Abstract
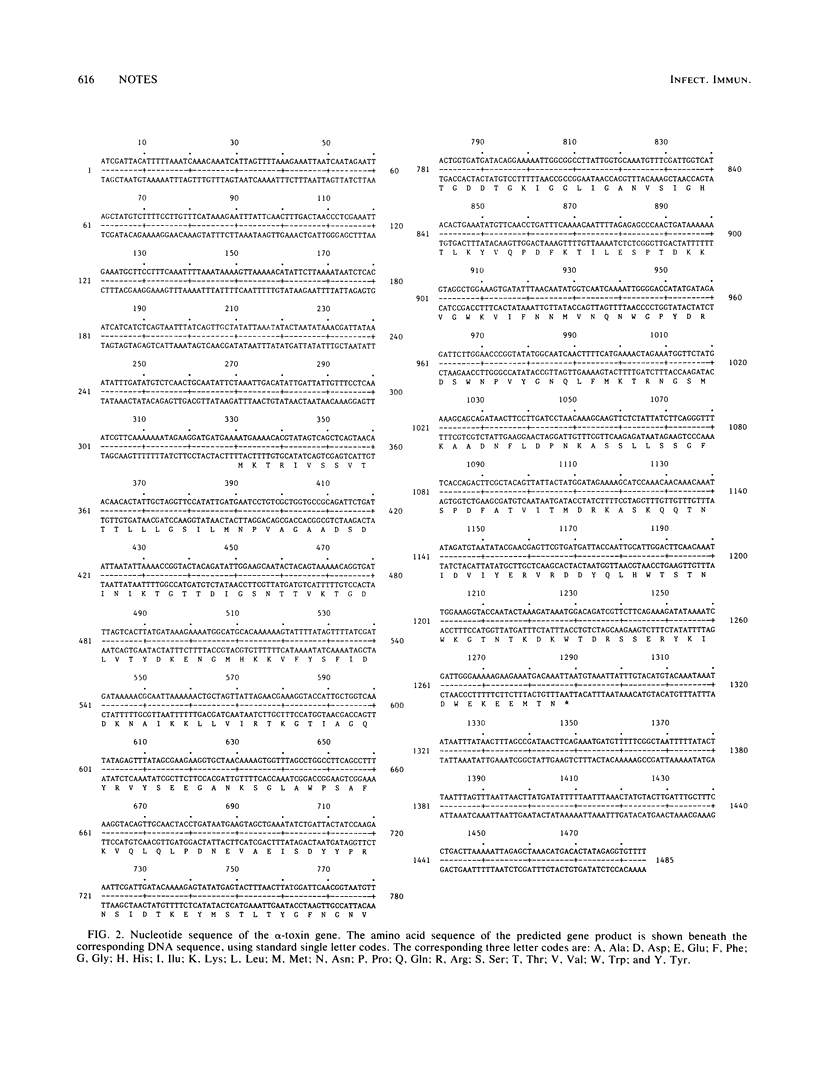
The complete DNA sequence of a cloned alpha-toxin gene from Staphylococcus aureus was determined. The amino acid sequence of the alpha-toxin protein, predicted from the DNA sequence, was described and compared with published data. The primary product of the cloned alpha-toxin gene contained a 26-amino-acid leader sequence which possessed characteristic features of a signal sequence involved in secretion. The mature alpha-toxin protein had a molecular size of 33,000 and contained only three short regions of high hydrophobicity in addition to a number of short, weakly hydrophobic regions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cassidy P., Harshman S. Studies on the binding of staphylococcal 125I-labeled alpha-toxin to rabbit erythrocytes. Biochemistry. 1976 Jun 1;15(11):2348–2355. doi: 10.1021/bi00656a016. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Silhavy T. J. Molecular components of the signal sequence that function in the initiation of protein export. J Cell Biol. 1982 Dec;95(3):689–696. doi: 10.1083/jcb.95.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather N., Kennedy S., Foster T. J., Kehoe M., Dougan G. Expression of a cloned Staphylococcus aureus alpha-hemolysin determinant in Bacillus subtilis and Staphylococcus aureus. Infect Immun. 1983 Sep;41(3):1112–1117. doi: 10.1128/iai.41.3.1112-1117.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer J. H., Arbuthnott J. P., Bernheimer A. W. Interaction of staphylococcal alpha-toxin with artificial and natural membranes. J Bacteriol. 1968 Mar;95(3):1153–1168. doi: 10.1128/jb.95.3.1153-1168.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer J. H., Arbuthnott J. P. Toxins of Staphylococcus aureus. Pharmacol Ther. 1982;19(1):55–106. doi: 10.1016/0163-7258(82)90042-0. [DOI] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Soberon X., Franceschini T., Nakamura K., Itakura K., Inouye M. Role of positive charge on the amino-terminal region of the signal peptide in protein secretion across the membrane. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3438–3441. doi: 10.1073/pnas.79.11.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I., Watanabe M. Chemical studies on staphylococcal alpha-toxin and its fragments. Toxicon. 1980;18(3):361–365. doi: 10.1016/0041-0101(80)90018-5. [DOI] [PubMed] [Google Scholar]
- Kehoe M., Duncan J., Foster T., Fairweather N., Dougan G. Cloning, expression, and mapping of the Staphylococcus aureus alpha-hemolysin determinant in Escherichia coli K-12. Infect Immun. 1983 Sep;41(3):1105–1111. doi: 10.1128/iai.41.3.1105-1111.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Six H. R., Harshman S. Purification and properties of two forms of staphylococcal toxin. Biochemistry. 1973 Jul 3;12(14):2672–2677. doi: 10.1021/bi00738a019. [DOI] [PubMed] [Google Scholar]
- Thelestam M., Jolivet-Reynaud C., Alouf J. E. Photolabeling of staphylococcal alpha-toxin from within rabbit erythrocyte membranes. Biochem Biophys Res Commun. 1983 Mar 16;111(2):444–449. doi: 10.1016/0006-291x(83)90326-1. [DOI] [PubMed] [Google Scholar]
- Tweten R. K., Christianson K. K., Iandolo J. J. Transport and processing of staphylococcal alpha-toxin. J Bacteriol. 1983 Nov;156(2):524–528. doi: 10.1128/jb.156.2.524-528.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Kato I. Purification and some properties of staphylococcal alpha-toxin. Jpn J Exp Med. 1974 Apr;44(2):165–178. [PubMed] [Google Scholar]


