Abstract
Lactation represents the greatest postnatal energetic expenditure for mammalian mothers, and a mother's ability to sustain the costs of lactation is influenced by her physical condition. Mothers in good condition may produce infants who weigh more, grow faster, and are more likely to survive than the infants of mothers in poor condition. These effects may be partially mediated through the quantity and quality of milk that mothers produce during lactation. However, we know relatively little about the relationships between maternal condition, milk composition, milk yield, and infant outcomes. Here, we present the first systematic investigation of the magnitude, sources, and consequences of individual variation in milk for an Old World monkey. Rhesus macaques produce dilute milk typical of the primate order, but there was substantial variation among mothers in the composition and amount of milk they produced and thus in the milk energy available to infants. Relative milk yield value (MYV), the grams of milk obtained by mammary evacuation after 3.5–4 h of maternal-infant separation, increased with maternal parity and was positively associated with infant weight. Both milk gross energy (GE) and MYV increased during lactation as infants aged. There was, however, a trade-off; those mothers with greater increases in GE had smaller increases in MYV, and their infants grew more slowly. These results from a well-fed captive population demonstrate that differences between mothers can have important implications for milk synthesis and infant outcome.
Keywords: milk composition and yield, parity, infant growth, maternal effects, life history
For mammalian females, lactation is the most costly form of parental investment. Mothers vary in their ability to meet the costs of lactation, and maternal effects on infant outcomes are common among mammals. As such, maternal influences on infant phenotype contribute to the variation on which natural selection acts (Mosseau and Fox, 1998; Räsänen and Kruuk, 2007). In nonhuman primates, infant growth and survival have been linked to variation in maternal weight, parity, age, and social rank as well as the timing of birth (in Chlorocebus aethiops; age and weight Fairbanks and McGuire, 1995; in Papio sp maternal age; Cheney et al., 2004; rank and parity; Altmann and Alberts, 2005; in Macaca mulatta maternal age and adiposity; Johnson and Kapsalis, 1995; birth-timing, Drickamer, 1974; for review, see Fairbanks, 1996 and Maestripieri, 2009). It is likely that these maternal effects are at least partially mediated through the quantity or quality of milk that mothers provide their infants during lactation, but currently little is known about interindividual differences in milk synthesis.
To develop a full understanding of parent-offspring conflict and life-history theory in primates, it is important to identify the sources, magnitude, and consequences of variation in milk composition and output (Trivers, 1974; Bowman and Lee, 1995; Johnson and Kapsalis, 1995; Lee, 1996; Johnson, 2003). Although variation in mother's milk has been well studied in seals (Oftedal et al., 1993; Oftedal et al., 1996; Mellish et al., 1999; Lang et al., 2005;McDonald and Crocker, 2006; Wheatley et al., 2006), deer (Landete-Castillejos et al., 2001; Gomez et al., 2002; Gjøstein et al., 2003), and rodents (Nagasawa et al., 1989; Derrickson et al., 1996; Rogowitz, 1996; Künkele and Kenagy, 1997; Künkele, 2000; Król and Speakman, 2003; Landete-Castillejos et al., 2005; Hayes and Solomon, 2006), these taxa have different life history strategies than primates. In comparison with other mammals, primates produce dilute milks over an extended lactation period resulting in low daily costs of investment. For this reason, a mother's ability to synthesize milk is less vulnerable to fluctuating food resources during lactation (for reviews, see Oftedal, 1984; Oftedal and Iverson, 1995; Sellen, 2007; Power et al., 2008).
Indirect measures of lactational output, such as nursing frequency and time spent in ventral contact, vary in relation to maternal condition. In rhesus macaques, infants of low-ranking mothers nurse more frequently but for shorter bouts than infants of high-ranking mothers (Gomendio, 1990). In vervet monkeys, mothers of both high rank and “good condition”—prime reproductive age and nonmarginal weight—spend less time in ventral contact with their infants than midranking or low-ranking mothers in “poor condition” (Fairbanks and McGuire, 1995). Infants of primiparous mothers nurse more often (Macaca fuscata; Tanaka, 1997) and are more likely to suckle from both nipples during a single suckling bout (Macaca mulatta; Wilson et al., 1988; Gomendio, 1989) than infants of multiparous mothers. These observations suggest that mothers with fewer resources for lactation produce lower energy milk and/or lower milk yield than do mothers in good condition, requiring greater nursing effort by infants (Gomendio, 1996; Tanaka, 1997). However, we must be cautious in drawing conclusions about milk transfer from behavioral data (Clutton-Brock, 1991; Cameron, 1998; Cameron et al., 1999).
Our knowledge about milk production in primates is mainly restricted to species-level descriptions, and interspecific comparisons of major milk constituents (reviewed in Oftedal and Iverson, 1995). Anthropoid primate milk is relatively dilute, generally consisting of <15% dry matter, with about 7% sugar, 1–5% fat, and 1–3% protein concentrations (Oftedal and Iverson, 1995). There is considerable individual variation in milk composition, particularly milk fat concentration, among samples collected from nonhuman primates (Macaca fuscata; Ota et al., 1991; M. mulatta, Lonnerdal et al., 1984; Hinde, 2007a,b; Callithrix jacchus; Power et al., 2002; Power et al., 2008; Samiri boliviensis; Milligan et al., 2008; Varecia variegata, Otolemur garnettii and crassicaudatus, Nycticebus coucang; Tilden and Oftedal, 1997), but the factors that underlie this variation are unclear.
Some evidence in primates indicates that the variation in the amount or composition of milk that females produce reflects maternal condition. Nutritional restriction in three captive baboons reduced milk yield and stalled infant growth, but did not affect the composition of milk (Roberts et al., 1985). In common marmosets, New World monkeys that typically produce singletons or twins, small mothers rearing twins produced milk with lower fat concentration than larger mothers of twins or small mothers of singletons. Twins born to small mothers grew more slowly than other infants and began to eat solid food at an earlier age (Tardif et al., 2001). Small mothers who reared twins lost more weight, were less likely to conceive in the subsequent year, and were more likely to become ill than other mothers (Tardif et al., 2001). In captive rhesus macaques, mothers with lower intestinal parasites produced milk with significantly lower fat concentration than mothers without parasites (Hinde, 2007a). For humans, milk production is largely invulnerable to maternal or environmental conditions (reviewed in Prentice et al., 1994; Lonnerdal, 2000; Sellen, 2007). However, milk yield increases with maternal parity (Motil et al., 1997), and milk composition is positively associated with maternal body composition and parity (Nommsen et al., 1991, reviewed in Dewey, 1997). Still, our understanding of the relationship between maternal condition, milk production, and infant outcomes in humans is complicated by cultural variation in nursing behavior, the introduction of supplementary foods during breastfeeding, and the prevalent use of formula.
To better understand how maternal and environmental conditions influence physiological aspects of lactation, we conducted a systematic investigation of the magnitude, sources, and consequences of individual variation in milk composition and production for an Old World monkey, the rhesus macaque (Macaca mulatta). Previous work on this population showed that mothers of sons produce milk with higher energy density than mothers rearing daughters, and this effect is especially pronounced for primiparous mothers (Hinde, 2007b). Here, we test the prediction that heavier and higher ranking mothers will produce higher quality and/or larger quantities of milk than lighter or lower ranking females, and we provide a more complete analysis of the effects of parity on milk production. The timing of parturition within the annual birth season may influence milk production as rhesus infants born late in the birth season have reduced survivorship (Drickamer, 1974). In red deer, the only mammal for which data are available, birth timing is associated with milk synthesis (Landete-Castillejos et al., 2001). Although constraints on cervid milk production poorly reflect the selective pressures that have shaped primate lactation strategies, the infant mortality data suggest that birth timing in rhesus might influence lactational performance. We predicted that mothers who give birth early or during the peak of the birth season would produce more and/or higher quality milk than females that gave birth late in the season. We expected characteristics such as milk gross energy (GE; energy density) and milk yield to be correlated with infant weight and growth rates during infancy. Finally, as infants' energetic requirements for behavioral activity and growth increase from the early postnatal period to peak lactation, we predicted that milk energy provided by the mother would increase during lactation, through changes in milk energy density or yield or both.
Methods
Subjects
Data were obtained from 58 mother–infant pairs at the California National Primate Research Center (CNPRC) during the 2006 birth season. All reproductive females from three social groups were recruited into the lactation study for an initial total of 70. Twelve subjects were dropped from this analysis: four were removed, because they showed evidence of mastitis, and milk could not be collected from one mammary; one subject's infant died midway through the study; one subject simultaneously nursed both her newborn and her yearling; and six subjects did not undergo all procedures because of colony management and scheduling limitations. Excluded females did not differ in maternal characteristics from the final study sample. All subjects were housed in large outdoor corrals (0.2 hectare) with numerous perches and structures that provided environmental enrichment and protection from the elements. Subjects received a commercial diet twice daily (Outdoor Monkey Lab Diet, PMI Nutrition Int'l, Brentwood, Missouri), supplemented semiweekly with fresh fruit and vegetables, and water was available ad libitum.
Maternal and infant measures
Mothers and infants were temporarily removed from the social group for milk collection and morphometric measurements at two time points: once when the infants were ∼1 month of age and again when infants were 3–4 months of age. For each mother, we measured weight (kg), abdominal circumference (cm), upper arm circumference (cm), abdominal skin fat folds (mm), and crown-rump length (cm). Body mass index was calculated as maternal weight/crown-rump length2. Maternal age at parturition (mean ± SEM = 8.4 ± 0.6 years; range, 2.9–22.2) and parity (5 ± 0.6 pregnancies; range, 1–18) were available from colony records. For the purposes of these analyses, the female hierarchy was determined for each social group based on colony records of the outcome of dyadic agonistic interactions among females. The hierarchy was divided into thirds for each group, and individuals were classified as high, medium, or low ranking. Individuals that were at the cusp of rank categories were grouped with their matriline. Thirty-eight percent of infants was male (22/58). Infants were weighed at 1 month of age and again between 3 and 4 months of age. Infant growth was calculated as grams of weight gain per day between the two time points. Birth timing was a continuous variable centered on the peak birth date for the outdoor colony at the CNPRC for the 2006 birth season. Birth dates for the subjects of this study ranged from February to August.
Milk collection procedure
Milk was collected at 1 month of infant age and again between 3 and 4 months of infant age. At the first time point, mother–infant pairs were captured in their home corral between 7:45 and 9:00 and separated for transport to temporary indoor housing. Mothers were lightly sedated (5 mg ketamine hydrochloride per kg body mass administered by intramuscular [IM] injection) and placed in mesh jackets (ProMed-Tec, Bellingham, MA) to prevent nursing and to allow milk accumulation for 3.5–4 h. Infants were weighed and then housed in holding cages with their mothers during the period of milk accumulation. Between 11:30 and 13:00, mothers were sedated with ketamine hydrochloride (5–10 mg/kg IM). Nipple areas were cleaned and the surrounding hair was trimmed. Mothers were administered exogenous oxytocin for myoepithelial cell contraction and milk let down [2 IU/kg (0.1 ml/kg) IM]. Milk was collected separately from each mammary by gentle hand stripping of the nipple. To minimize sampling bias (Oftedal, 1984), each mammary was fully evacuated as indicated by the transition from streaming milk to solitary droplets of milk during hand collection. A single researcher (KH) collected all milk samples for the duration of the study using these methods consistently throughout. Full mammary evacuation occurred within 10–15 min for all subjects. The samples were stored frozen at −20°C until milk composition analysis. After the mothers recovered from sedation, mother–infant pairs were returned to their home corrals.
At the second time point (3–4 months of infant age), mother–infant pairs were again captured between 7:45–9:00 and relocated. Milk was collected between 11:30 and 13:00. Milking procedures were identical to the first time point with one exception. Most of the infants (n = 55) were subjects of an on-going biobehavioral assessment program, which required infants to be separated from their mothers for a short period (Capitanio et al., 2005; Capitanio et al., 2006). For these mothers, jacketing during milk accumulation was unnecessary. For three mothers who were not part of the biobehavioral assessment program, the procedure used at the first time period was used at the second time period.
Milk composition analysis and relative milk yield values
Composition analyses of proximate milk constituents were conducted in the Nutrition Laboratory at the Smithsonian National Zoological Park (SNZP) in Washington DC using standard methods (Oftedal and Iverson, 1995; Hinde, 2007b; Milligan et al., 2008; Power et al., 2008). Dry matter was measured gravimetrically; samples were weighed to 0.001 mg, before and after 3 h at 100°C in a forced air-drying oven. The amount of nitrogen in each sample was determined using a CHN elemental gas analyzer (Model 2400, PerkinElmer, Norwalk, CT) using a 2-s oxygen burst to promote complete combustion. Crude protein was estimated as 6.38*nitrogen. To determine fat, total lipids were measured by sequential extractions with ethanol, diethyl ether, and petroleum ether by a micromodification of the Rose-Gottleib procedure. Sugar was assayed by the phenol-sulfuric acid method, using lactose monohydrate as the standard (Dubois et al., 1956; Marier and Boulet, 1959), with the results expressed on an anhydrous lactose basis. Assays were performed in duplicate and checked for agreement. Milk fat, sugar, and protein values were totaled and compared with absolute dry matter value to assess assay accuracy; mean ± SD was 0.97 ± 0.02; range, 0.90–1.04, at both 1 month and 3–4 months of infant age. All milk analysis methods have been validated at the SNZP Nutrition Laboratory using both fresh cow milk and powdered cow milk from the National Institute of Standards and Technology. Milk fat and protein concentration represent the average of right and left mammary for each mother at both time points, and sugar concentration is the value from the left mammary.
GE, also referred to as energy density, was calculated as per Oftedal (1984); 9.11 kcal/g for fat, 3.95 kcal/g for sugars, and 5.86 kcal/g for protein, which allowed for both an estimate of the total energy density of milk (kcal/g) and the percent energy contribution of fat, protein, and sugar. The value for milk GE likely is a slight overestimate, because it does not account for nonprotein nitrogen (Oftedal, 1984), however, assuming similarities in nonprotein nitrogen between humans and nonhuman primates, the estimated inflation is <0.01 kcal/g (Lonnerdal and Atkinson, 1995; Milligan et al., 2008). To verify this method of calculating GE, bomb calorimetry was conducted on a subset of milk samples. These values significantly correlated with GE calculations from proximate assays (r = 0.991, P < 0.0001, N = 10) and were not significantly different (paired sample t test; t = 1.387; P = 0.199). The values of fat, protein, sugar, and GE in the milk were used to calculate the grams of fat, protein, and sugar in 1 kilocalorie of milk for each mother at each time point.
A relative measure of milk production and milk yield value (MYV) was determined as the total weight in grams of the milk sample obtained by mammary evacuation after a standard time of maternal-infant separation (3.5–4 h). This method of estimating relative differences in milk production among individuals has been used for other primates (Ota et al., 1991; Tardif et al., 2001). More precise methods of measuring milk yield include isotope dilution procedures, repeated milking over a defined time period, or weighing infants before and after nursing (reviewed in Oftedal, 1984), but none were considered suitable for rhesus monkeys housed under social conditions that allow for species-typical behavior. The other methods normally require multiple disruptions of the mother–infant dyad and social group and/or require that infants be restricted from access to the food and water that is available for adults. Our measure of MYV was used as an indicator of relative differences in milk production among subjects under standardized conditions but should not be considered an estimate of the absolute or daily milk yield of rhesus macaques. Terms and definitions of milk measures are provided in Table 1.
TABLE 1. Terms and definitions of milk variables.
| Term | Definition |
|---|---|
| Milk composition | Percentage of fat, protein, and sugars in whole milk samples. |
| Gross energy/energy density (GE) | The kilocalories per gram of milk, the “quality” of the milk. |
| Percent energy | The contribution of milk constituents (fat, protein, and sugar) to the gross energy of milk as a percent. |
| Milk yield value (MYV) | The grams of milk that mothers produced in a 3.5–4-h period of time. MYV is a relative measure of the quantity of milk produced among individuals. |
Data analysis
Repeated measures ANCOVA were used to measure within subject changes in milk composition, GE, and MYV between 1 month and 3–4 months of infant age, corrected for the number of days between measures (range, 61–99 days). Multivariate correlation matrices were used to measure relationships among milk constituents and percent energy contributions among milk constituents. We used ANOVA to look at differences in maternal condition between high, middle, and low-ranking mothers. We fitted multiple regression models to the continuous outcome variables of GE, MYV, infant weight, and infant growth. Predictor variables for the regression models of dependant milk variables initially included maternal parity, weight, age, social rank, and birth timing. Maternal weight and parity are correlated (r = 0.5, N = 58, P < 0.0001), and interactions between the variables were included in the regression models. Infant sex has been associated with milk synthesis in this population (Hinde, 2007b) and was included in regression models of milk outcomes. Predictor variables for the regression models of infant weight initially included maternal parity, weight, age, social rank, and birth timing, infant sex, and GE and MYV. Infant age was a covariate in all models. For regression models of infant growth or changes in milk between the two time points, infant age at time point one and the number of days between time points were included as covariates. Final model selection was determined by comparison of models using Akaike's information criterion (AIC) to measure goodness of fit after forward and backward stepwise inclusion of predictor variables (Akaike, 1974). Models presented reflect the end product of this process, and all covariates are listed in text and/or tables. Significance was set at P ≤ 0.05, and statistical tests were conducted in JMP 7.0 (SAS Institute, 2002, Cary, NC).
Results
Maternal condition was highly variable among mothers, but did not change substantially between the two time periods (Table 2). Maternal weight was significantly correlated with the other morphometric measures at each time point (P < 0.001); abdominal circumference (r = 0.92 at 1 month, r = 0.94 at 3–4 months), upper arm circumference (0.78, 0.88), abdominal skin fat fold (0.53, 0.55), and crown-rump length (0.74, 0.72). Because of the high correlation between these parameters, weight was used as the primary maternal morphometric characteristic in the analyses that follow. There was no relationship between social rank and mothers' weight, age, parity, or body mass index (all P > 0.35, adjR2 < 0.01).
TABLE 2. Mean ± SEM for maternal and infant characteristics at one and 3.5 months of infant age (N = 58).
| 1 month | 3.5 months | |||
|---|---|---|---|---|
| Mean ± SE | Range | Mean ± SE | Range | |
| Maternal characteristics | ||||
| Weight (kg) | 8.6 ± 0.2 | 5.4–13.2 | 8.9 ± 0.2 | 5.5–13.7 |
| Upper arm circ (cm) | 17.9 ± 0.2 | 14.2–21.6 | 17.6 ± 0.2 | 14.9–20.8 |
| Abdominal circ (cm) | 41.4 ± 1.0 | 29.4–63.1 | 42.5 ± 1.0 | 29.6–60.7 |
| Abd. skin fat fold (mm) | 7.0 ± 0.3 | 2.9–13.3 | 8.2 ± 0.4 | 3.3–14.1 |
| BMI | 32.3 ± 0.6 | 21.0–44.4 | 32.8 ± 0.7 | 24.5–46.8 |
| Infant characteristics | ||||
| Age (days) | 33.2 ± 0.3 | 29–39 | 103.7 ± 0.9 | 91–118 |
| Weight (kg) | 0.64 ± 0.01 | 0.37–0.85 | 1.0 ± 0.02 | 0.54–1.32 |
There was variation among individuals for all milk constituents at both time periods, but the variation in milk fat concentration among samples from different individuals was most notable (range, 3% to >14%; Table 3). Milk composition changed significantly as infants aged; both milk fat and protein concentrations increased. Because of the increase in fat and protein concentrations, the energy density of the milk (milk GE, kcal/g) increased by 20% on average during this time period. Mean MYVs increased by 50% between the two time points from 11.4 ± 0.6 g to 17.0 ± 1.0 g. The percent energy from fat increased between 1 month and 3–4 months, whereas percent energy from sugar and to a lesser extent protein decreased (Table 4). On a per calorie basis, infants nursing from mothers that produced milk of low energy density received more grams of sugar and fewer grams of fat than did infants nursing from mothers that produced milk high in energy density at both time points (see Fig. 1).
TABLE 3. Mean ± SE for milk composition, gross energy, and milk yield value at one and 3.5 months of infant age (N = 58).
| 1 month | 3.5 months | |||||
|---|---|---|---|---|---|---|
| Mean ± SE | Range | Mean ± SE | Range | t | P | |
| Milk composition | ||||||
| Fat (%) | 4.6 ± 0.2 | 2.2–11.7 | 6.2 ± 0.2 | 3.2–14.2 | 8.32 | <0.0001 |
| Protein (%) | 1.8 ± 0.03 | 1.4–2.5 | 2.1 ± 0.04 | 1.6–3.0 | 7.58 | <0.0001 |
| Sugars (%) | 7.6 ± 0.04 | 6.6–8.2 | 7.5 ± 0.04 | 6.3–8.1 | −3.08 | 0.003 |
| GE (kcal/g) | 0.83 ± 0.02 | 0.6–1.5 | 0.99 ± 0.02 | 0.7–1.7 | 8.72 | <0.0001 |
| Milk yield value (g) | 11.4 ± 0.6 | 3.8–22.6 | 17.0 ± 1.0 | 4.9–33.8 | 8.24 | <0.0001 |
Milk composition, energy density, and yield changed significantly between the two time points.
TABLE 4. Mean ± SE and ranges for the percent energy per kcal/g from fat, protein, and sugar at one and 3.5 months of infant age (N =58).
| 1 month | 3.5 months | |||||
|---|---|---|---|---|---|---|
| Mean ± SE | Range | Mean ± SE | Range | t | P | |
| Energy (%) | ||||||
| Fat | 49.6 ± 0.9 | 39–72 | 56.5 ± 0.8 | 41–75 | 7.8 | <0.0001 |
| Protein | 13.1 ± 0.2 | 10–17 | 12.6 ± 0.2 | 9–15 | −2.4 | 0.02 |
| Sugars | 37.3 ± 0.8 | 18–52 | 30.9 ± 0.7 | 14–43 | −8.6 | <0.0001 |
Percent energy from fat, protein, and sugar changed significantly between the two time points.
Fig. 1.
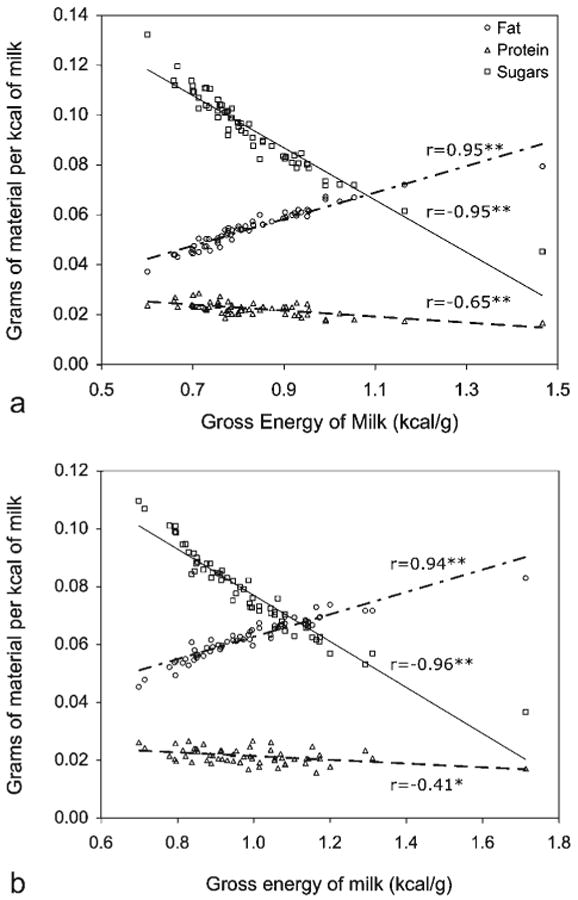
On a per calorie basis, infants nursing from mothers that produced low-energy milk received more grams of sugar and protein and fewer grams of fat than did infants nursing from mothers that produced high-energy milk at 1 month (a) and 3.5 months (b) of infant age. Results remained significant (P < 0.01) even after removing the high GE outlier. *P = 0.001, **P < 0.0001.
Maternal parity influenced MYV, but not milk GE. In the early postnatal period at 1 month of infant age, maternal parity was significantly associated with MYV (Table 5). There was an interaction with maternal weight: less experienced, lower weight mothers produced less milk than predicted by parity alone. At 3–4 months of infant age, maternal parity remained positively associated with MYV (see Fig. 2), controlling for maternal weight, infant age, and sex, but there was no longer a significant interaction between parity and weight. Maternal age, social rank, and birth timing had no significant effects on MYV in either the early postnatal period or at peak lactation. Including these predictors produced higher AIC values and poorly fitted models; however, maternal parity remained significantly associated with MYV even when including these other maternal variables (P < 0.05). None of the maternal characteristics measured in this study were associated with variation in milk GE.
TABLE 5. Multiple regression results for relative milk yield value (MYV) among mothers at 1 month and 3–4 months of infant age (N = 58).
| Out come: MYV (g) | ||||||
|---|---|---|---|---|---|---|
| 1 month | 3–4 months | |||||
| Predictors | β | SE | P | β | SE | P |
| Intercept | −2.5 | 8.6 | – | −2.8 | 16.9 | – |
| Parity | 0.3 | 0.2 | 0.05 | 0.8 | 0.3 | 0.005 |
| Maternal weight (kg) | 0.6 | 0.4 | – | 0.5 | 0.6 | 0.01 |
| Parity × maternal weight (kg) | −0.3 | 0.1 | 0.008 | |||
| Infant | ||||||
| Age | 0.2 | 0.3 | – | 0.1 | 0.1 | – |
| Sex (M) | −0.9 | 0.6 | – | −0.1 | 0.1 | – |
| F5,52 = 5.52, P = 0.0006, adiR2 = 0.27 | F4,53 = 4.44, P < 0.004, adiR2 = 0.19 | |||||
Estimated effects are presented for MYV in grams. For infant sex estimated effects are presented for males.
Fig. 2.
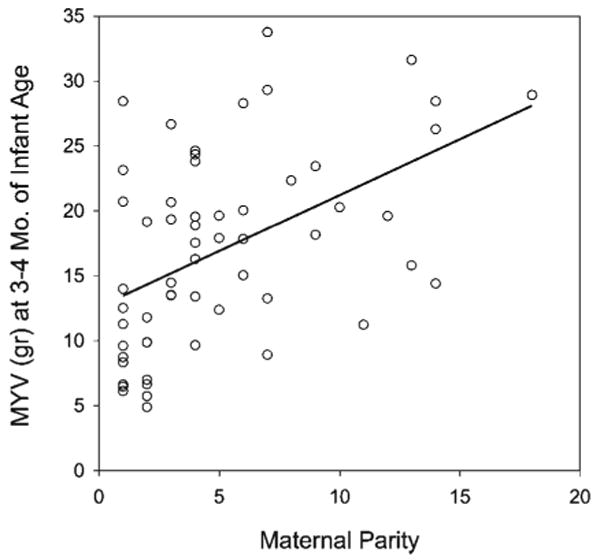
Milk yield value was associated with maternal parity at 3.5 months of infant age even accounting for infant age, sex, and maternal weight.
Although in the majority of mothers, both milk GE and MYV increased as their infants matured, the magnitude of the change in GE was negatively associated with the magnitude of the change in MYV (β ± SE = −17.0 ± 0.4.6, P = 0.0005) even accounting for infant age at the first time point and number of days between samples (Model: F3,54 = 5.9, adjR2 = 0.21, P = 0.002). Mothers whose milk greatly increased in GE had smaller increases or decreases in the amount of milk produced, whereas mothers in which MYV greatly increased had smaller increases in milk GE.
Infants that received more energy from milk, due to higher GE and/or yield, were expected to weigh more and grow faster than infants who received less energy. As expected, MYV was positively associated with infant weight; milk GE was not significantly associated with infant weight, increased AIC values, and was removed from the models. At both time points, infants weighed more if they were older on the date of milk collection, were male, had heavier mothers, and if their mothers produced more milk during the 4-h separation period (Table 6). For each added gram of milk produced by the mother during milk accumulation, infants' weighed an additional 6 g at 1 month of age and 10 g at 3–4 months of age. Maternal parity, age, social rank, and birth timing were not associated with infant weight at either time point. Furthermore, inclusion of these predictors increased AIC values and were not included in the final multiple regression models presented earlier and in Table 6.
TABLE 6. Multiple regression results for infant weight at 1 month and 3–4 months of infant age (N = 58).
| Outcome: infant weight | ||||||
|---|---|---|---|---|---|---|
| 1 month | 3–4 months | |||||
| Predictors | β | SE | P | β | SE | P |
| Intercept | 28.8 | 17.5 | – | 57.9 | 35.9 | – |
| Milk yield value | 6.1 | 2.6 | 0.025 | 9.6 | 2.8 | 0.001 |
| Infant | ||||||
| Sex (male) | 30.6 | 12.0 | 0.014 | 47.8 | 19.9 | 0.02 |
| Age (days) | 11.7 | 5.1 | 0.025 | 6.2 | 3.0 | 0.05 |
| Maternal weight (kg) | 18.7 | 6.9 | 0.009 | 28.8 | 11.9 | 0.02 |
| F4,53 = 8.78, P < 0.0001, adiR2 = 0.35 | F4,53 = 8.08, P < 0.0001, adiR2 = 0.33 | |||||
Estimated effects are presented for infant weight in grams. For infant sex estimated effects are presented for males.
On average, infants were 55% ± 2% heavier at 3–4 months of age than they were at 1 month of age, but some infants' weight increased by <10% and other infants increased by >100%. Infant growth rate was positively associated with the increase in MYV from 1 to 3–4 months (β ± SE = 0.08 ± 0.03, P = 0.03) even accounting for baseline MYV and infant weight at the first time point (Model: F3,54 = 6.52, adjR2 = 0.23, P = 0.0008) (see Fig. 3). Infants gained an additional 0.08 g/day for each additional gram of MYV the mother produced between the two time points.
Fig. 3.
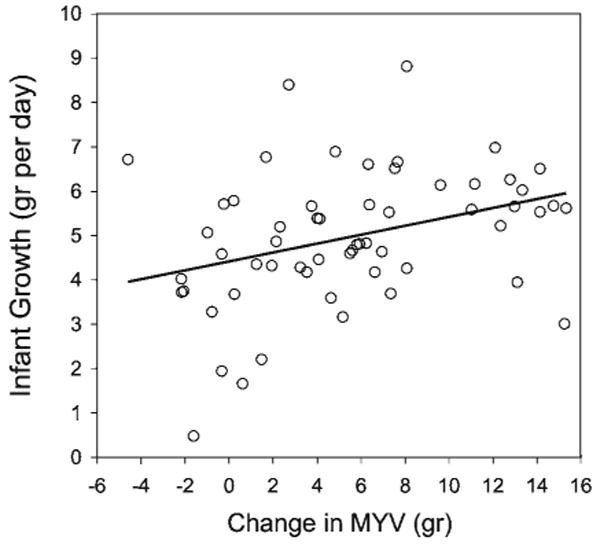
Infant growth rate (grams per day) was positively associated with the increase in milk yield value (MYV) from 1 month to 3.5 months of infant age after controlling for infant age and baseline MYV at 1 month of infant age.
Discussion
The concentration of milk constituents and their relative contribution to milk energy in this population correspond closely to previous descriptions of macaque milk (Lonnerdal et al., 1984; Ota et al., 1991; Hinde, 2007a,b) and fit the dilute milk pattern found broadly among primates (Oftedal and Iverson, 1995). Milk fat concentrations showed the highest interindividual variation and sugar the lowest. As predicted, the energy density of milk increased over lactation as the energetic requirements of infants became greater. This was largely due to significant increases in fat concentration between 1 and 3.5 months.
The relatively low energy contribution from protein (∼13%) in rhesus macaques conforms to predictions that species with slower infant growth rates will have lower protein concentrations in milk (Powers, 1933; Bernhart, 1961; Oftedal, 1986). Energy from protein in milk was lower than that for fast-growing prosimians and New World monkeys but above that of slower-growing humans (reviewed in Power et al., 2008). Although concentration of protein increased between the two time points from 1.8 to 2.1%, the percent energy from protein decreased as a result of the relatively greater increase in energy contribution from fat. This contrasts with the pattern documented in New World monkeys for which the percent energy from protein remains constant during lactation and is independent of the energy density of the milk (Callathrix jacchus, Power et al., 2008; Samiri boliviensis, Milligan et al., 2008). Marmosets and squirrel monkeys have faster growth rates than macaques, and selection may have favored constant levels of energy from protein to sustain infant growth during lactation (Power et al., 2002; Milligan et al., 2008; Power et al., 2008). However, as we have no measure of the variability with which protein was metabolized versus deposited into lean tissue in the infants, the biological relevance of the changes in protein energy over lactation in rhesus macaques remains to be determined.
Our relative measure of milk yield was associated with maternal parity. Under these standard conditions, mothers of higher parities produced more milk than mothers of lower parities in both the early postnatal period and at peak lactation. As there was no effect of parity on milk composition, our data suggest that the infants of mothers with higher parities received more milk nutrients, and hence more energy than infants of mothers with lower parities. This may have important long-term effects, because milk yield was positively associated with infant weight and growth. In semi-free-ranging macaques, infants' weight at one year of age is positively correlated with their weight at eight years of age, and heavier infants have higher reproductive success than lighter males (Bercovitch et al., 2000).
Young macaque mothers initiate reproduction before attaining adult size (Lipkin et al., 2001; Cerroni et al., 2003) and like other young mammalian mothers face tradeoffs between investing in their own growth and reproduction (Clutton-Brock, 1991; Stearns, 1992). Primiparous mothers are especially constrained in their reproductive ability during pregnancy and lactation (reviewed in Robbins et al., 2006). In rodents, primiparous mothers are less efficient in lactational performance than experienced mothers (Künkele and Kenagy, 1997; Künkele, 2000). It is not clear whether these results can be generalized to primates, because small rodents are “income breeders,” dietary energy primarily supports lactation (Jönsson, 1997), whereas primates are characterized by a mixed income/capital strategy, sustaining lactation through body reserves as well as dietary intake and possibly increased metabolic efficiency (Roberts et al., 1985; Nievergelt and Martin, 1999). Primiparous monkey mothers generally have fewer body reserves available to sustain lactation than multiparous mothers. Additionally, infants of primiparous macaque mothers are significantly bigger relative to maternal size at peak lactation than are infants of multiparous mothers (Hinde, 2007b). Gomendio (1989) hypothesized that primiparous mothers nursed more frequently to compensate for poor milk output. Our data suggest that primiparous macaque females may adjust their nursing behavior to offset deficits in the quantity, rather than quality, of milk that they produce for their offspring.
There was a linear increase in our measure of milk yield with increasing parity, not simply a difference between multiparous and primiparous mothers; for each additional parity, mothers produced an extra third of a gram of milk at 1 month and three-fourths of a gram of milk at 3–4 months of infant age. This finding corresponds to predictions derived from the residual reproductive value hypothesis (Williams, 1966; Trivers, 1974; Pianka and Parker, 1975; reviewed in Clutton-Brock, 1991; Silk et al., 1993; Cameron et al., 2000). As females age and their future reproductive value declines, they are expected to gradually increase investment in each infant. Others have argued, however, that reproductive performance improves with maternal experience, because mothers become more physiologically efficient and target care during critical periods of infant development (Fairbanks, 1996; Cameron et al., 2000). Functional development of the mammary occurs during each pregnancy prior to lactation (Akers, 2002), as such individual reproductive history, rather than chronological age, may exert greater influence on lactational performance.
The amount of milk that mothers produced was associated with infant weight, independent of maternal weight, and infant age and sex throughout the study. Mothers that produced greater quantities of milk had heavier infants at both 1 and 3–4 months of age. At 1 month of infant age, infant weight was strongly associated with infant sex and maternal weight, suggesting that infant weight in the early neonatal period may be more influenced by prenatal factors. However, at 3–4 months of age, during peak lactation, milk yield was the most significant predictor of infant weight. The causal processes underlying the relationship between infant weight and milk yield are not well understood. Infants that receive greater amounts of milk may grow more than infants that receive less milk. Alternatively, bigger infants may be able to elicit more milk from their mothers, or there may be a bidirectional feedback system between infant size and milk yield (Ono and Boness, 1996).
Interestingly, mothers in which milk yield increased between 1 month and 3.5 months had a limited increase in milk energy density, suggesting that there is a tradeoff and mothers are unable to substantially increase yield and energy density simultaneously. Nursing behavior may be a contributing factor in this tradeoff. An increase in milk energy density may allow longer periods between nursing bouts as infants more quickly become satiated by high fat concentration milk (Jenson, 1999), whereas increasing the yield but keeping milk dilute may allow the infant to have frequent access to the nipple without depleting the mother. Frequent nursing bouts may keep the infant in closer proximity to the mother, thereby inhibiting risky social situations such as infant kidnapping or aggression directed toward the infant (Gomendio, 1996). More frequent nursing, however, may be costly for the mother by increasing inter-birth intervals (Gomendio, 1989; Johnson et al., 1993) although multiparous mothers are less sensitive to suckling-suppressed fertility (Wilson et al., 1988). There were negative consequences in this study associated with increasing milk energy density at the expense of increasing milk yield. Infants whose mothers increased milk yield over lactation had higher growth rates, whereas limited increases in milk yield were associated with slower rates of infant growth. The results of this study indicate that, for macaques, the amount of milk produced by the mothers is associated with infant weight and growth to a much greater extent than is the GE of milk.
We failed to detect any effect of maternal weight, social rank, or birth timing on milk composition or our measure of milk yield. This may be because the captive animals that we studied were all well nourished, and their food supplies did not vary seasonally. In fact, maternal rank was not associated with differences in maternal weight or adiposity in this sample. Macaques likely use a mixed income/capital lactation strategy relying on food intake and body reserves to sustain lactation depending on environmental and maternal conditions. Body reserves buffer lactation from environmental fluctuations, and for these captive macaques with ample access to food, mothers are less reliant on reserves to sustain lactation and instead rely on an “income” strategy. This may account for the paucity of maternal body condition effects on milk composition in Old World monkeys (baboons; Roberts et al., 1985, macaques; Hinde, 2007a) and humans (reviewed in Prentice et al., 1994; Lonnerdal, 2000; Sellen, 2007). Additionally, the magnitude of variance in captive primates, where the highest milk energy density values are observed, may exceed the variation in wild populations (Power et al., 2008). In the wild, mothers may be food-limited and prevented from producing energetically dense milks, constraining the range of variation in milk composition.
Conclusions
The results of this study indicate that there is a marked variation in milk yield and milk composition among females in a well-fed captive population. This variation has significant effects on infant growth during lactation. Future work should examine the long-term effects of variation in milk quality and quantity on infant survival and behavioral development and assess the impact of lactational investment on mothers' future reproductive performance. To date, the sources of the observed variation in milk composition in this population remain unknown. It is possible that factors not measured in this study, such as the health of the mother, may influence milk production (Hinde, 2007a), but it seems likely that much of the variation in milk composition among mothers reflects underlying genetic or epigenetic factors.
Acknowledgments
The UCLA and UC Davis Institutional Care and Use Committees approved the research protocol for the study. The authors thank John Capitanio, Laura Del Rosso, Brett Farnham, Dale Sussdorf, and Michael Jakubasz for providing integral logistical support and Mark Grote for statistical advice. Joan Silk, Lynn Fairbanks, and two anonymous reviewers provided valuable comments that greatly improved this manuscript. KH thanks her father Jim Hinde.
Grant sponsor: NSF; Grant number: DDIG 0525025 awarded to K.H. and Joan Silk; Grant sponsor: NIH; Grant numbers: RR019970, RR000169 awarded to John Capitanio and the California National Primate Research Center, DK77639 awarded to MLP; Grant sponsor: American Society of Primatologists awarded to K.H.
Literature Cited
- Akaike H. A new look at the statistical model identification. IEEE Trans Automat Control. 1974;19:716–723. [Google Scholar]
- Akers RM. Lactation and the mammary gland. Iowa: Blackwell Publishing; 2002. [Google Scholar]
- Altmann J, Alberts SC. Growth rates in a wild primate population: ecological influences and maternal effects. Behav Ecol Sociobiol. 2005;57:490–501. [Google Scholar]
- Bercovitch FB, Widdig A, Nürnberg P. Maternal investment in rhesus macaques (Macaca mulatta) reproductive costs and consequences of raising sons. Behav Ecol Sociobiol. 2000;48:1–11. [Google Scholar]
- Bernhart FW. Correlation between growth-rate of the suckling of various species and the percentage of total calories from protein in the milk. Nature. 1961;191:358–360. [Google Scholar]
- Bowman JE, Lee PC. Growth and threshold weaning weights among captive rhesus macaques. Am J Phys Anthropol. 1995;96:159–175. doi: 10.1002/ajpa.1330960205. [DOI] [PubMed] [Google Scholar]
- Cameron EZ. Is suckling behavior a useful predictor of milk intake? A review. Anim Behav. 1998;56:521–532. doi: 10.1006/anbe.1998.0793. [DOI] [PubMed] [Google Scholar]
- Cameron EZ, Linklater WL, Stafford KJ, Minot EO. Aging and improving reproductive success in horses: declining residual reproductive value or just older and wiser? Behav Ecol Sociobiol. 2000;47:243–249. [Google Scholar]
- Cameron EZ, Stafford KJ, Linklater WL, Veltman CJ. Suckling behaviour does not measure milk intake in horses. Equus caballus. Anim Behav. 1999;57:673–678. doi: 10.1006/anbe.1998.0997. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mason WA, Mendoza SP, DelRosso L, Roberts JA. Nursery Rearing and biobehavioral organization. In: Sackett GP, Ruppenthal GC, Elias K, editors. Nursury rearing of non-human primates in the 21st century. New York: Springer; 2006. pp. 191–214. [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Dev Psychobiol. 2005;46:318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Cerroni AM, Tomlinson GA, Turnquist JE, Grynpas MD. Effect of parity on bone mineral density in female rhesus macaques from Cayo Santiago. Am J Phys Anthropol. 2003;121:252–268. doi: 10.1002/ajpa.10238. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM, Fischer J, Beehner J, Bergman T, Johnson SE, Kitchen DM, Palombit RA, Rendell D, Silk JB. Factors affecting reproduction and mortality among babbons in the Okavango Delta. Botswana. Int J Primatol. 2004;25:401–428. [Google Scholar]
- Clutton-Brock TH. The evolution of parental care. Princeton, NJ: Princeton University Press; 1991. [Google Scholar]
- Derrickson EM, Jerrard N, Oftedal OT. Milk composition of two precocial, arid-dwelling rodents. Kerodon rupestris and Acomys cahirinus. Physiol Zool. 1996;69:1402–1418. [Google Scholar]
- Dewey KG. Energy and protein requirements during lactation. Annu Rev Nutr. 1997;17:19–36. doi: 10.1146/annurev.nutr.17.1.19. [DOI] [PubMed] [Google Scholar]
- Drickamer L. A 10-year summary of reproductive data for free-ranging Macaca mulatta. Folia Primatol. 1974;21:61–80. doi: 10.1159/000155596. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- Fairbanks LA. Individual differences in maternal style: causes and consequences for mothers and offspring. Adv Study Behav. 1996;25:579–611. [Google Scholar]
- Fairbanks LA, McGuire MT. Maternal condition and the quality of maternal care in vervet monkeys. Behaviour. 1995;132:733–754. [Google Scholar]
- Gjøstein H, Holand Ø, Weladji RB. Milk production and composition in reindeer (Rangifer tarandus): effect of lactation stage. Comp Biochem Physiol A. 2003;137:649–656. doi: 10.1016/j.cbpb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Gomendio M. Suckling behaviour and fertility in rhesus macaques (Macaca mulatta) J Zool Lond. 1989;217:449–467. [Google Scholar]
- Gomendio M. The influence of maternal rank and infant sex on maternal investment trends in rhesus macaques: birth sex ratios, inter-birth intervals and suckling patterns. Behav Ecol Sociobiol. 1990;27:365–375. [Google Scholar]
- Gomendio M. Maternal styles in Old World primates; their adaptive significance. In: Pryce CR, Martin RD, Skuse D, editors. Motherhood in human and non-human primates. Basel: Karger; 1996. pp. 59–68. [Google Scholar]
- Gomez JA, Landete-Castillejos T, Garcia AJ, Gallego L. Effect of calving advance on milk production and composition, and calf growth in Iberian deer (Cervus elaphus hispanicus) Small Rumin Res. 2002;44:213–218. [Google Scholar]
- Hayes LD, Solomon NG. Mechanisms of maternal investment by communal prairie voles. Microtus ocrogaster. Anim Behav. 2006;72:1069–1080. [Google Scholar]
- Hinde K. Milk composition varies in relation to the presence and abundance of Balantidium coli in the mother in captive rhesus macaques (Macaca mulatta) Am J Primatol. 2007a;69:625–634. doi: 10.1002/ajp.20373. [DOI] [PubMed] [Google Scholar]
- Hinde K. First-time macaque mothers bias milk composition in favor of sons. Curr Biol. 2007b;17:R58–R959. doi: 10.1016/j.cub.2007.09.029. [DOI] [PubMed] [Google Scholar]
- Jenson RG. Lipids in human milk. Lipids. 1999;34:1243–1271. doi: 10.1007/s11745-999-0477-2. [DOI] [PubMed] [Google Scholar]
- Johnson SE. Life history and the competitive environment: trajectories of growth, maturation, and reproductive output among chacma baboons. Am J Phys Anthropol. 2003;120:83–98. doi: 10.1002/ajpa.10139. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Berman CM, Malik I. An integrative model of the lactational and environmental control of mating in rhesus monkeys. Anim Behav. 1993;46:63–78. [Google Scholar]
- Johnson RL, Kapsalis E. Determinants of post-natal weight in infant rhesus monkeys: implications for the study of interindividual differences in neonatal growth. Am J Phys Anthropol. 1995;98:343–353. doi: 10.1002/ajpa.1330980307. [DOI] [PubMed] [Google Scholar]
- Jönsson KI. Capital and income breeding as alternative tactics of resource use in reproduction. Oikos. 1997;78:57–66. [Google Scholar]
- Król E, Speakman JR. Limits to sustained energy intake VII: milk energy output in laboratory mice at thermoneutrality. J Exp Biol. 2003;206:4267–4281. doi: 10.1242/jeb.00675. [DOI] [PubMed] [Google Scholar]
- Künkele J. Does primiparity affect the efficiency of converting energy to offspring production in the guinea-pig? Can J Zool. 2000;78:300–306. [Google Scholar]
- Künkele J, Kenagy GJ. Inefficiency of lactation in primiparous rats: the costs of first reproduction. Physiol Biochem Zool. 1997;70:571–577. doi: 10.1086/515862. [DOI] [PubMed] [Google Scholar]
- Landete-Castillejos T, Garcia A, Gallego G. Calf growth in captive Iberian red deer (Cervus elaphus hispanicus): effects of birth date and hind milk production and composition. J Anim Sci. 2001;79:1085–1092. doi: 10.2527/2001.7951085x. [DOI] [PubMed] [Google Scholar]
- Landete-Castillejos T, García A, López-Serrano FR, Gallego L. Maternal quality and differences in milk production and composition for male and female Iberian red deer calves (Cervus elaphus hispanicus) Behav Ecol Sociobiol. 2005;57:267–274. [Google Scholar]
- Lang SLC, Iverson SJ, Bowen WD. Individual variation in milk composition over lactation in harbor seals (Phoca vitulina) and the potential consequences on intermittent attendance. Can J Zool. 2005;83:1525–1531. [Google Scholar]
- Lee PC. The meanings of weaning: growth, lactation, and life history. Evol Anthropol. 1996;5:87–96. [Google Scholar]
- Lipkin EW, Aumann CA, Newell-Morris LL. Evidence for common controls over inheritance of bone quantity and body size from segregation analysis in a pedigreed colony of non-human primates (Macaca nemestrina) Bone. 2001;29:249–257. doi: 10.1016/s8756-3282(01)00508-7. [DOI] [PubMed] [Google Scholar]
- Lonnerdal B. Breast milk: a truly functional food. Nutrition. 2000;16:509–511. doi: 10.1016/s0899-9007(00)00363-4. [DOI] [PubMed] [Google Scholar]
- Lonnerdal B, Atkinson S. Nonprotein nitrogen factors in human milk. In: Jensen RG, editor. Handbook of milk composition. San Diego: Academic Press; 1995. pp. 351–387. [Google Scholar]
- Lonnerdal B, Keen CL, Glazier CE, Anderson J. A longitudinal study of rhesus monkey (Macaca mulatta) milk composition: trace elements, minerals, protein, carbohydrate, and fat. Pediatr Res. 1984;18:911–914. doi: 10.1203/00006450-198409000-00023. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Maternal influences on offspring growth, reproduction, and behavior in primates. In: Maestripieri D, Mateo JM, editors. Maternal effects in mammals. Chicago: The University of Chicago Press; 2009. [Google Scholar]
- Marier JR, Boulet M. Direct analysis of lactose in milk and serum. J Dairy Sci. 1959;42:1390–1391. [Google Scholar]
- McDonald BI, Crocker DE. Physiology and behavior influence lactation efficiency in northern elephant seals (Mirounga angusirostris) Physiol Biochem Zool. 2006;79:484–496. doi: 10.1086/501056. [DOI] [PubMed] [Google Scholar]
- Mellish JE, Iverson SJ, Bowen WD. Variation in milk production and lactation performance in grey seals and consequences for pup growth and weaning characteristics. Physiol Biochem Zool. 1999;72:67–690. doi: 10.1086/316708. [DOI] [PubMed] [Google Scholar]
- Milligan LA, Gibson SV, Williams LE, Power ML. The composition of milk from Bolivian squirrel monkeys (Samiri boliviensis boliviensis) Am J Primatol. 2008;70:35–43. doi: 10.1002/ajp.20453. [DOI] [PubMed] [Google Scholar]
- Mosseau TA, Fox CW. The adaptive significance of maternal effects. Trends Ecol Evol. 1998;13:403–407. doi: 10.1016/s0169-5347(98)01472-4. [DOI] [PubMed] [Google Scholar]
- Motil KJ, Kertz B, Thotathuchery M. Lactational performance of adolescent mothers shows preliminary differences from that of adult women. J Adolesc Health. 1997;20:442–449. doi: 10.1016/S1054-139X(97)00036-0. [DOI] [PubMed] [Google Scholar]
- Nagasawa H, Naito T, Kataoka K. Relationship between milk composition and pup's growth in mice. Proc Soc Exp Biol Med. 1989;191:78–81. doi: 10.3181/00379727-191-42892. [DOI] [PubMed] [Google Scholar]
- Nievergelt CM, Martin RD. Energy intake during reproduction in captive common marmosets (Callithrix jacchus) Physiol Behav. 1999;65:849–854. doi: 10.1016/s0031-9384(98)00249-2. [DOI] [PubMed] [Google Scholar]
- Nommsen LA, Lovelady CA, Heinig MJ, Lönnerdal B, Dewey KG. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: the DARLING Study. Am J Clin Nutr. 1991;53:457–465. doi: 10.1093/ajcn/53.2.457. [DOI] [PubMed] [Google Scholar]
- Oftedal OT. Milk composition, milk yield, and energy output at peak lactation: a comparative review. Symp Zool Soc Lond. 1984;51:33–85. [Google Scholar]
- Oftedal OT. Growth rate and milk composition: a critical appraisal. The breast fed infant: a model for performance. Report of the Ninety-First Ross Conference on Pediatric Research; Columbus, OH: Ross Laboratories; 1986. pp. 50–58. [Google Scholar]
- Oftedal OT, Bowen WD, Boness DJ. Energy transfer by lactating hooded seals and nutrient deposition in their pups during the four days from birth to weaning. Physiol Zool. 1993;66:412–436. [Google Scholar]
- Oftedal OT, Bowen WD, Boness DJ. Lactation performance and nutrient deposition in pups of the harp seal (Phoca groenlandica) on ice floes off southeast Labrador. Physiol Zool. 1996;69:635–657. [Google Scholar]
- Oftedal OT, Iverson SJ. Comparative analysis of nonhuman milks: a phylogenetic variation in the gross composition of milks. In: Jensen RG, editor. Handbook of milk composition. San Diego: Academic Press; 1995. pp. 749–789. [Google Scholar]
- Ono KA, Boness DJ. Sexual dimporphism in sea lion pups: differential maternal investment, or sex-specific differences in energy allocation? Behav Ecol Sociobiol. 1996;38:31–41. [Google Scholar]
- Ota K, Makino Y, Kimura M. Lactation in the Japanese monkey (Macaca fuscata): yield and composition of milk and nipple preference of young. Primates. 1991;32:35–48. [Google Scholar]
- Pianka ER, Parker WS. Age specific reproductive tactics. Am Nat. 1975;109:4453–464. [Google Scholar]
- Power ML, Oftedal OT, Tardiff SD. Does the milk of callitrichid monkeys differ from that of a larger anthropoids? Am J Primatol. 2002;56:117–127. doi: 10.1002/ajp.1068. [DOI] [PubMed] [Google Scholar]
- Power ML, Verona CE, Ruiz-Miranda C, Oftedal OT. The composition of milk from free-living common marmosets (Callithrix jacchus) in Brazil. Am J Primatol. 2008;70:78–83. doi: 10.1002/ajp.20459. [DOI] [PubMed] [Google Scholar]
- Powers GF. The alleged correlation between the rate of growth of the suckling and the composition of the milk of the species. J Pediatr. 1933;3:201–217. [Google Scholar]
- Prentice AM, Goldberg GR, Prentice A. Body mass index and lactation performance. Eur J Clin Nutr. 1994;48:S78–S89. [PubMed] [Google Scholar]
- Räsänen K, Kruuk LEB. Maternal effects and evolution at ecological time-scales. Funct Ecol. 2007;21:408–421. [Google Scholar]
- Robbins AM, Robbins MM, Gerald-Steklis N, Steklis HD. Age-related patterns of reproductive success among female mountain gorillas. Am J Phys Anthropol. 2006;131:511–521. doi: 10.1002/ajpa.20474. [DOI] [PubMed] [Google Scholar]
- Roberts SB, Cole TJ, Coward WA. Lactational performance in relation to energy intake in the baboon. Am J Clin Nutr. 1985;41:1270–1276. doi: 10.1093/ajcn/41.6.1270. [DOI] [PubMed] [Google Scholar]
- Rogowitz GL. Trade-offs in energy allocation during lactation. Am Zool. 1996;36:197–204. [Google Scholar]
- Sellen DW. Infant and young child feeding practices: evolution, recent cross cultural variation and contemporary public health challenges. Annu Rev Nutr. 2007;27:123–148. doi: 10.1146/annurev.nutr.25.050304.092557. [DOI] [PubMed] [Google Scholar]
- Silk JB, Short J, Roberts J, Kusnitz J. Gestation length in rhesus macaques (Macaca mulatta) Int J Primatol. 1993;14:95–104. [Google Scholar]
- Stearns SC. The evolution of life-histories. Oxford: Oxford University Press; 1992. [Google Scholar]
- Tanaka I. Parity related differences in suckling behavior and nipple preference among free-ranging Japanese macaques. Am J Primatol. 1997;42:331–339. doi: 10.1002/(SICI)1098-2345(1997)42:4<331::AID-AJP8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Power ML, Oftedal OT, Power RA, Layne DG. Lactation, maternal behavior and infant growth in common marmoset monkeys (Callithrix jacchus): effects of maternal size and litter size. Behav Ecol Sociobiol. 2001;51:17–25. [Google Scholar]
- Tilden CD, Oftedal OT. Milk composition reflects pattern of maternal care in prosimian primates. Am J Primatol. 1997;41:195–211. doi: 10.1002/(SICI)1098-2345(1997)41:3<195::AID-AJP3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Trivers R. Parent-offspring conflict. Am Zool. 1974;14:249–264. [Google Scholar]
- Wheatley KE, Bradshaw CJA, Davis LS, Harcourt RG, Hindell MA. Influence of maternal mass and condition on energy transfer in Weddell seals. J Anim Ecol. 2006;75:724–733. doi: 10.1111/j.1365-2656.2006.01093.x. [DOI] [PubMed] [Google Scholar]
- Williams GC. Adaptation and natural selection. Princeton, NJ: Princeton University Press; 1966. [Google Scholar]
- Wilson ME, Walker ML, Pope NS, Gordon TP. Prolonged lactational infertility in adolescent rhesus monkeys. Biol Reprod. 1988;38:163–174. doi: 10.1095/biolreprod38.1.163. [DOI] [PubMed] [Google Scholar]


