Summary
Objective
Growth hormone (GH) influences bone mass maintenance. However, the consequences of lifetime isolated GH deficiency on bone are not well established. We assessed the bone status and the effect of 6 months of GH replacement in GH-naïve adults with IGHD due to a homozygous mutation of the GHRH-R gene.
Patients and methods
We studied 20 individuals (10 men) with IGHD at baseline, after six months of depot GH treatment, and six and 12 months after GH discontinuation. Quantitative heel ultrasound (QUS) was performed and serum osteocalcin (OC) and cross-linking telopeptide of type I collagen (ICTP) were measured. QUS was also performed at baseline and one year later in a group of 20 normal control individuals (CO), who did not receive GH treatment.
Results
At baseline, the IGHD group had lower QUS than CO (T-score: IGHD = -1.15 ± 0.9 vs. C = -0.07 ± 0.9 p< 0.001). GH treatment improved this parameter, with improvement persisting for one year post-treatment (12 months: IGHD = -0.59 ± 0.9 p< 0.05). GH also caused an increase in serum OC (baseline vs. pGH, p< 0.001) and ICTP (baseline vs. pGH p< 0.01). The increase in OC was more marked during treatment and its reduction was slower after GH discontinuation than in ICTP.
Conclusions
These data suggest that lifetime severe IGHD is associated with significant reduction in QUS parameters, which are partially reverse by short-term depot GH treatment which induces a biochemical pattern of bone anabolism that persists for at least 6 months after treatment discontinuation.
Keywords: growth hormone deficiency, bone density, bone ultrasound, IGF-I
Introduction
There is evidence that the GH-IGF-I axis plays a pivotal role in maintaining bone health, and that its lack may predispose to loss of bone1,2 and to fractures3. Serum IGF-I levels have been shown to correlate with bone mineral density (BMD) in various conditions associated with bone mass loss4,5, and GH deficiency (GHD) is associated with low BMD6,7. Although there is evidence of beneficial effect of long-term GH treatment on bone, there is no consensus on the most appropriate treatment length. While some studies indicate that stabilization of bone mass occurs after 5 years of treatment8, others suggest that the gain continues for up to 10 years9.
Most adults with acquired GHD have a combination of pituitary hormones deficits1,2,10,11. As a consequence, many of these patients take hormone replacements that can themselves influence bone metabolism. These problems can be obviated by studying patients with isolated GHD (IGHD). However, this disease is rare12, and IGHD children are often no longer deficient when re-tested as adults13. We have identified a large extended pedigree with approximately 100 IGHD individuals (over several generations) residing in Itabaianinha County, in the Brazilian state of Sergipe14,15. All the affected individuals carry a homozygous null mutation (IVS1+1G→A) in the GHRH receptor (GHRHR) gene (GHRHR). The adult patients have proportionate severe short stature (−4 to −8 SD score for height) and otherwise normal pituitary function. Previously, a small number of subjects with a different mutation in the same gene were reported to have low BMD but near-normal volumetric BMD, leading to the conclusion that IGHD has little impact on bone mineralization16.
The objective of the present study was to assess the bone status of IGHD adults from Itabaianinha, and the response to six months of GH therapy. Although dual-energy x-ray absorptiometry (DXA) is commonly used to determine bone density, it is not suitable for field research. We therefore used quantitative ultrasound (QUS). This method predicts fracture risk17,18, is safe, easy to use, portable, and radiation-free, rendering it an ideal tool use in a rural area as the one where our subjects live.
Subjects and Methods
Subjects
The study group included 20 IGHD adults, 10 men 46.1 ± 14.3 years and 10 women 47.7 ± 15, 3 years (5 in menopause) from the Itabaianinha kindred recruited by advertising in the local health clinic and by word of mouth. They were all homozygous for the IVS1+1G→A GHRHR mutation. None had previously received GH replacement therapy. GHD was diagnosed as reported previously 14.
A control group (CO) consisted of 20 normal subjects, 10 men 47.1±16.0 years, and 10 women 45.8 ± 16.7 years (4 in menopause) residing in the same community and of similar socioeconomic level as the IGHD group, matched to for age and body mass index (Table 1). They were recruited from volunteers for a study aimed to analyze the phenotype of individuals heterozygous for the GHRH-R mutation. All the CO subjects were homozygous for the wild type allele. The CO group served to control the quality of the measurement of bone ultrasonography. The subjects were selected with exclusion of individuals with history of osteometabolic disease, alcohol abuse, smoking, hepatic or renal disease, or using medications known to interfere with mineral metabolism (anabolic steroids, glucocorticoids, anticonvulsants, diuretics or medication for the treatment of osteoporosis).
Table 1.
Clinical characteristics of the individuals in the control group (CO) and in the group with isolated growth hormone deficiency (IGHD). Values are means ± SD. BMI=body mass index.
| CO (n = 20) | IGHD (n = 20) | p | |
|---|---|---|---|
| Sex (M/F) | 10/10 | 10/10 | 1 |
| Post-menopause | 4/10 | 5/10 | 0.754 |
| Age (years) | 46.5 ± 16.0 | 46.1 ± 14.5 | 0.94 |
| Weight (kg) | 67.3 ± 14.7 | 36.8 ± 4.0 | <0.001 |
| Height (cm) | 167.3 ± 9.5 | 123.9 ± 5.7 | <0.001 |
| Height Z-score | 0.10 ± 1.3 | -6.06 ± 0.5 | <0.001 |
| BMI (Kg/m2) | 23.9 ± 3.4 | 23.9 ± 3.4 | 0.97 |
Experimental design
Twenty IGHD individuals were treated with depot GH (Nutropin depot, Genentech®, South San Francisco, CA, USA) administered every two weeks for 6 months. Blood samples were collected and QUS was performed at baseline and at 6 month intervals throughout the 18 months of the study: baseline, post GH (pGH) and at 12 and 18 months (6 mo and 12mo) washout periods. The initial and final GH doses were 0.33 and 0.38 mg/kg in women and 0.25 and 0.35 mg/kg in men. Dosing was based on published data showing increase in GH and IGF-1 lasting 14-17 days in adults after a single 0.25 or 0.5 mg/kg dose19. A similar starting dose (0.3 mg/kg) was used in a trial that showed efficacy of this preparation in adults20. Titration of the dose according to serum IGF-I levels could not be done due to the limited drug availability (whose manufacturing was discontinued before the start of this study). After the 4th injection, the same dose (1 vial, 13.5 mg) was administered to all subjects, corresponding to an average of 27.1 and 25 μg /kg/day in women and men, respectively. None of the female subjects was taking oral or transdermal estrogen therapy, which, in addition to influence bone metabolism, may cause resistance to GH21. Injections were given subcutaneously at the subject's residence by the same operator. Side effects were all of mild intensity and transient, and included local pain and large joint pain in 25% of the patients, muscle pain and headache in 10% of individuals. Both the University of Sergipe and the Johns Hopkins University Institutional Review boards approved these studies, and all subjects gave written informed consent.
Methods
Height and weight standard deviation scores were calculated using UK standards22. Blood was collected in the morning between 7:00 and 9:00, after a 10-12 hour fast, and samples were kept in an ice-chilled container until centrifugation. Serum aliquots were stored at −40° C until the time of measurements, all carried out in a single assay.
IGF-I was measured in duplicate by the DSL-5600 immunoradiometric method (IRMA) (Diagnostic Systems Laboratories, Webster, TX, USA). Osteocalcin was measured by DSL-7600 IRMA. Serum levels of the C-terminal cross-linking telopeptide of type I collagen (ICTP) were measured by radioimmunoassay (Orion Diagnostic, Oy, Espoo, Finland). The intra-assay coefficients of variation for the determination of IGF-I, osteocalcin and ICTP were 4.5% 6.4% and 8.2%, respectively.
Heel QUS was performed with rigorous standardization of subject positioning using the Achilles Insight device (Lunar/GE, Madison, WI, USA). This is a water-based system that uses transmission of an US wave in a temperature-controlled water bath (37° C) through the heel. QUS measures the stiffness by the equation: stiffness = (0.67 × coefficient of Ultrasound Broad Band attenuation in dB/MHz + 0.28 × speed of sound in m/second) − 420. Stiffness index (SI) was expressed as the percentage of the values obtained by the manufacturer in a young adult population. The exams were performed following manufacturer's instructions. The menu of the apparatus allows choosing the reference population, and a normal South American population was chosen as reference. The coefficient of variation of the stiffness measurement, using the equation of Gluer et al 23, was 2.84%.
Laboratory and bone QUS data were analyzed statistically by the Multilevel test (random and fixed effects) and regression models were constructed using the PROC MIXED software (SAS version 8.0, San Diego, CA, USA)24. The data regarding physical characteristics (weight, height, BMI and stature Z-score) were analyzed by the parametric Student t-test. The level of significance was set at 5% in all analyses.
Results
Table 1 shows that the groups were matched for age, sex and BMI, although weight and height were, as expected, lower in the IGHD group. The Z-score for height was -6.0 SD for the IGHD and 0.1 SD for the control group (p< 0.001).
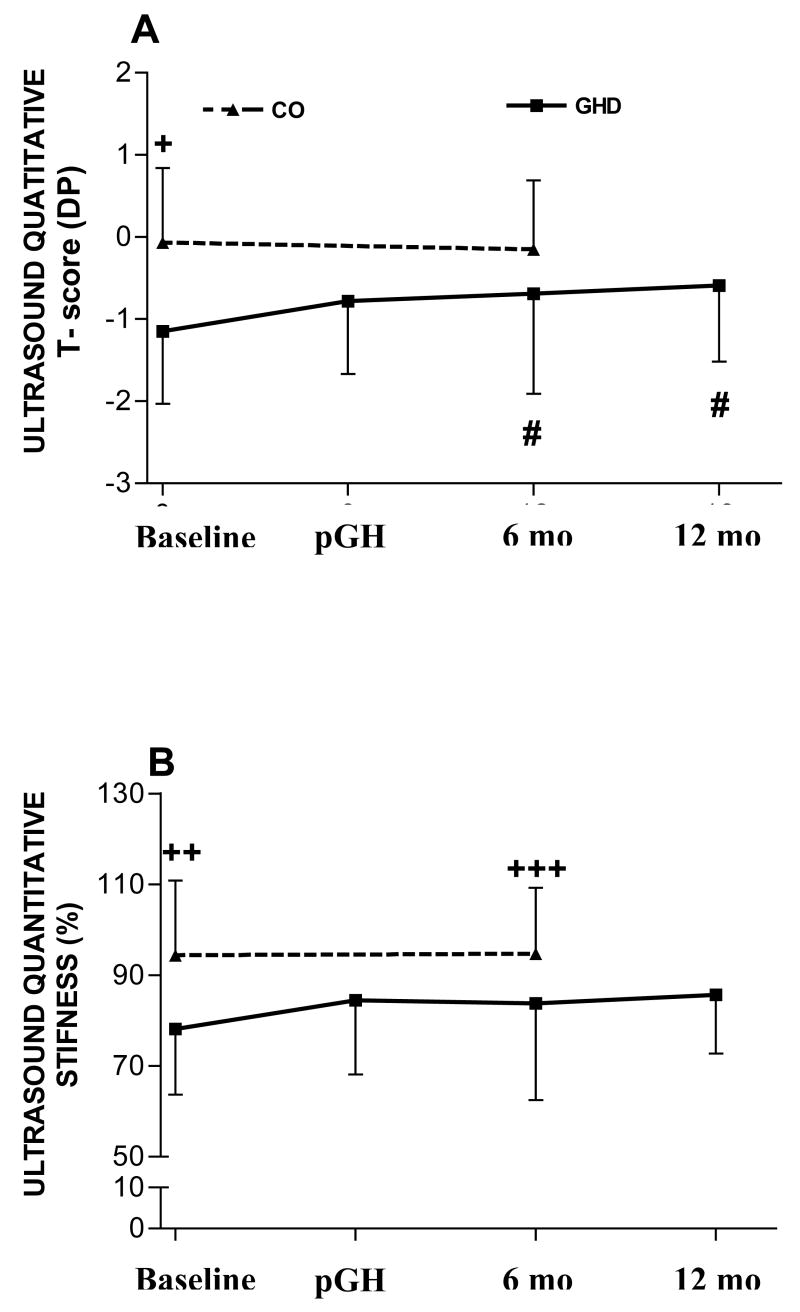
Basal stiffness values (IGHD = 78.15 ± 14.47 vs. C = 94.30 ± 16.54 %, p< 0.05) and T-scores (IGHD = -1.15 ± 0.88 vs. C= -0.07 ± 0.91 SD, p<0.001) were significantly lower in the IGHD group when compared to normal controls (Figure 1).
Figure 1.
Values of quantitative ultrasound parameters (A: T-score; B: stiffness) of normal control individuals (CO) obtained at baseline and after one year (no intervention), and of GH deficient (GHD) individuals obtained at baseline, after 6 months of treatment with depot GH (pGH) and 6 months (6mo) and 12 months (12mo) after treatment discontinuation. +p < 0.001 for CO vs. GHD at baseline; ++p <0.04 for CO vs. GHD at baseline; +++p < 0.04 CO vs. GHD at 6mo; # p < 0.04 for GHD at 6mo vs. baseline and 12mo vs. baseline.
Trough (2 weeks after last injection) serum IGF-I increased significantly from basal (0.13 ± 0 nmol/L, -3.1 ± 0.54 SDS) to the end of 6 months GH treatment (3.53 ± 2.81 nmol/L, −2.59 ± 0.62 SDS p< 0.001), without normalization, and returned to basal values at 6 months (0.13 ± 0.32 nmol/L. −3.1 ± 0.54 SDS) and 12 months (0.13 ± 0 nmol/L, −3.1 ± 0.54) of washout. The increase of IGF-I at 6 months of GH treatment in comparison to basal was significant in both sexes (females: median difference = 4.58 nmol/L, p = 0.006 and males: median difference = 1.83 nmol/L, p = 0.036), indicating no GH resistance in our female group.
After six months of treatment (pGH), the ultrasonometric parameters tended to increase (stiffness: pGH = 84.45 ± 16.30, T-score= -0.78 SD). This trend towards T score elevation was maintained for 6 and 12 months after the end of treatment. Thus the T-score value became significantly higher at these time points compared to baseline (Basal= -1.15 vs. 6mo= -0.69 vs. 12mo= -0.59 SD, p< 0.05) (Figure 1). At 6mo the T-score of the IGHD group was not different from the CO group in the same interval.
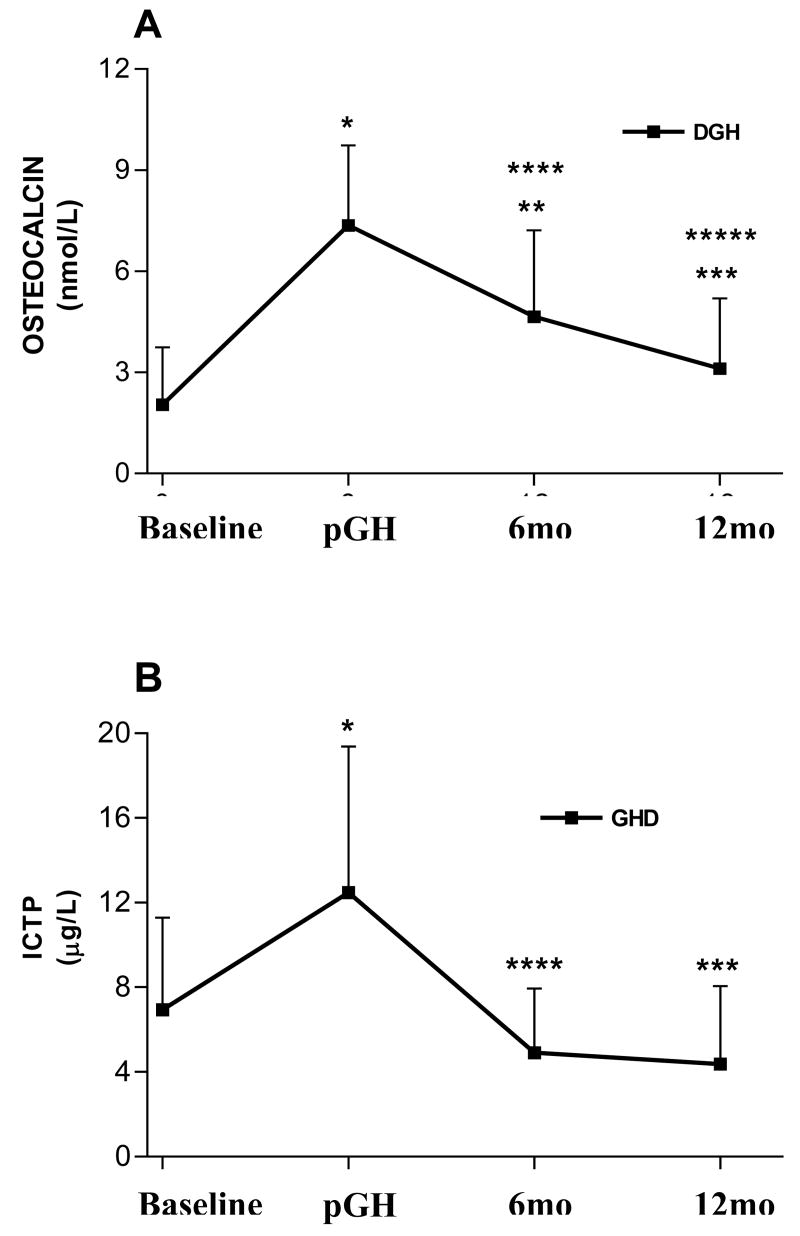
GH replacement caused a marked elevation of the biochemical remodeling parameters. However, while the increase OC was about 3.5 times (Basal = 2.05 ± 1.69 vs. pGH= 7.36 ± 2.38 nmol/L, p< 0.001), the increase in ICTP was less marked (Basal = 6.95 ± 4.35 vs. pGH= 12.48 ± 6.90 μg/L, p< 0.001) (Figure 2). A difference was also observed in the reduction of these metabolites after GH discontinuation. While the fall in OC was slow, remaining significantly higher than baseline at 6mo (4.65 ± 2.56 nmol/L, p< 0.001), ICTP fell more rapidly, returning to baseline at 6 mo (T12= 4.91 μg/L).
Figure 2.
Serum osteocalcin and cross-linking telopeptide of type I collagen (ICTP) in GH deficient individuals (GHD) obtained at baseline, after 6 months of treatment with depot GH (pGH) and 6 months (6mo) and 12 months (12mo) after treatment discontinuation. *p< 0.001 for pGH vs. baseline; **p< 0.001 for 6mo vs. baseline; ***p< 0.001 for 12mo vs. pGH; ****p< 0.001 for 6mo vs. pGH. *****p< 0.01 for 12mo vs. 6 mo.
Discussion
Most previous studies have shown that GHD is associated with a moderate reduction of bone mass in adults 8-10,25, 26. However, GHD is frequently associated with multiple pituitary hormones deficits, whose replacement cannot fully mimic physiological secretion. Particularly, prednisone doses as low as 2.5 mg/day can lead to bone loss27. Similarly, thyroid hormone replacement may not be accurate, as in hypopituitary patients one cannot rely on TSH to guide dosing, and even mild hyperthyroidism may reduce BMD28.
In contrast, any abnormality observed in the IGHD subjects can be attributed to GHD. We found that the adult IGHD subjects have reduced QUS T scores compared to controls, and that six months of GH treatment induced positive changes, that reached significance after 6 months from the completion of treatment. In addition, GH led to a predominant state of bone anabolism, and the beneficial effects continued for at least 6 months after the end of treatment.
An important aspect to be considered in the present study is the influence of stature, i.e., bone length, on the measurement of the structural parameters of the skeleton. It has been well established that the measurement of areal BMD by DXA underestimates bone mass in individuals with short stature and overestimates it in high stature29-31. Indeed, when Maheshwari et al.16 studied 4 young males with IGHD due to a GHRH-R mutation, they found reduced areal, but normal volumetric bone density. Although some studies have indicated that size may influence the results of bone ultrasonomtery32,33, QUS instruments from different manufacturers have significant differences in their calibration methods and analysis software as well as in the design of the transducers34. Cheng et al.34 verified that heel width influences Achilles assessments and that calcaneal length and soft tissue thickness have a substantial effect on measurements performed with another QUS devices, the Sahara (Hologic, Bedford, MA). Nevertheless, it should be emphasized that we observed that GH therapy had a positive effect on QUS parameters, supporting a probable causative effect of GHD on reduced bone ultrasonometry. It is also important to remember that Maheshwari et al. studied young males, at the time of peak bone mass. It is possible that congenital IGHD does not influence peak mass, but causes bone loss after peak mass is attained. Indeed, reduced bone density occurs in a mouse model of GH deficiency (little mouse) that carries a homozygous mutation on the same gene (GHRH-R) as our patients. Interestingly, in this model, the magnitude of the effect of GH replacement on BMD varies at different ages35. On the other hand, it is important to keep in mind that we have no report from the Itabaianinha community that indicates that the GHD subjects have an increased incidence of fractures.
Previous studies have reported contradictory results regarding the changes in BMD caused by GH replacement. Some have shown a tri-phasic course, with an initial loss, a gain after the first year of treatment, and subsequent stability after 5 years of treatment8. Others have reported a gain that persisted for about five 26 and ten years of treatment9. Our data are not directly comparable to those obtained in these studies if we consider that DXA and QUS, although both correlate with the fracture risk36-38, evaluate different aspects of bone structure39-40. Two studies have assessed the effect of GH replacement on bone by ultrasonography. They showed a period of 6 months decline followed by 6 years of gradual improvement8, 36. In both studies the site for assessment was the phalanx, and the patients had acquired GHD. In addition, although we could not obtain peak levels (all the study subjects live in a very rural area, and we could only meet them to inject the GH depot), it is likely that in our study even peak serum IGF-I did not reach normal values. It is therefore possible that submaximal GH replacement has a preferential or earlier effect on bone anabolism when compared to more intense treatment. In addition, the depot formula delivers GH with a pharmacokinetic that is different from daily injections.
Similar to other studies that have aimed to evaluate GH influence on bone, we did not supplement the individuals with calcium and vitamin D (VD)9. However, we have not detected VD deficiency in individuals living in rural areas of Brazil, even in those with unfavorable socioeconomic conditions (i.e. patients with leprosy)41,42. Solar exposure is naturally responsible for about 80% of the supply of 25(OH)D. All subject were rural workers, exposed to the sun throughout the year, as the annual average temperature in the state of Sergipe (tropical climate) is around 26°C (78.8°F), making VD deficiency unlikely.
Recent studies suggested that low bone mass in GHD may also be consequence of abnormalities in PTH circadian rhythm together with reduced parathyroid gland and target-organ sensitivity43. Further, it seems that GH treatment can restore PTH sensitivity in kidney decreasing calcium wasting44. In the present study, we have not evaluated calcium intake or elimination.
The experimental design of a 6-month period of treatment followed by one year of washout revealed interesting aspects. Treatment discontinuation caused return of ICTP to baseline by the first wash out visit (6 mo), while the effect on OC continued for at least six months, suggesting a prolonged anabolic effect. Accordingly, we observed an ascending curve of ultrasonometric T-score values, which persisted until the 2 month washout. The mechanism of this continued effect is to be determined, but it is unlikely to be related to persistence of GH as shown in pharmacokinetic studies with depot injections45.
Our results suggest that a relatively short-term treatment may be beneficial on bone of individuals with lifelong IGDH. This is particularly important if we consider the cost of treatment and its potential adverse effects on other parameters46. It is important to notice that we used a long-acting preparation, which results in sustained GH elevation. This may raise concerns for side effects, by analogy to acromegaly, in which serum GH levels are not always high, but never dip to the very low levels seen in normal individuals47. The anabolic effect of GH on bone tissue persists in acromegalic individuals even after remission48. Further studies are needed to test whether this mean of GH delivery results in a more positive impact on bone tissue than the daily treatment.
In summary, we have shown that subjects with lifetime IGHD have lower heel QUS T scores than age-matched controls, and that a 6-month treatment with a submaximal dose of depot GH increases T scores and induces a biochemical pattern of bone anabolism that persists for at least 6 months after treatment discontinuation.
Acknowledgments
The authors wish to thank Sebastião Brandão Filho for technical help with the laboratory assays and Ivanilde Santana for secretarial assistance.
Support: NIH Grant 1 R01 DK065718, by a grant from the Genentech Center for Clinical Research in Endocrinology (both to R.S.), and by grants from CAPES and FAEPA
Footnotes
Clinical trial.gov identifier NCT00149708 (Registration date September 6, 2005
References
- 1.Patel MB, Arden NK, Masterson LM, Phillips DI, Swaminathan R, Syddall HE, Byme CD, Wood PJ, Cooper C, Holt RI. Hertfordshire Cohort Study Group Investigating the role of the growth hormone-insulin-like growth factor (GH-IGF) axis as a determinant of male bone mineral density (BMD) Bone. 2005;37:833–841. doi: 10.1016/j.bone.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Yakar S, Rosen CJ, Beamer WG, Arkert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frustyk J, Boisclair YR, LeRoith D. Circulating levels of IGF-1 directly regulate bone growth and density. Journal of Clinical Investigation. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmer H, Svensson J, Rylander L, Johannsson G, Rosén T, Bengtsson B, Thorén M, Höybye C, Degerblad M, Bramnert M, Hägg E, Engström BE, Ekman B, Thorngren KG, Hagmar L, Erfurth EM. Fracture Incidence in GH-Deficient Patients on Complete Hormone Replacement Including GH. Journal of Bone and Mineral Research. 2007;22:1842–1850. doi: 10.1359/jbmr.070811. [DOI] [PubMed] [Google Scholar]
- 4.Gillberg P, Olofsson H, Mallmin H, Blum WF, Ljunghall S, Nilsson AG. Bone mineral density in femoral neck is positively correlated to circulating insulin-like growth factor (IGF)-I and IGF-binding protein (IGFBP)-3 in Swedish men. Calcified Tissue International. 2002;70:22–29. doi: 10.1007/s002230020048. [DOI] [PubMed] [Google Scholar]
- 5.Taveira ATA, Fernandes MIM, Galvão LC, Sawamura R, Vieira EM, Paula FJA. Impairment of bone mass development in children with chronic cholestatic liver disease. Clinical Endocrinology. 2007;66:518–523. doi: 10.1111/j.1365-2265.2007.02765.x. [DOI] [PubMed] [Google Scholar]
- 6.Sneppen SP, Hoeck HC, Kollerup G, Sorensen OH, Laurberg P, Rasmussen UF. Bone mineral content and bone metabolism during physiological GH treatment in GH-deficient adults – an 18-month randomised, placebo-controlled, double blinded trial. European Journal of Endocrinology. 2007;146:187–195. doi: 10.1530/eje.0.1460187. [DOI] [PubMed] [Google Scholar]
- 7.Snyder PJ, Biller BM, Zagar A, Jackson I, Arah BM, Nippoldt TB, Cook DM, Mooradian AD, Kwan A, Scism-Bacon J, Chipman JJ, Hartman ML. Effect of growth hormone replacement on BMD in adult-onset growth hormone deficiency. Journal of Bone and Mineral Research. 2007;22:762–770. doi: 10.1359/jbmr.070205. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm B, Kann PH. Long-term effects of 7-year growth hormone substitution on bone metabolism, bone density, and bone quality in growth hormone-deficient adults. Medical Klinisch. 2004;99:569–577. doi: 10.1007/s00063-004-1088-4. [DOI] [PubMed] [Google Scholar]
- 9.Götherström G, Bengtsson BA, Bosaeus I, Johannsson G, Svensson J. Ten-year GH replacement increases bone mineral density in hypopituitary patients with adult onset GH deficiency. European Journal of Endocrinology. 2007;156:55–64. doi: 10.1530/eje.1.02317. [DOI] [PubMed] [Google Scholar]
- 10.Arafah BM, Prunty D, Ybarra J, Hlavin ML, Selman WR. The dominant role of increased intrasellar pressure in the pathogenesis of hypopituitarism, hyperprolactinemia, and headaches in patients with pituitary adenomas. Journal of Clinical Endocrinology and Metabolism. 2000;85:1789–1793. doi: 10.1210/jcem.85.5.6611. [DOI] [PubMed] [Google Scholar]
- 11.Webb SM, Rigla M, Wägner A, Oliver B, Bartumeus F. Recovery of hypopituitarism after neurosurgical treatment of pituitary adenomas. Journal of Clinical Endocrinology and Metabolism. 1999;84:3696–3700. doi: 10.1210/jcem.84.10.6019. [DOI] [PubMed] [Google Scholar]
- 12.Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah growth study: growth standards and the prevalence of growth hormone deficiency. Journal of Pediatrics. 1994;125:29–35. doi: 10.1016/s0022-3476(94)70117-2. [DOI] [PubMed] [Google Scholar]
- 13.Jull A, Kastrup KW, Pedersen SA, Skakkebaek NE. Growth hormone (GH) provocative retesting of 108 young adults with childhood-onset GH deficiency and the diagnostic value of insulin-like growth factor (IGF-I) and IGF-binding protein-3. Journal of Clinical Endocrinology and Metabolism. 1997;82:1195–1201. doi: 10.1210/jcem.82.4.3892. [DOI] [PubMed] [Google Scholar]
- 14.Salvatori R, Hayashida CY, Aguiar-Oliveira MH, Phillips JA, Souza AH, Gondo RG, Toledo SP, Conceição MM, Prince M, Maheshwari HG, Baumann G, Levine MA. Familial dwarfism due to a novel mutation of the growth hormone-releasing hormone receptor gene. Journal of Clinical Endocrinology and Metabolism. 1999;84:917–923. doi: 10.1210/jcem.84.3.5599. [DOI] [PubMed] [Google Scholar]
- 15.Aguiar-Oliveira MH, Gill MS, Barreto ES, Alcantara MR, Miraki-Moud F, Menezes CA, Souza AH, Martinelli CE, Pereira FA, Salvatori R, Levine MA, Shalet SM, Camacho-Hubner C, Clayton PE. Effect of severe growth hormone (GH) deficiency due to a mutation in the GH-releasing hormone receptor on insulin-like growth factor (IGFs), IGF-binding proteins and ternary complex formation throughout life. Journal of Clinical Endocrinology and Metabolism. 1999;84:4118–4126. doi: 10.1210/jcem.84.11.6133. [DOI] [PubMed] [Google Scholar]
- 16.Maheshwari HG, Bouillon R, Nijs J, Oganov VS, Bakulin AV, Baumann G. The impact of congenital, severe, untreated growth hormone (GH) deficiency on bone size and density in young adults: insights from genetic GH-releasing hormone receptor deficiency. Journal of Clinical Endocrinology and Metabolism. 2003;88:2614–2618. doi: 10.1210/jc.2002-021120. [DOI] [PubMed] [Google Scholar]
- 17.Marin F, Gonzalez-Macias J, Diez-Perez A, Palma S, Delgado-Rodriguez M. Relationship between bone quantitative ultrasound and fractures: a meta-analysis. Journal of bone and mineral research. 2006;21:126–135. doi: 10.1359/jbmr.060417. [DOI] [PubMed] [Google Scholar]
- 18.Khaw KT, Reeve J, Luben R, Bingham S, Welch A, Wareham N, Oakes S, Day N. Prediction of total and hip hip fractures risk in men and women by quantitative ultrasound of the calcaneous: Epic-Norfalk prospective population study. Lancet. 2004;363:197–202. doi: 10.1016/S0140-6736(03)15325-1. [DOI] [PubMed] [Google Scholar]
- 19.Cook DM, Biller BM, Vance ML, Hoffman AR, Phillips LS, Ford KM, Benziger DP, Illeperuma A, Blethen SL, Attie KM, Dao LN, Reimann JD, Fielder PJ. The pharmacokinetic and pharmacodynamic characteristics of a long-acting growth hormone (GH) preparation (nutropin depot) in GH-deficient adults. Journal of Clinical Endocrinology and Metabolism. 2002;87:4508–4514. doi: 10.1210/jc.2002-020480. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman AR, Biller BMK, Cook D, Baptista J, Silverman BL, Dao L, Attie KM, Fielder P, Maneatis T, Lippe B, Lippe B Genetech Adult Growth Hormone Deficiency Study Group. Efficacy of a long-acting growth hormone (GH) preparation in patients with adult GH deficiency. Journal of Clinical Endocrinology and Metabolism. 2005;90:6431–6440. doi: 10.1210/jc.2005-0928. [DOI] [PubMed] [Google Scholar]
- 21.Wolthers T, Hoffman DM, Nugent AG, Duncan MW, Umpleby M, Ho KK. Oral estrogen antagonizes the matabolic actions of growth hormone in growth hormone-deficient women. American Journal of Physiology Endocrinology and Metabolism. 2001;281:E1191–E1196. doi: 10.1152/ajpendo.2001.281.6.E1191. [DOI] [PubMed] [Google Scholar]
- 22.Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, Preece MA. Cross-sectional stature and weight reference curves for the UK 1990. Archives of Disease in Childhood. 1995;73:17–24. doi: 10.1136/adc.73.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glüer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporosis International. 1995;5:262–270. doi: 10.1007/BF01774016. [DOI] [PubMed] [Google Scholar]
- 24.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Education in Behavior of Statistic. 1998;24:323–355. [Google Scholar]
- 25.Toogood A, Adams J, O'neill P, Shalet S. Elderly patients with adult-onset growth hormone deficiency are not osteopenic. Journal of Clinical Endocrinology and Metabolism. 1997;82:1462–1466. doi: 10.1210/jcem.82.5.3932. [DOI] [PubMed] [Google Scholar]
- 26.Koranyi J, Svensson J, Götherström G, Sunnerhagen KS, Bengtsson B, Johannsson G. Baseline characteristics and the effects of five years of GH replacement therapy in adults with GH deficiency of childhood or adulthood onset: a comparative, prospective study. Journal of Clinical Endocrinology and Metabolism. 2001;86:4693–4699. doi: 10.1210/jcem.86.10.7896. [DOI] [PubMed] [Google Scholar]
- 27.Van Staa TP. The pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosis. Calcified Tissue International. 2006;79:129–137. doi: 10.1007/s00223-006-0019-1. [DOI] [PubMed] [Google Scholar]
- 28.Tauchmanovà L, Nuzzo V, Del Puente A, Fonderico F, Esposito-Del Puente A, Padulla S, Rossi A, Bifulco G, Lupoli G, Lombardi G. Reduced bone mass detected by bone quantitative ultrasonometry and DEXA in pre- and postmenopausal women with endogenous subclinical hyperthyroidism. Maturitas. 2004;48:299–306. doi: 10.1016/j.maturitas.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Carter DR, Louxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. Journal of Bone and Mineral Research. 1992;7:137–145. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 30.Leonard MB, Propert KJ, Zemel BS, Stallings VA, Feldman HI. Discrepancies in pediatric bone mineral density reference data: potential for misdiagnosis of osteopenia. The Journal of Pediatrics. 1999;135:182–188. doi: 10.1016/s0022-3476(99)70020-x. [DOI] [PubMed] [Google Scholar]
- 31.Janz K. Physical activity and bone development during childhood and adolescence: implications for the prevention of osteoporosis. Minerva Pediatric. 2002;54:93–104. [PubMed] [Google Scholar]
- 32.Hans D, Schott AM, Arlot ME, Sornay E, Delmas PD, Meunier PJ. Influence of anthropometric parameters on ultrasound measurements of Os calcis. Osteoporosis International. 1995;5:371–376. doi: 10.1007/BF01622259. [DOI] [PubMed] [Google Scholar]
- 33.van den Bergh JP, Noordam C, Ozyilmaz A, Hermus AR, Smals AG, Otten BJ. Calcaneal ultrasound imaging in healthy children and adolescents: relation of the ultrasound parameters BUA and SOS to age, body weight, height, foot dimensions and pubertal stage. Osteoporosis International. 2000;11:967–976. doi: 10.1007/s001980070036. [DOI] [PubMed] [Google Scholar]
- 34.Cheng S, Njeh CF, Fan B, Cheng X, Hans D, Wang L, Fuerst T, Genant HK. Influence of region of interest and bone size on calcaneal BMD: implications for the accuracy of quantitative ultrasound assessments at the calcaneus. British Journal of Radiology. 2002;75:59–68. doi: 10.1259/bjr.75.889.750059. [DOI] [PubMed] [Google Scholar]
- 35.Kasukawa Y, Baylink DJ, Guo R, Mohan S. Evidence that sensitivity to growth hormone (GH) Is growth period and tissue type dependent: studies in GH-deficient lit/lit Mice. Endocrinology. 2003;144:3950–3957. doi: 10.1210/en.2002-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kann P, Piepkorn B, Schehler B, Andreas J, Lotz J, Prellwitz W, Beyer J. Effect of long-term treatment with GH on bone metabolism, bone mineral density and bone elasticity in GH-deficient adults. Clinical Endocrinology. 1998;48:561–568. doi: 10.1046/j.1365-2265.1998.00439.x. [DOI] [PubMed] [Google Scholar]
- 37.Hans D, Dagent-Molina P, Schott AM, Sebert JL, Cormier C, Kotzki PO, Delmas PD, Pouilles JM, Breart G, Meunier PJ. Ultrasonographic heel measurements to predict hip fracture in elderly women: the EPIDOS prospective study. Lancet. 1996;348:511–514. doi: 10.1016/s0140-6736(95)11456-4. [DOI] [PubMed] [Google Scholar]
- 38.Bauer DC, Gluer CC, Cauley JA, Vogt TM, Ensrud KE, Genant HKL, Black DM. Broadband ultrasound attenuation predicts fractures strongly and independently of densitometry in older women. A prospective study. Study of Osteoporotic Fractures Research Group. Archives of Internal Medicine. 1997;157:629–634. [PubMed] [Google Scholar]
- 39.Varenna M, Sinigaglia L, Adami S, Giannini S, Isaia G, Maggi S, Filipponi P, Munno O, Mangeri D, de Feo D, Crepaldi G. Association of quantitative heel ultrasound with history of osteoporotic fractures in elderly men: The ESOPO study. Osteoporosis International. 2005;16:1749–1754. doi: 10.1007/s00198-005-1914-4. [DOI] [PubMed] [Google Scholar]
- 40.Haïat G, Padilla F, Peyrin F, Laugier P. Variation of ultrasonic parameters with microstructure and material properties of trabecular bone: a 3d model simulation. Journal of Bone and Mineral Research. 2007;22:665–674. doi: 10.1359/jbmr.070209. [DOI] [PubMed] [Google Scholar]
- 41.Pereira FA, de Castro JA, dos Santos JE, Foss MC, de Paula FJA. Impact of marked weight loss induced by bariatric surgery on bone mineral density and remodeling. Brazilian Journal of Medical and Biological Research. 2007;40:509–517. doi: 10.1590/s0100-879x2007000400009. [DOI] [PubMed] [Google Scholar]
- 42.Ribeiro FB, Pereira FA, Muller E, Foss NT, Paula FJA. Evaluation of bone mineral metabolism in patients recently diagnosed with leprosy. The American Journal of the Medical Sciences. 2007;334:322–326. doi: 10.1097/MAJ.0b013e318142bafb. [DOI] [PubMed] [Google Scholar]
- 43.White HD, Ahmad AM, Durham BH, Peter R, Prabhakar VKB, Colett P, Vora JP, Fraser WD. PTH circadian rhythm and PTH target-organ sensitivity is altered in patients with adult growth hormone deficiency with low BMD. Journal of Bone and Mineral Research. 2007;22:1798–1807. 2007. doi: 10.1359/jbmr.070715. [DOI] [PubMed] [Google Scholar]
- 44.Joseph K, Ahmad AM, Ul-Haq M, Durham BH, Whittingham P, Fraser WD, Vora JP. Effects of growth hormone administration on bone mineral metabolism, PTH sensitivity and PTH secretory rhythm in postmenopausal women with established osteoporosis. Journal of Bone and Mineral Research. 2008;23:721–729. doi: 10.1359/jbmr.071117. [DOI] [PubMed] [Google Scholar]
- 45.Jones AJ, Putney S, Johnson OL, Cleland JL. Recombinant human growth hormone poly(lactic-co-glycolic acid) microsphere formulation development. Advanced Drug Delivery Reviews. 1997;28:71–84. doi: 10.1016/s0169-409x(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira JLM, Aguiar-Oliveira MH, D'Oliveira A, Jr, Pereira RMC, oliveira CRP, Farias CT, Barreto-Filho JA, Anjos-Andrade FD, Marques-Santos C, Nascimento-Junior AC, Alves EA, Oliveira FT, Campos VC, Ximenes R, Blackford A, Parmigiani G, Salvatori R. Congenital growth hormone (GH) deficiency and atherosclerosis effects of GH replacement in GH-naive adults. Journal of Clinical Endocrinology and Metabolism. 2007;92:4664–4670. doi: 10.1210/jc.2007-1636. [DOI] [PubMed] [Google Scholar]
- 47.Merriam GR, Wyatt FG. Diagnosis and treatment of growth hormone deficiency in adults: current perspectives. Current Opinion in Endocrinology and Diabetes. 2006;13:362–368. [Google Scholar]
- 48.Biermasz NR, Hamdy NA, Pereira AM, Romijn JA, Roelfsema F. Long-term maintenance of the anabolic effects of GH on the skeleton in successfully treated patients with acromegaly. European Journal of Endocrinology. 2005;152:53–60. doi: 10.1530/eje.1.01820. [DOI] [PubMed] [Google Scholar]