compared to the scAAV-2/8 and -2/rh10 cohorts (p=0.0002, one way ANOVA). For each serotype there was a roughly linear relationship between the number of vector particles administered and hFIX levels in murine plasma. Despite its comparatively lower hepatocyte gene transfer efficiency, the mean plasma hFIX level (0.06±0.01μg/mL) observed after tail vein administration of the lowest dose of scAAV2/7-LP1-hFIXco (4×108vg/kg) was significantly above the threshold (0.05μg/ml, 1% of normal) required to ameliorate the bleeding phenotype in patients with severe haemophilia B.
Figure 1. In-vivo expression of hFIX from AAV pseudotyped vectors.
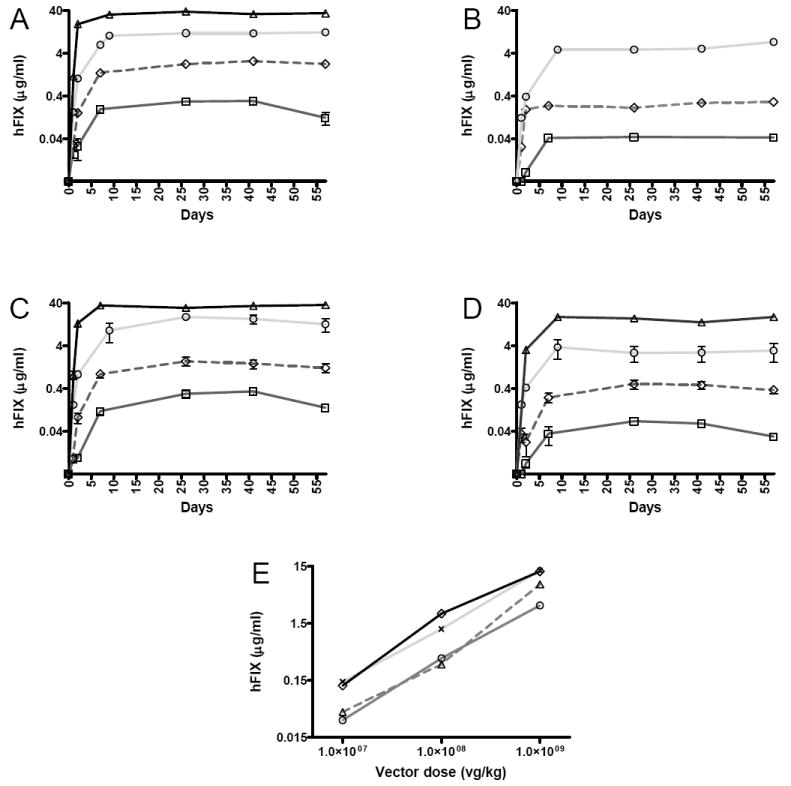
Plasma human FIX (hFIX) levels (mean ± SEM) measured by ELISA between days 1 and 57 in mice after tail vein injection of scAAV-LP1-hFIX packaged with capsid derived from serotype -rh.10 (Panel A), -9, (Panel B), -8 (Panel C) and 7 (Panel D) and at different particle doses consisting of 2×1011 (triangle), 4×1010 (circle), 4×109 (diamond) and 4×108 (square) vg/kg. Panel E shows the relationship between vector dose and transgene expression on day 57 for scAAV-LP1-hFIX packaged with capsid derived from serotype -rh.10 (◇), -9, (△), -8 (×) and 7 (○).
Biodistribution of AAV vectors of alternate serotypes
We next evaluated the differences in biodistribution of the vector genome following tail vein administration of 4×109vg/kg of each pseudotyped scAAV vector in mice. The proviral copy number as assessed by qPCR analysis of genomic DNA extracted from liver, spleen, heart, kidneys, lungs and testes of mice (N=3) sacrificed 8 weeks after vector administration was consistent for each set of tissues within serotype grouping but varied substantially between different tissue sites and serotypes. The liver was most efficiently transduced with at least 40 fold more vector genome copies per cells (vg/cell) when compared to non-hepatic tissues (Table 1 and Figure 2). This is consistent with previous reports of preferential transduction of the liver following tail vein administration of AAV vectors. The average transgene copy number in the liver of mice transduced with vector pseudotyped with serotype 8 and rh.10 capsid was 3±1 and 3±0.5 transgene copies per cell respectively. This is significantly higher (p<0.001 two way ANOVA) than the levels observed in the liver of animals transduced with scAAV-2/7 (0.7±0.2vg/cell) and -2/9 (1±0.2vg/cell). Notably, the ratio of plasma hFIX level to transgene copy number in the liver was similar for scAAV vectors pseudotyped with serotype 7, 8 and rh.10 capsid protein but was two fold higher for scAAV2/9. This suggests that AAV9 capsid may influence efficiency of transgene expression (Table 1).
Table 1.
Transgene expression per proviral copy in murine liver after tail vein administration of 4×1010 vg/kg of scAAV-LP1-hFIXco
| Pseudotyped vectors | Mean steady state hFIX antigen level (% of normal) | Transgene copy number per diploid Genome (c/dg)§ | Transgene expression per proviral genome copy (% of normal) |
|---|---|---|---|
| scAAV2/7 | 3 | 0.7 | 4 |
| scAAV2/8 | 13 | 3.0 | 4 |
| scAAV2/9 | 7 | 1.0 | 8 |
| scAAV2/rh.10 | 12 | 3.0 | 4 |
Transgene copy number estimated by qPCR analysis
Figure 2. Biodistribution of scAAV vector.
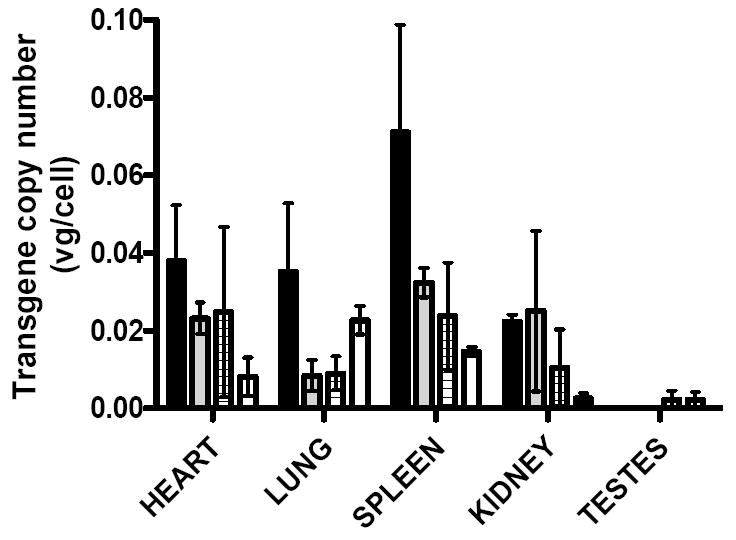
Genomic DNA was isolated from three individual mice in each of the four serotype cohorts (serotype -rh.10 [Black filled bar], -9, [Grey filled bar], -8 [Bar with criss-crosses] and 7 [Clear bar]) following tail vein administration of 4×1010vg/kg from the liver (see Table 1) and nonhepatic tissues (panel B). Transgene copy number in each tissue was quantified by a vector specific qPCR assay. Shown is transgene copy number per diploid genome corrected for variation in loading and amplification efficiency using GAPDH primers.
Biodistribution of the vector to the non-hepatic tissues was also influenced by the AAV capsid proteins (Figure 2). scAAV2/rh.10 appeared to be most efficient at transducing the spleen (0.08±0.03vg/cell) with moderate levels of gene transfer into the heart and lungs (0.04±0.01vg/cell) and a relatively low copy number in the kidneys (0.02±0.002vg/cell). In comparison, scAAV-2/9 and 2/8 vectors were less efficient at transducing the lungs, but moderate level of gene transfer was observed into the heart and spleen (average of 0.03±0.001vg/cell). The lungs (0.02±0.005vg/cell) were the most efficiently transduced with AAV serotype-7 pseudotyped vectors.
Switching serotype capsid protein enables efficient transduction in mice with pre-existing humoral immunity to AAV
The kinetics of the humoral response to viral capsid proteins was identical for the 4 different pseudotypes of scAAV assessed. Anti-AAV capsid IgG was detectable within 7-10 days of tail vein administration of vector. The antibody titres reached peak levels within 3 weeks which were then stably maintained for the duration of the study. The neutralising potential of these antibodies was confirmed using a transduction inhibition assay (data not shown). Eight weeks after gene transfer with 4×1010vg/kg of scAAV-2/8 or −2/rh.10, mice from each of these two cohorts were challenged with a second tail vein administration of AAV (4×1010vg/kg), this time encoding human coagulation factor VII (rAAV-hFVII) under the control of the LP1 promoter pseudotyped with either serotype 8 or rh.10 capsid protein. Secondary challenge with vectors based on the same serotype did not result in any gene transfer as assessed by plasma expression of hFVII (Figure 3). Mice previously exposed to scAAV2/rh.10-LP1-hFIXco were successfully transduced with rAAV-hFVII pseudotyped with serotype 8 capsid resulting in mean steady state plasma hFVII levels of 4±0.4% of normal, which is comparable to transgene expression achieved in naïve, control animals (5±0.4% of normal) not previously sensitised to AAV. Similar results were obtained when mice previously exposed to scAAV2/8-LP1-hFIXco were challenged with rAAV-hFVII pseudotyped with rh.10 capsid protein. Thus despite the close (93%) amino-acid sequence homology between AAV8 and rh.10 capsid, vectors pseudotyped with alternative serotype can overcome serotype specific antibodies.
Figure 3.
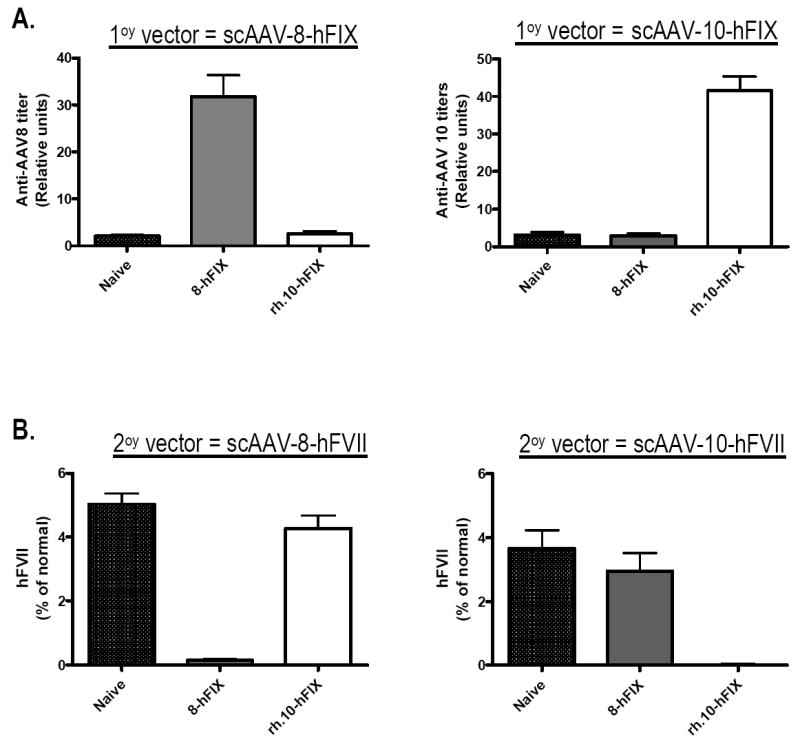
A. Anti-AAV 8 (left panel) and rh.10 (right panel) antibody titers in mice 57 days after tail vein administration of 4×1010vg/kg of scAAV-LP1-hFIX packaged with capsid from AAV serotype 8 or rh.10 compared to naïve untransduced animals. Shown are relative anti-AAV antibody titers from 3 animals. B. Re-administration of rAAV vector pseudotyped with serotype 8 (left panel) or rh.10 (right panel) capsids encoding human FVII. Shown are plasma levels (mean ± SEM) of human FVII protein measured by ELISA 42 days after tail vein administration of LP1-hFVII.
Bortezomib influences AAV mediated gene transfer especially in female mice
We have previously shown that gender significantly influences liver transduction with single-stranded AAV through the binding of a nuclear protein to the Rep binding site on AAV-2 ITR.(15) It was hypothesised that this interaction facilitated de-novo second strand synthesis. Since, scAAV vectors dispense with the need for second strand synthesis, we assessed the influence of gender on hepatocyte gene transfer with this vector configuration. As shown in Figure 4, tail vein administration of 4×1010vg/kg scAAV2/8-LP1-hFIXco into 8-10 week old C57Bl/6 mice resulted in systemic expression of hFIX in male mice that was almost 2 fold higher (7±1μg/ml, N=7) when compared to the levels achieved in the female cohort (4±0.6μg/ml, N=8). This is significantly less than the previously reported 10 fold difference in transgene expression between males and females following tail vein administration of single stranded rAAV vectors.(15) We next investigated if bortezomib, a clinically available proteosome inhibitor, could augment scAAV mediated transduction of the murine liver. Tail vein injection of bortezomib (1 μg/mg), 24 hours prior to administration of scAAV2/8-LP1-hFIXco increased hFIX expression in female mice by 2 fold to 9±2μg/ml (N=8, p= 0.02). Male mice treated with bortezomib had a more modest but significant (p=0.04) increase in transgene expression with steady state hFIX levels of 10±1μg/ml (N=7). Notably, the level of hFIX observed in male and female mice conditioned with bortezomib was comparable. Re-administration of bortezomib at 24 hours after transduction with scAAV2/8-LP1-hFIXco did not result in a significant further increase in hFIX expression in male (11±2μg/ml) or female mice (11±1μg/ml) conditioned with this agent prior to vector administration. In contrast, a single administration of bortezomib after vector administration (at 24 hours) did not abrogate the gender difference (data not shown).
Figure 4. Influence of gender and proteasome inhibition on scAAV mediated transgene expression.
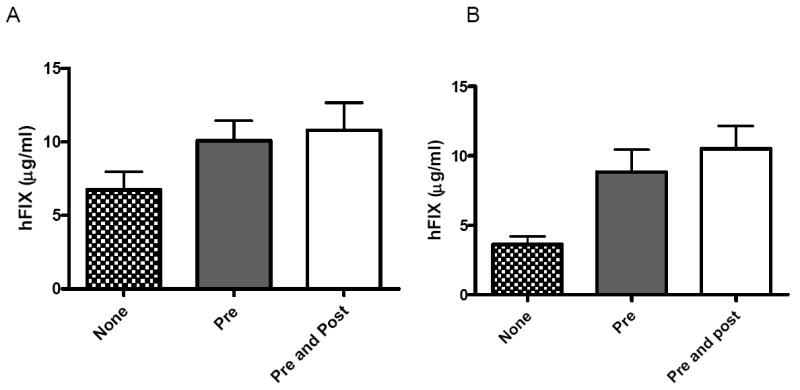
Human FIX concentration (hFIX) in murine plasma after tail vein administration of 4 × 109 vg/kg of scAAV2/8-LP1-hFIXco in cohorts (n=6) of male (Panel A) and female (Panel B) C57Bl/6 mice. A proportion of these animals were treated with a single dose of bortezomib 24 hours (Grey bar) before or with two doses of bortezomib given 24 hours before and again 24 hours (Clear Bar) after scAAV administration and their hFIX expression compared with mice that received excipient (Crisscross bar) alone. All transgene expression values are depicted as average together with the standard error of the mean.
Delayed administration of E1/E3 deleted adenoviral vectors enhances stable rAAV mediated transgene expression
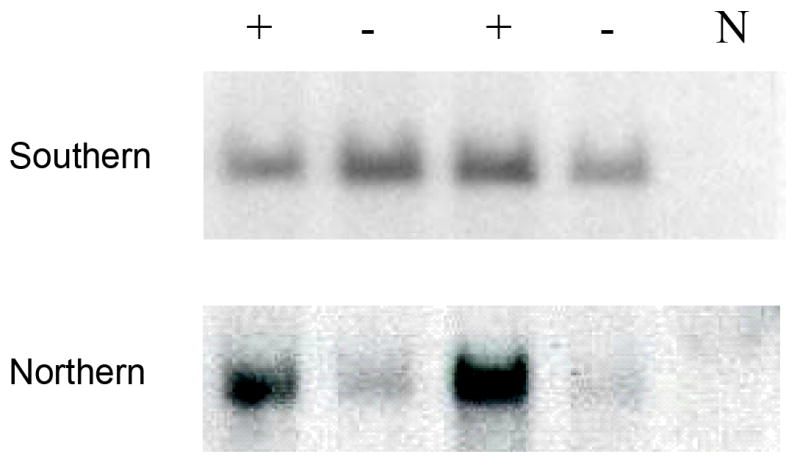
Previously it had been demonstrated that co-administration of single-stranded rAAV vectors with adenovirus significantly enhanced conversion to a double stranded, more stable form, thereby increasing transgene expression.(14) In order to determine the consequences of delayed administration of adenovirus, at a time when all the genome of a single-stranded rAAV has been converted to a double stranded form, we used our previously described single stranded vector consisting of CMV-IE enhancer, β-actin promoter (CAGG) directing the expression of hFIX cDNA. (23) A dose of 2×1012 vg/kg of rAAV2-CAGG-FIX was injected into the portal vein of two groups of 8-10 week old male mice. The kinetic of expression in these animals was consistent with that reported previously with stable levels of hFIX (0.12±0.02μg/ml) being established by 10 weeks after administration of rAAV2-CAGG-FIX (Figure 5). 4×109vg/kg of AV5LacZ, an E1/E3 deleted adenovirus serotype-5 vector particles encoding the β-galactosidase gene under the control of the CMV promoter was administered in a proportion of mice at 10 weeks after administration of rAAV2-CAGG-FIX. This resulted in a rapid increase in hFIX levels which peaked at 0.28±0.02μg/ml prior to declining to steady state levels of 2±0.01μg/ml. The increase in transgene expression following late administration of adenovirus was highly significant (p=0.004 by student T test). The plasma hFIX levels in the control group treated with PBS (excipient) remained unchanged during this period. Southern blot analysis of genomic DNA extracted from the liver at 24 weeks after gene transfer with rAAV2-CAGG-FIX demonstrated that the hFIX transgene copy number was not significantly altered by administration of AV5LacZ and ranged between 0.1 to 0.25 copies per cell, which was comparable to that seen in control animals. This contrasts with the increase in transgene copy number that occurs when adenovirus is administered concurrently with single-stranded AAV.(14) Northern blot analysis revealed 10-fold higher hFIX mRNA levels in the liver of rAAV2-CAGG-FIX transduced animals that were exposed to adenovirus when compared to controls, consistent with an increase in transcription or stability of the rAAV2-CAGG-FIX mRNA.
Figure 5.
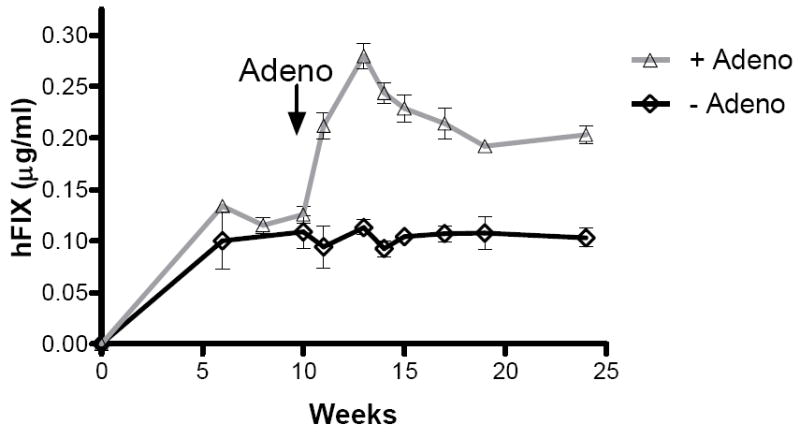
A. Human FIX expression (mean ± SEM) following tail vein administration of 2 × 1012 vg/kg rAAV2 CAGG-FIX vector particles in C57Bl/6 mice (n=6/cohort). 4 × 109vg/kg of AV5LacZ, an E1/E3 deleted adenoviral vector encoding the β galactosidase gene was administered into one cohort (△) at 10 weeks after rAAV2 CAGG-FIX mediated gene transfer. The second cohort received an equal volume of PBS (◇).
B. Genomic DNA (top panel) and total cellular RNA (bottom panel) were extracted from the liver of mice that had been sequentially transduced with rAAV2 CAGG-FIX and AV5LacZ adenoviral particles (as described above). rAAV transgene copy number was determined by digesting 10mg of DNA with NheI followed by Southern blot transfer and hybridization with a hFIX specific probe. Northern blot transfer of 10mg of total hepatocellular RNA onto nylon membrane followed by hybridization with a hFIX specific probe. N = samples from naïve mouse, - = samples from mice exposed to rAAV2 CAGG-FIX only and + = mice sequentially transduced with rAAV2 and adenovirus.
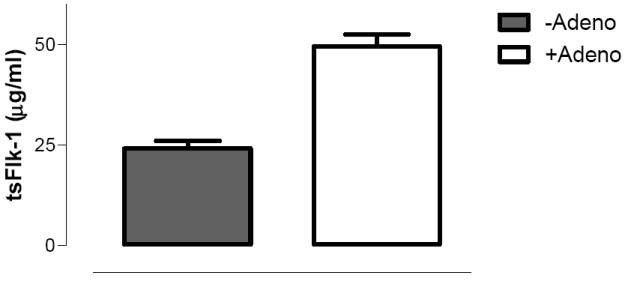
C. Truncated soluble vascular endothelial growth factor receptor (tsFlk-1) levels (mean ± SEM) in mice transduced with rAAV2 CAGG-tsFlk-1 alone (filled bar, N=14) or sequentially with rAAV2 CAGG-tsFlk-1 followed 15 weeks later by 4 × 109 vg/kg of AV5LacZ (clear bar, N=10).
D. Plasma levels of hFIX as determined by ELISA 4 weeks after a bolus tail vein injection of 4 × 109 vg/kg AV5GFP (clear bar, N=4) or PBS (filled bar, N=4) into BalB/c mice, that were 20 weeks previously transduced with 4 × 1012 vg/kg of rAAV2 LSP-FIX.
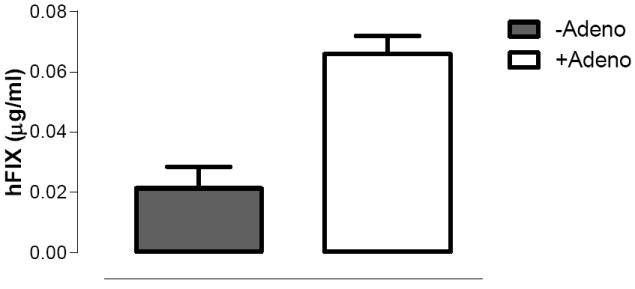
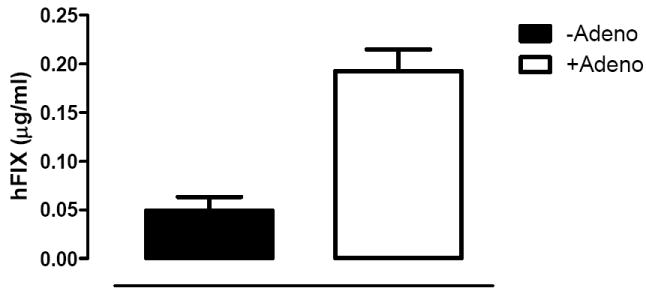
E. Plasma levels of hFIX as determined by ELISA 10 days after a bolus tail vein injection of 4 × 109 vg/kg AV5GFP (clear bar, N=3) or PBS (filled bar, N=3) into C56Bl/6 mice, that were 8 weeks previously transduced with 4×108 vg/kg of scAAV2/8-LP1-hFIXco.
We next determined if the increase in AAV proviral mRNA by adenovirus was influenced by AAV transgene. AV5LacZ (4×109vg/kg) was, therefore, injected into the tail vein of B6.CB17-Prkdc-SzJ mice at 15 weeks following portal vein administration of 4×1012vg/kg of rAAV-2 CAGG-tsFlk, a vector in which the hFIX cDNA has been replaced with the truncated soluble vascular endothelial growth factor receptor (tsFlk-1) cDNA. (24) Delayed administration of AV5LacZ resulted in an increase in tsFlk-1 plasma levels from 24±2μg/ml to 50±3μg/ml, a result that is highly significant (P<0.0001 by student T test). These studies in immunodeficient mice suggest that augmentation of AAV transgene expression observed following late administration of adenovirus is likely to be mediated by specific proteins expressed by the adenoviral vector as opposed to a non-specific consequence of inflammation that often follows the systemic administration of this virus in immunocompetent animals. Next the influence of AAV promoter was assessed using AV5GFP, which is identical to AV5LacZ except that the β-galactosidase gene has been replaced with the green fluorescent protein cDNA.(25) 4×109vg/kg of AV5GFP was administered into the tail vein of mice at 20 weeks after gene transfer with rAAV2-LPS-FIX, a vector in which the CAGG proviral promoter has been replaced with the α1-microglobulin / bikunin enhancer and thyroid hormone-binding globulin promoter (also referred to as liver specific promoter, LSP).(24;26) A three-fold increase in steady state hFIX from 0.02±0.007μg/ml to 0.07±0.006μg/ml (p=0.005 by student T test) was observed following delayed administration of recombinant adenovirus. These results indicate that the enhancement in rAAV transgene expression observed after late administration of adenovirus is not influenced by the vector transgene, proviral promoter, mouse strain, timing of adenovirus administration or the adenoviral vector cDNA.
Next, 4×108vg/kg of scAAV2/8-LP1-hFIXco was injected into the tail vein of two groups of 6-8 week old C57Bl/6 male mice (N=3/group). Eight weeks after scAAV transduction one group received 4×109vg/kg of AV5GFP via the tail vein, whilst the other received excipient. Administration of AV5GFP increased plasma hFIX levels by almost 4 fold from 0.05±0.01μg/ml to 0.19± 0.02μg/ml (Figure 5E). This difference in expression is highly significant (p=0.02, by student T test) and suggests that the late adenovirus effect is not influenced by AAV genome configuration or capsid protein.
Discussion
Several distinct factors that influence AAV mediated gene transfer of the murine liver were identified in this study including AAV capsid protein, gender, proteasome inhibition and late infection with adenoviral vectors. Comparison of the liver transduction efficiency of scAAV pseudotyped with capsids from four recently isolated nonhuman primate serotypes showed an almost linear relationship between vector dose and transgene expression. Expression of hFIX at therapeutic levels was documented with scAAV vector doses as low as 1×107vg/mouse (4×108vg/kg). Irrespective of vector dose, scAAV vectors pseudotyped with serotype 8 or rh.10 capsid were more efficient at transducing the liver than scAAV2/7. Capsid proteins appeared to influence the efficiency with which the transgene was expressed with scAAV2/9 vectors mediating at least 2 fold higher plasma levels of hFIX per copy of the transgene in hepatocytes when compared to the other serotypes evaluated. Gender had a significant impact on scAAV mediated transgene expression from the liver with two fold higher levels observed in male compared to female mice. Pre-treatment of mice with bortezomib, a proteosome inhibitor that is in clinical use, increased scAAV mediated transgene expression by at least 50%, with its effect more marked in female than male mice. Unexpectedly, administration of an early generation recombinant adenoviral vector at 10-20 weeks after AAV gene transfer resulted in a 2-3 fold increase in steady state AAV transgene expression through a mechanism that augmented AAV proviral transcription or mRNA stability. Hence, multiple steps of the AAV gene transfer pathway, ranging from binding to the target cells, endosomal escape and molecular processing of the proviral DNA can be further optimized to enhance the potency of AAV.
The ability to pseudotype the AAV2 proviral genome with naturally occurring or synthetic capsid has generated a large series of new chimeric rAAV vectors with distinct biological properties. (10;11;22;27-29) Amongst these, AAV2/8 vectors have received the most attention with numerous studies demonstrating highly efficient transduction of the murine liver with vectors based on this serotype when compared to rAAV2. This stems, at least in part, from the ability of rAAV2/8 to uncoat more rapidly resulting in a distinct profile of transgene expression with earlier establishment of steady state levels (within 1 week) when compared with AAV2 where expression peaks at 4-6 weeks in mice.(7;17) Identical transgene expression kinetics were observed with vectors pseudotyped with capsid from serotypes 7, 9 and rh.10 suggesting that these nonhuman primate isolates may also have similar uncoating properties. The differences in transgene expression between the various pseudotyped scAAV vectors and their biodistribution profile suggest that other factors such as viral binding and uptake by target cells are also important. Cellular binding of AAV-8 and -9 appears to be mediated by the 37/67-kDa laminin receptor (LamR) but the receptors for serotypes 7 and rh.10 remain undefined.(30) Capsid proteins may also influence the efficiency of transgene expression, as noted in the cohort transduced with scAAV2/9. Consistent with previous reports, the mean plasma hFIX levels in the scAAV-2/8 and -2/9 groups of mice were not significantly different because of the two fold higher level of transgenic protein per hepatocyte proviral copy in the mice transduced with scAAV2/9.(10;31) The mechanism by which serotype 9 capsid mediates higher expression per proviral copy needs to be defined.
An important observation of our study is the similar high transduction efficiency observed with scAAV vectors pseudotyped with either serotype 8 or rh.10 capsid in mice. In addition, despite over 90% amino acid sequence homology between the capsids protein, there appeared to be little humoral immune cross-reactivity between these two AAV serotypes in mice indicating that the neutralizing antibodies were directed against specific epitopes that are not common to the two serotypes. This encourages us to believe that patients who have been sensitized to serotype 8 vectors as a result of exposure to wild type virus or recombinant vector can be effectively treated at a later time point with similar doses of vector pseudotyped with AAV-rh.10.
Previously we have demonstrated a ten fold higher transduction of the liver in male mice compared to females when using single stranded AAV vectors.(15) This gender effect appeared to be mediated by an interaction between the rep binding site (RBS) within the AAV ITRs and a distinct androgen dependent hepatocellular nuclear protein, which increased the hepatocyte transgene copy number and expression of the transgenic protein in males. We hypothesized that that this gender difference, which has also been reported in dogs, was a result of enhanced AAV second strand synthesis that was catalyzed by the interaction between the AAV ITR and the androgen dependent RBS binding nuclear protein.(15;22;32-34) The fact that we and others have observed a similar gender influences with scAAV mediated transgene expression, though to a lesser extent than single-stranded AAV vectors, suggests that the gender difference is due to a more complex mechanism that likely involves multiple pathways.(35) Interestingly, pre-treatment of mice with bortezomib diminished the gender difference following scAAV mediated gene transfer. This to our knowledge is the first report of the gender specific effects of bortezomib on scAAV mediated gene transfer to the liver. Previously peptide aldehyde inhibitors of the proteasome pathway have been shown to augment AAV mediated transduction of fibroblasts, endothelial and pulmonary epithelial cells by preventing ubiquitination of viral particles.(16;36) We speculate that in addition to reducing proteasomal degradation of scAAV2/8 vector particles, bortezomib may reduce ubiquitination of the RBS binding nuclear protein, thus facilitating stable retention of the AAV genome in the target cells. Further studies are necessary to explore this and other possibilities as the gender difference in AAV mediated transduction of the liver has significant implications for gene therapy of autosomal and acquired disorders affecting the liver.
An unexpected finding was the enhancement of AAV transgene expression following administration of adenovirus at 10-20 weeks after AAV gene transfer. This is mediated through a mechanism that is independent of the mouse strain, AAV transgene and proviral promoter. Late administration of adenovirus does not increase the rAAV transgene copy number but instead enhances proviral transcription or mRNA stability by almost 10 fold. We observed a similar effect with scAAV vectors pseudotyped with serotype 8 capsid suggesting that this late effect is not influenced by capsid protein or genome configuration. Therefore, it appears that adenovirus has disparate effects on the AAV gene transfer pathway depending on the stage at which it is administered. Previous studies have shown that adenovirus influences the nuclear location of the AAV genome.(37) We, therefore, hypothesize that expression of adenoviral proteins 10-20 weeks after AAV mediated gene transfer changes the nuclear location of the AAV genome to a nuclear location that is more conducive to transgene expression. Irrespective of the mechanism, these observations may have significant implications for the use of AAV vectors in the clinic. For disorders such as diabetes, where the therapeutic window is narrow, adenoviral infection may tip therapeutic AAV mediated transgene expression into the toxic range. Alternatively, adenoviral infection may help to restore flagging AAV expression into the therapeutic range. This may be important for chronic disorders such for haemophilia, where natural turnover of hepatocytes over time may lead to a decline in transgene expression.
In summary we have identified several different factors that augment hepatocyte gene transfer in mice. These results have significant implications for the translation of gene therapy to the clinic and merit further investigation in a context relevant to humans.
Methods and Materials
AAV and adenoviral vector production and purification
The CAGG-FIX, CAGG-tsFlk and LPS-FIX plasmids have been described before.(15) rAAV-hFVII consist of the full-length 2.4kb human FVII cDNA under the control of the LP1 promoter and is flanked by AAV2 ITRs. The production of rAAV and scAAV vectors pseudotyped with capsid from alternative serotypes has been previously described. (7;8) In brief, an adenoviral helper plasmid and chimeric AAV2 Rep-7Cap, -8Cap, -9Cap or rh.10Cap packaging plasmids were used to generate AAV-7, -8, -9, -rh.10 pseudotyped vector particles.(10;11;22) The vectors stocks were purified using iodixanol gradient, the vector genome (vg) titers were determined by our qPCR method using linear plasmid DNA as standards. The purified vector stocks were consistently free of contamination with wild type AAV and cellular and adenoviral proteins as judged by our previously described methods.(38),(23) Adenoviral vectors, AV5LacZ and AV, vector stocks were prepared as described previously.(25)
Animal studies and Bio-assays
All procedures were performed in accordance with institutional guidelines under protocols approved by the Institutional Biosafety Committee and the Institutional Animal Care and Use Committee at St Jude Children’s Research Hospital, Memphis. All animal work carried out in the UK was performed under the authority of the UK Home Office Project and Personal Licences regulations and was compliant with the guidelines of the University College London ethical review committee. C57Bl/6 and Balb/c immunocompetent mice and B6.CB17-Prkdc-SzJ mice were obtained from either Charles Rivers, Harlan or Jackson Laboratories and tail vein administration of AAV and adenoviral vector particles was performed as described before.(7;8) Blood samples were collected at regular intervals and assayed for the appropriate transgene (hFIX hFVII or ts-Flk-1) by the ELISA. Methods for detection of hFIX and ts-Flk-1 have been described before.(23;24) Human FVII levels in mouse plasma were assessed using the Asserachrom human FVII ELISA kit (Axis-shield, UK). The probability of statistical differences between experimental groups was determined by one-way ANOVA and paired student t test using GraphPad Prizm version 4.0 software (GraphPad, San Diego, CA).
Molecular studies
To determine AAV transgene copy number, high molecular weight genomic DNA (10μg) extracted from murine tissue samples, using our previously described method(39),(23) was digested with BsrDI (for scAAV-LP1-hFIXco) or NheI (for CAGG-hFIX), electrophoresed through a 0.8% agarose gel, transferred to a nylon membrane (Hybond-N+; Amersham Biosciences, U.K) and then hybridized with a α32P-labelled 400bp hFIX fragment at 42°C. The intensity of the hybridization was determined using the STORM phosphorimager and ImageQuant software (Molecular Dynamics, Sunnyvale, CA). A quantitative real-time polymerase chain reaction (qPCR) assay, based on 2X IQ Supermix (Bio-Rad, Hercules, California) SYBR Green detection, was used to evaluate the biodistribution of scAAV-FIX vectors in genomic DNA extracted from various murine tissues using the following primers: forward primer, 5’GGGCAAGTATGGCATCTACA3’; and reverse primer, 5’AAAGCATCGAGTCAGGTCAG3’. Integrity of the murine DNA was assessed using the following GAPDH primers: forward: 5’GGAGTCCACTGGCGTCTTCAC3’ and reverse: 5’GAGGCATTGCTGATGATCTTGAGG3’. The lower limit of sensitivity for this assay was determined to be 10 viral copies/μg genomic DNA. Total cellular RNA was isolated from 20-30 mg of liver tissue derived from cohorts transduced with AAV followed by late exposure to adenoviral vector using RNA STAT-60 (TEL TEST, Inc, Friendswood TX). Approximately 10μg of total RNA from each sample was subjected to denaturing agarose electrophoresis, transferred to nylon membrane (Hybond-N+; Amersham Biosciences, Arlington Heights, IL) by Northern blotting procedure. The blots were hybridized with a α32P-labelled FIX probe and the intensity of hybridization was determined as described above.
Detection of anti-AAV antibodies
An immunocapture assay was used to detect anti-AAV specific antibodies in murine plasma as described before.(23) Results were expressed as the endpoint titer, defined as the reciprocal of the interpolated dilution with an absorbance value equal to five times the mean absorbance background value. Neutralizing antibody titers were assessed by determining the ability of murine serum to inhibit transduction of 293T cells by pseudotyped scAAV vector containing the enhanced green fluorescent protein (GFP) cDNA under the control of the CMV promoter (scAAV CMV-GFP) as previously described.(39),(40) The neutralizing antibody titer is expressed as the dilution that inhibited transduction of 293T cells by 50%.
Acknowledgments
We wish to thank Dr Arthur Nienhuis and Professor David Linch for critically reviewing this manuscript. We would also like to thank Dr James Wilson for providing the AAV packaging plasmids.
This work was supported by The Katharine Dormandy Trust, U.K., Medical Research Council, U.K, Department of Health’s NIHR Biomedical Research Centres funding award to UCLH/UCL, U.K, The ASSISI Foundation of Memphis, the American Lebanese Syrian Associated Charities (ALSAC), National Heart, Lung, and Blood Institute (NHLBI) grant HL073838,
Reference List
- 1.Nathwani AC, Benjamin R, Nienhuis AW, Davidoff AM. Current status and prospects for gene therapy. Vox Sang. 2004 Aug;87(2):73–81. doi: 10.1111/j.1423-0410.2004.00543.x. [DOI] [PubMed] [Google Scholar]
- 2.Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003 Apr 15;101(8):2963–72. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 3.Manno CS, Arruda VR, Pierce GF, Glader B, Ragni M, Rasko J, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006 Feb 12; doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 4.Flotte TR, Brantly ML, Spencer LT, Byrne BJ, Spencer CT, Baker DJ, et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus alpha 1-antitrypsin (rAAV2-CB-hAAT) gene vector to AAT-deficient adults. Hum Gene Ther. 2004 Jan;15(1):93–128. doi: 10.1089/10430340460732490. [DOI] [PubMed] [Google Scholar]
- 5.Stedman H, Wilson JM, Finke R, Kleckner AL, Mendell J. Phase I clinical trial utilizing gene therapy for limb girdle muscular dystrophy: alpha-, beta-, gamma-, or delta-sarcoglycan gene delivered with intramuscular instillations of adeno-associated vectors. Hum Gene Ther. 2000 Mar 20;11(5):777–90. doi: 10.1089/10430340050015671. [DOI] [PubMed] [Google Scholar]
- 6.Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007 Jun 23;369(9579):2097–105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 7.Davidoff AM, Gray JT, Ng CY, Zhang Y, Zhou J, Spence Y, et al. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5 and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Mol Ther. 2005;11(6):875–888. doi: 10.1016/j.ymthe.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN, et al. Self complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006 Apr 1;107(7):2653–61. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y, et al. Safe and efficient transduction of the liver after peripheral vein infusion of self complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007 Feb 15;109(4):1414–21. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002 Sep 3;99(18):11854–9. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004 Jun;78(12):6381–8. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackus WJ, Frakking FN, Grummels A, Gamadia LE, De Bree GJ, Hamann D, et al. Expansion of CMV-specific CD8+CD45RA+ Blood. 2003 Aug 1;102(3):1057–63. doi: 10.1182/blood-2003-01-0182. [DOI] [PubMed] [Google Scholar]
- 13.Yan Z, Zak R, Luxton GW, Ritchie TC, Bantel-Schaal U, Engelhardt JF. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J Virol. 2002 Mar;76(5):2043–53. doi: 10.1128/jvi.76.5.2043-2053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher KJ, Gao GP, Weitzman MD, DeMatteo R, Burda JF, Wilson JM. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996 Jan;70(1):520–32. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidoff AM, Ng CY, Zhou J, Spence Y, Nathwani AC. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood. 2003 Jul 15;102(2):480–8. doi: 10.1182/blood-2002-09-2889. [DOI] [PubMed] [Google Scholar]
- 16.Duan D, Yue Y, Yan Z, Yang J, Engelhardt JF. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest. 2000 Jun;105(11):1573–87. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas CE, Storm TA, Huang Z, Kay MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol. 2004 Mar;78(6):3110–22. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003 Dec;10(26):2112–8. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Ma HI, Li J, Sun L, Zhang J, Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003 Dec;10(26):2105–11. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- 20.Fisher KJ, Jooss K, Alston J, Yang Y, Haecker SE, High K, et al. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997 Mar;3(3):306–12. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 21.Schwarzmeier JD, Hubmann R, Duchler M, Jager U, Shehata M. Regulation of CD23 expression by Notch2 in B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2005 Feb;46(2):157–65. doi: 10.1080/10428190400010742. [DOI] [PubMed] [Google Scholar]
- 22.De BP, Heguy A, Hackett NR, Ferris B, Leopold PL, Lee J, et al. High levels of persistent expression of alpha1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol Ther. 2006 Jan;13(1):67–76. doi: 10.1016/j.ymthe.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Nathwani AC, Davidoff A, Hanawa H, Zhuo F, Vanin EF, Nienhuis AW. Factors influencing in-vivo transduction by recombinant adeno-associated viral vectors expressing the human factor IX cDNA. Blood. 2001 Mar;97(5):1258–65. doi: 10.1182/blood.v97.5.1258. [DOI] [PubMed] [Google Scholar]
- 24.Davidoff AM, Ng CY, Zhang Y, Streck CJ, Mabry SJ, Barton SH, et al. Careful Decoy Receptor Titering is Required to Inhibit Tumor Angiogenesis While Avoiding Adversely Altering VEGF Bioavailability. Mol Ther. 2005 Feb;11(2):300–10. doi: 10.1016/j.ymthe.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Nathwani AC, Persons DA, Stevenson SC, Frare P, McClelland A, Nienhuis AW, et al. Adenovirus-mediated expresssion of the murine ecotropic receptor facilitates transduction of human hematopoietic cells with an ecotropic retroviral vector. Gene Ther. 1999 Aug;6(8):1456–68. doi: 10.1038/sj.gt.3300974. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Takabe K, Bidlingmaier SM, Ill CR, Verma IM. Sustained correction of bleeding disorder in hemophilia B mice by gene therapy. Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):3906–10. doi: 10.1073/pnas.96.7.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiorini JA, Yang L, Liu Y, Safer B, Kotin RM. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J Virol. 1997;71:6823–33. doi: 10.1128/jvi.71.9.6823-6833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiorini JA, Kim F, Yang L, Kotin RM. Cloning and characterization of adeno-associated virus type 5. J Virol. 1999 Feb;73(2):1309–19. doi: 10.1128/jvi.73.2.1309-1319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutledge EA, Halbert CL, Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1998 Jan;72(1):309–19. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006 Oct;80(19):9831–6. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fournier S, Delespesse G, Rubio M, Biron G, Sarfati M. CD23 antigen regulation and signaling in chronic lymphocytic leukemia. J Clin Invest. 1992 Apr;89(4):1312–21. doi: 10.1172/JCI115717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogura T, Mizukami H, Mimuro J, Madoiwa S, Okada T, Matsushita T, et al. Utility of intraperitoneal administration as a route of AAV serotype 5 vector-mediated neonatal gene transfer. J Gene Med. 2006 Aug;8(8):990–7. doi: 10.1002/jgm.916. [DOI] [PubMed] [Google Scholar]
- 33.Sarkar R, Mucci M, Addya S, Tetreault R, Bellinger DA, Nichols TC, et al. Long-term efficacy of adeno-associated virus serotypes 8 and 9 in hemophilia a dogs and mice. Hum Gene Ther. 2006 Apr;17(4):427–39. doi: 10.1089/hum.2006.17.427. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Calcedo R, Nichols TC, Bellinger DA, Dillow A, Verma IM, et al. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005 Apr 15;105(8):3079–86. doi: 10.1182/blood-2004-10-3867. [DOI] [PubMed] [Google Scholar]
- 35.Wu Z, Sun J, Zhang T, Yin C, Yin F, Van DT, et al. Optimization of Self-complementary AAV Vectors for Liver-directed Expression Results in Sustained Correction of Hemophilia B at Low Vector Dose. Mol Ther. 2007 Dec 4; doi: 10.1038/sj.mt.6300355. [DOI] [PubMed] [Google Scholar]
- 36.Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006 Jun;24(6):687–96. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 37.Humeau LM, Binder GK, Lu X, Slepushkin V, Merling R, Echeagaray P, et al. Efficient lentiviral vector-mediated control of HIV-1 replication in CD4 lymphocytes from diverse HIV+ infected patients grouped according to CD4 count and viral load. Mol Ther. 2004 Jun;9(6):902–13. doi: 10.1016/j.ymthe.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Davidoff AM, Ng CY, Sleep S, McIntosh J, Azam S, Zhao Y, et al. Purification of recombinant adeno-associated virus type 8 vectors by ion exchange chromatography generates clinical grade vector stock. Journal of Virological Methods. 2004;121(2):209–215. doi: 10.1016/j.jviromet.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Nathwani AC, Davidoff AM, Hanawa H, Hu Y, Hoffer FA, Nikanorov A, et al. Sustained high-level expression of human factor IX (hFIX) after liver- targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood. 2002 Sep 1;100(5):1662–9. doi: 10.1182/blood-2002-02-0589. [DOI] [PubMed] [Google Scholar]
- 40.Nathwani AC, Hanawa H, Vandergriff J, Kelly P, Vanin EF, Nienhuis AW. Efficient gene transfer into human cord blood CD34+ cells and the CD34+CD38- subset using highly purified recombinant adeno-associated viral vector preparations that are free of helper virus and wild-type AAV. Gene Ther. 2000 Feb;7(3):183–95. doi: 10.1038/sj.gt.3301068. [DOI] [PubMed] [Google Scholar]


