Abstract
Neurotoxicology is entering a new phase in how it views and practices risk assessment. Perhaps more than any of the other disciplines that comprise the science of toxicology, it has been compelled to consider a daunting array of factors other than those directly coupled to chemical and dose, and the age and sex of the subject population. In epidemiological investigations, researchers are increasingly cognizant of the problems introduced by allegedly controlling for variables classified as confounders or covariates. In essence, they reason, the consequence is blurring or even concealing interactions of exposure with modifiers such as the individual’s social ecology. Other researchers question the traditional practice of relying on values such as NOAELs when they are abstracted from a biological entity that in reality represents a multiplicity of intertwined systems. Although neurotoxicologists have come to recognize the complexities of assessing risk in all its dimensions, they still face the challenge of communicating this view to the health professions at large.
Keywords: Chemical intolerance, Early development, Intervention, Lifetime exposure, NOAEL, Risk assessment, Threshold
1. Introduction
Neurological diseases and disorders are rarely unidimensional or unifactorial. Even those whose etiologies seem closely linked to genetic predispositions, such as as autism, tend to be the product of multiple and intertwined risk factors, of which environmental chemical exposures may serve as one component. The list of such factors can be intimidating: age, sex, dietary practices, immune status, and intercurrent disease state comprise only a small portion of a much larger list.
Despite such complexities, which are widely recognized, traditional risk assessment practices manage to elude this reality. They tend to focus instead on exposures to single chemicals in isolation from other risk factors. Animal studies all too often examine the effects of a single chemical as though it were independent of age, sex, and early environment. Epidemiological and clinical studies tend to emphasize the main effects of environmental exposures, stripping away interactions by allegedly controlling for confounders. The result is a wide gulf between current models of diseases and disorders and the actual conditions under which they emerge.
The vulnerability of an organism to disease also depends upon its confrontation with ecological stressors and modifiers. Advances in our ability to interdict threats to neurobehavioral integrity require us to take such modifiers into account in addition to xenobiotic exposures. These modifiers are embedded in the individual’s social ecology, the web of factors such as class status, neighborhood and income that envelops that person in a particular social setting. We already possess substantial evidence of the health impact of single ecological factors. We lack the data required to model the interactions between these risk modifiers and chemical exposures and to translate them into risk policy.
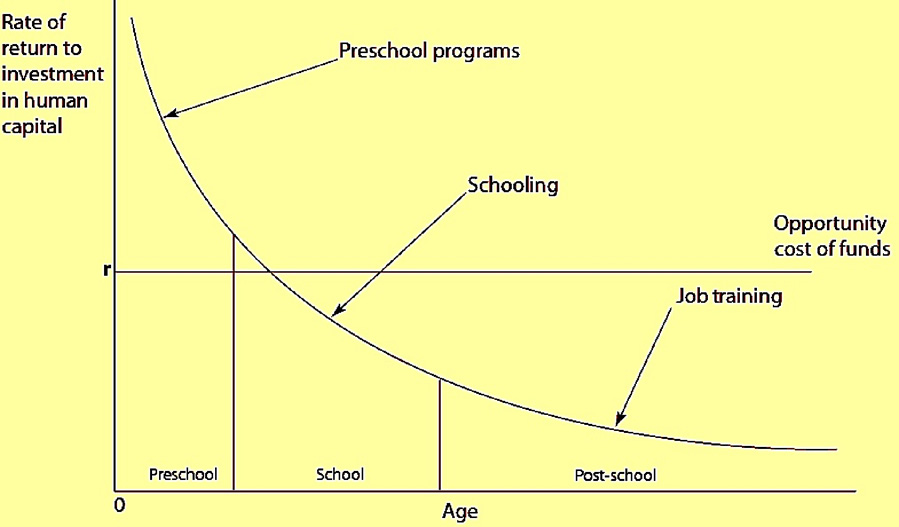
One way to exemplify the challenges is to view the current scene in neurotoxic risk assessment against the wider social ecological background. A useful frame of reference is offered by early education programs such as Head Start. Figure 1 presents the argument by economists such as James Heckman (Cunha, 2005) that the dividends from such an investment are highest when the investments are made during early development. Later investments in education and training, according to calculations from his group at the University of Chicago (Cunha et al, 2005), may fall below the opportunity costs.
Figure 1.
Rates of return to human capital investment initially setting investment to be equal across all ages (Cunha et al, 2005).
Early interventions, according to a now extensive literature, without question provide substantial benefits. Figure 2, based on the Abcedarian project (Barnett and Masse, 2002) testifies to their effectiveness.
Figure 2.
Academic and social benefits at school exit for CPC participants (Cunha et al, 2005).
Such early intervention programs have been aimed primarily at disadvantaged populations. In weighing costs and benefits, they unfortunately overlook an impediment to their success that, if accounted for, might demonstrate an even great scope of benefits. They do not take account of environmental chemical exposures, which almost always are greater in such populations. (Nor, in fact, do the bulk of the numerous studies examining the relationship between health and socioeconomic status).
Toxicology offers a complementary history from the perspective of neurotoxicology. Early developmental effects tended to be ignored until the thalidomide catastrophe (Weiss and Landrigan, 2000). Later, we recognized the cogency of the question posed by David Rall, the second director of NIEHS, at a neurotoxicology meeting in the 1980s: “Suppose that thalidomide, instead of causing the birth of children with missing limbs, had instead reduced their intellectual potential by 10%. Would we be aware, even today, of its toxic potency?” This is the kind of question that has animated developmental neurotoxicology for much of its life.
It also leads us to the presentations given in a session from the 24th International Neurotoxicology Conference held in 2006. It was convened to broadly examine the multifaceted nature of neurotoxic risk. It was structured around a series of four questions formulated to provoke a myriad of positions. Panelists were then requested to respond, on the basis of their own background, to the questions they had been assigned. Their answers represent a sample of the mutiplicity of views among those who practice neurotoxicology in one of its many forms. Rather than melding into a unified but narrow point of view, the respondents offered a personal account of where they see deficiencies in current practice and the direction toward which they believe the discipline needs to move to overcome these deficiencies. They demonstrate the diversity of perspectives reflected in a meeting such as the 24th International Neurotoxicology Conference and offer guidance for future conferences.
The questions and responsed are listed below.
1. We are supposed to be part of a science whose framework was constructed by the mantra that The Dose Makes the Poison. Is it snow time to bid farewell to the NOAEL as it is currently applied?
Deborah C. Rice
There are multiple issues embedded in the question of whether the NOAEL approach should be discarded. As is generally acknowledged, the NOAEL/LOAEL approach is dependent on the choice of doses rather than any real biological threshold. It is also dependent on the number of subjects and the variability of responses, which determines statistical power. This may be particularly relevant in animal studies, in which power is very low and yet only the dose group(s) statistically different from the control group are considered to be affected, even in the presence of an orderly dose-effect function that includes lower doses. The doses identified as a NOAEL or LOAEL are obviously also dependent on the endpoint(s) under consideration. The NOAEL associated with effects of developmental lead exposure may differ by orders of magnitude between studies and for different endpoints, for example (Rice et al., 1996). The issue of relevant endpoint is particularly relevant for endocrine disruptors such as bisphenol A, for which different biochemical mechanisms may be responsible for low-dose effects versus effects on more standard tests of endocrine activity that are observed at much higher doses (vom Saal and Hughes, 2005). One response to the recognition of the limitation of the NOAEL approach is the use of benchmark dose (BMD) analysis in conjunction with modeling of the dose-effect relationship. This allows the use of all the information on the relationship between dose and effect in animal studies, and the relationship between individual body burden and effect in human studies. BMD analyses require explicitly defining an acceptable risk level as well as defining an adverse effect level if using continuous data. In addition, it is typically assumed that the exposure-effect relationship is linear, which of course may not be the case. Rather, the actual shape of the relationship should be determined, as has been performed for methylmercury (Budtz-Jørgensen et al., 2000; Axtell et al., 2000). Another fallacy embedded in the statement that “[t]he dose makes the poison” is failure to take into account potentially sensitive populations. This perhaps most obvious with respect to lifestage, for which there is overwhelming evidence for multiple chemicals that the fetus may be severely and permanently affected at doses or body burdens that have little or no effect on the mother. It is being increasingly recognized, however, that aging, disease states, genetic polymorphisms, and the greater social environment may also result in differential toxicity. The assertion that “[t]he dose makes the poison” is therefore an incomplete characterization of the relationship between exposure and effect, and may be seriously misleading in some circumstances.
Ted Schettler
What kinds of data are risk assessors willing to use, what are the rules of evidence, and what is “the real world”?
We know of many different variables that can influence risk. Some are intrinsic to an individual or community and some are external. Ecologists have been dealing with complexity in natural systems for a long time. When trying to understand system dynamics, establishing and defining system conditions is extremely important.
What happens when we think of the brain and intimately related immune, endocrine, and gastrointestinal systems as an ecosystem—and at times, like a more or less vulnerable ecosystem?
The “state space” of a system is defined by the state variables that constitute the system. It’s a three dimensional space of all the possible combinations of these variables. A “basin of attraction” is a region in state space in which the system tends to remain. For our purposes, we might think of an individual living within a community as a complex system nested within its own complex system—living within a kind of “basin of attraction”.
The trajectory of early development and ongoing functioning will be influenced by both intrinsic and extrinsic variables. An extrinsic factor of particular interest to a risk assessor must be considered within the particulars of the state space of the system and the nature of the basin of attraction.
The preponderance of neurodevelopmental and neurodegenerative disease is a result of complex interactions among numerous variables. To be sure, there are occasions when some single factor is overwhelmingly responsible, but this is generally an exception.
Within individuals there are multiple determinants of risk, and within populations the causes of neurodevelopmental and neurodegenerative conditions are heterogeneous. They include genetic makeup and a wide range of both current and historical variables. Attributes of populations are the context for the distribution of risk factors in individuals within those populations."
Multiple mechanisms and multiple pathogenic pathways are woven together in various combinations to result in similar appearing phenotypes and neuropathology. For example, the pathogenic cascade leading to cell death in Parkinson’s disease can include mitochondrial dysfunction, oxidative stress, excitotoxicity, inflammation, and abnormal degradation of abnormal cellular proteins in the ubiquitin-proteasome system. Combinations of these mechanisms undoubtedly vary from person to person.
The end result of this complexity is marked system-condition heterogeneity—varying from one individual to another AND one community to another, depending also on time and place.
Resilience, adaptability, transformability:
System resilience and adaptability are critical concepts. Resilience is the capacity of a system to absorb disturbance and reorganize while undergoing change so as to retain essentially the same function. In biology this corresponds to a homeostatic response.
Adaptability is the capacity of actors in a system to influence resilience. There are four ways to do this:
move thresholds for transformative change away from the current state of the system. Transformative change means creating a fundamentally new system when current conditions make the existing system untenable. In neurobiology, a transformative change might be acquisition of new system operating conditions that lead inexorably to clinical disease.
move the current state of the system away from the threshold
make the threshold more difficult to reach
manage cross-scale interactions to avoid loss of resilience at the largest and most catastrophic scales.
We can see counterparts of each of these in medicine and public health. Ultimately, when clinical disease does become manifest, system operating conditions may well have undergone transformative change.
In Alzheimer’s disease, for example, it may be unclear whether inflammation is a cause or consequence of the cascade of events seen at the outset or during progression. But there’s no reason why it can’t be both. With changing system conditions, a consequence can become a cause as it is caught up in a positive feedback loop, perpetuating itself and its downstream effects. Now the system has been transformed and is operating in a different “basin of attraction”. Resilience and adaptability may now look very different from the earlier system operating conditions. The old rules may not apply.
This represents the complexity of some neurodegenerative and multi-system diseases of concern. This is the real world. Now, we wonder if we can translate “risk assessment” into this world —to assess the risk of introducing a single external variable into this system complexity and heterogeneity.
If we are to do it at all, we should do it with great humility. And perhaps even with a sense of humor.
Whether risk assessment will be a valid exercise in this complexity will depend on an honest evaluation of existing system conditions. Those conditions will vary and depend on factors intrinsic and extrinsic to the individual and community of interest. NOAELs from traditional animal testing protocols provide only a single datum point among many which are also determinative of outcomes. We should find little comfort in 10-fold safety factors without re-visiting their justification. The “rules of evidence” to be considered by a risk assessor must be re-considered, and the burden should be on the risk assessment community to show transparently how and why specific choices were made—including their simplifications and assumptions.
Donna Mergler
The concepts of LOAEL and NOAEL have been very useful for our scientific understanding of toxic substances and for deriving regulations that limit human exposure to harmful chemicals. Developed at a time when human studies were mostly limited to epidemiologic assessment of the relation between exposure and disease, the animal studies, from which the LOAELs and NOAELs were determined, could target metabolic and behavioral changes with respect to increasing doses of different chemicals. There are, however, many drawbacks to extrapolation to the human situation, amongst which are the following:
Chemicals are mostly examined one at a time, when real life situations involve multiple exposures with substances, both beneficial and harmful, that can influence each other’s absorption, metabolism and/or effect;
Animal models seek to limit genetic heterogeneity, relying on strains of rats, mice or other animals that have been bred specifically for laboratory purposes, whereas humans are characterized by an amazing genetic variability;
Although some studies have demonstrated the importance of stress on laboratory animals’ response to toxic substances, for the most part, the social environment of laboratory animals is limited and can in no way be compared to the complex human social situations, which affects resilience and the capacity to respond to toxic assault;
The consideration of gender and effects throughout the lifespan (and for females, not solely in terms of reproduction) is relatively recent;
The choice of the outcome(s) assessed is not necessarily the most appropriate to humans. A striking anecdote of this can be found in a letter from a worker in the microelectronics industry who had been chronically exposed to hot solvent vapors and, like her co-workers, was suffering from cognitive and sensory deficits, as well severe neuropsychiatric symptoms (Bowler et al, 1991a; Bowler et al, 1991b; Mergler et al, 1991). When informed that rats exposed to this same substance only displayed a slight reduction in grip strength, she stated: “The rat couldn’t speak, but we can.” (Messing and Mergler, 1995)
The area of human toxicology and neurotoxicology has brought to the fore methods for examining early changes in structure and function in human populations exposed to potentially harmful chemicals. The growth and development of this area has been oftentimes painful since existing paradigms about pre-conceived notions of wellbeing and disease and individual and collective health are continually questioned. Studies on early alterations in biological and psychological functioning in human populations have revealed that even small modifications can have important social and public health consequences, as demonstrated by the extensive literature on lead and children’s neurodevelopment (for review see: Toscano and Guilarte, 2005), the growing literature on neurobehavioral effects of mercury exposure (for review see: Mergler et al, 2007), and more recently for manganese, pesticides and some of the emerging chemicals. The study of human populations at different periods throughout the lifespan and through longitudinal cohort studies is increasing our knowledge on the complexity of the relation between exposure and effects and the importance of considering contextual factors (nutritional and health status, housing conditions, exposure profile, cultural practices…) and genetic variability, not only as potential confounders in linear models, but also in interaction with exposure (Bellinger 2001; Grandjean, 2004)
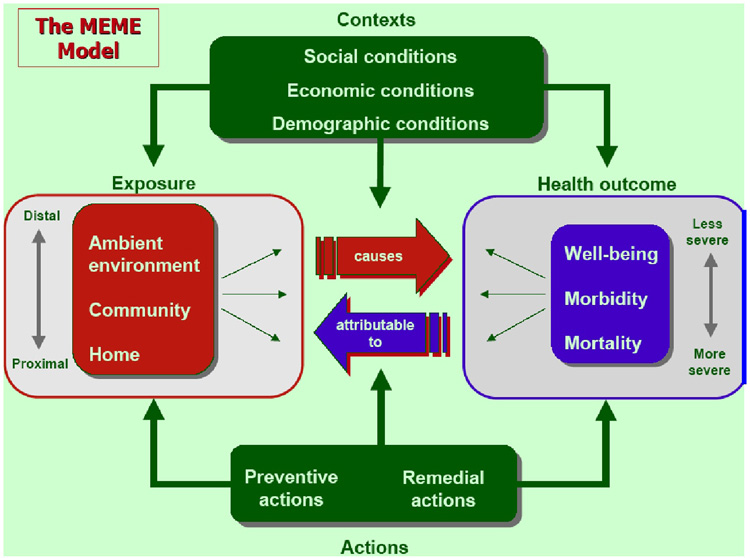
The Multiple Exposure/Multiple Effects (MEME) model that was developed by the Committee on Indicators of Children’s Health of the World Health Organization provides some insight into this complexity (Figure 3). The model illustrates the influence of social, economic and demographic contexts on exposure parameters and health, as well as on the relation between exposure and effects. It incorporates the notions of multiple exposures in the home, community and region and the continuum of effects from well-being to mortality. It does not however include the influence of health on context, whereby persons, whose capacities and health have been diminished by toxic exposure, are less capable of functioning, with possible effects on their social, economic and demographic reality.
Figure 3.
Multiple Exposures/Multiple Effects Model. (from: Briggs, D. 2003. Making a difference: Indicators to improve children’s environmental health. Prepared for the World Health Organization. Geneva, Switzerland: WHO)
There are, of course, many methodological limits to studying the complexity of the human situation, most notably are the difficulties in identifying the relevant variables and having sufficient statistical power through study design or analysis to examine multiple effects and interactions. The challenge to our capacity to study this complexity and reduce the harm caused by exposure to toxic chemicals lies in our ability to carry out collaborative interdisciplinary research, which combines animal and human studies and incorporates the social and natural sciences, as well as the health sciences.
2. We agree that exposures take place in a complex societal setting whose features help determine how the consequences of those exposures will be expressed. So what does all this mean for what is labeled as translational research? Does it mean viewing disease as an astute clinician might see it? Such a clinician would not undertake to diagnose and treat a patient without knowing the patient’s home environment, his or her workplace, dietary practices, family situation, economic status, and other components of the individual’s life. Is our situation parallel?
Claudia S. Miller
Before I became a physician, ultimately becoming board-certified in Internal Medicine and Allergy/Immunology, I was an Industrial Hygienist. I was in the business of assessing risk, and I learned to do it by identifying hazards and quantifying exposures. I was also trained to expect that the dose and target tissue of a toxicant would help predict pathology and to apply common exposure thresholds designed to protect the majority of healthy working males exposed 8 hours a day over a 40-hour workweek. Of course, our understanding of toxicology has matured and yielded paradigm-shifting insights: we now accept that there is no risk-free exposure to mutagens, that some exposure-response curves are U-shaped, and that individual susceptibility to environmental agents can vary by orders of magnitude. Each of these paradigm shifts began with someone’s observation. What follows here is the story of observations made by myself and others about a new pattern of illness and the strong relationship of chronic health problems—ranging from asthma and autoimmune disorders to ADHD and mood disorders—to common environmental exposures. Many of us in environmental health now believe that we have overlooked the most prevalent exposure-related disease mechanism and its attendant health conditions. Although this workshop is entitled “Translating Risk Assessment into the Real World,” risk assessment is, in fact, a two-way street. There is an equally compelling and urgent need for translating real world observations into new risk assessment strategies, including clinical studies that make use of environmentally controlled hospital units.
Darwin said that all science starts with observation.
In the late 1980s, the EPA headquarters building in Washington, DC, underwent remodeling and recarpeting. Hundreds of EPA workers subsequently reported symptoms of "sick building" syndrome. Several dozen employees (some with graduate degrees in environmental health) reported becoming ill and remained ill, with multi-system symptoms and new-onset intolerances for structurally unrelated chemicals, foods, drugs, caffeine and alcohol (Ashford and Miller 1998). The problem didn't fit our existing paradigms for toxicology or allergy.
In 1995, a paper appeared in Archives of Environmental Health describing 75 people who said they became ill—again, with multi-system symptoms and new-onset intolerances—following remodeling of their home or workplace, and another 37 who said they became ill following an organophosphate or carbamate exposure (Miller and Mitzel 1995). Despite having different initial exposures—pesticides and remodeling volatile organic chemicals—both groups reported similar symptoms and intolerances for structurally unrelated substances.
In 1999, a validated questionnaire for assessing chemical intolerance called the QEESI (Quick Environmental Exposure and Sensitivity Inventory) appeared in the journal Toxicology and Industrial Health (Miller and Prihoda 11 1999a),b. It permitted the comparison of symptoms and intolerances of sick Gulf War veterans and people who had become ill following other exposures (indoor air pollutants, pesticides) or after receiving an implant. Subjects reported remarkably similar new-onset intolerances for structurally unrelated chemicals, foods, alcoholic beverages and medications. Since that time, several studies have estimated that this problem affects 4–6% of the US population. These estimates include only those individuals who recognize and report that they have intolerances, and likely miss those who are relatively “masked” (e.g., smokers, heavy fragrance users) (see Figure 4), thus potentially underestimating the problem by several-fold (Ashford and Miller 1998).
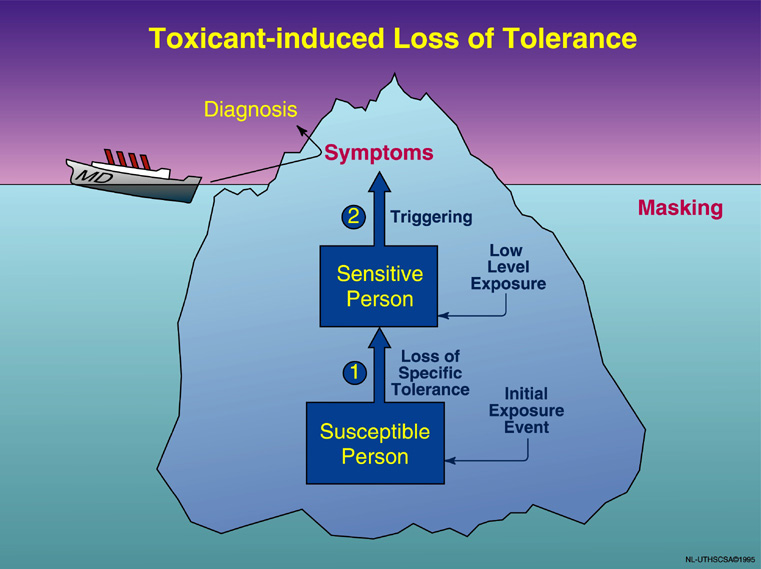
Figure 4.
Phenomenology of TILT. Illness appears to develop in two stages: (1) Initiation, i.e., loss of prior, natural tolerance resulting from an acute or chronic exposure (pesticides, solvents, indoor air contaminants, etc.), followed by (2) triggering of symptoms by small quantities of previously tolerated chemicals (traffic exhaust, fragrances), foods, drugs, and food/drug combinations (alcohol, caffeine). The physician sees only the tip of the iceberg—the patient’s symptoms—and formulates a diagnosis based on them (e.g., asthma, chronic fatigue, migraine headaches). Masking hides the relationship between symptoms and triggers. The initial exposure event causing breakdown in tolerance may go unnoticed (©UTHSCSA 1996).
Following publication of the first edition of the book Chemical Exposures: Low Levels and High Stakes (second edition 1998), the Japanese government decided to study the problem by building the world's first environmentally-controlled medical unit, an EMU. In 2001, delegates sponsored by the National Institute of Environmental Health Sciences (NIEHS) traveled to Japan to view the unit, the first of four EMUs to be built in that country.
In September 2005, the NIEHS and the National Institute of Alcoholism and Alcohol Abuse (NIAAA) co-sponsored a meeting to explore the relationship between chemical intolerance and addiction, and to entertain a new disease paradigm, possibly a new theory of disease, called “toxicant-induced loss of tolerance” or “TILT.” The background white paper for the meeting had appeared in the millennial edition of the journal Addiction, which focused on theories of addiction (Miller 2000).
What is the evidence for this theory?
TILT has been observed by researchers in more than a dozen countries internationally. Their reports are consistent: following an initial exposure event, such as a pesticide spraying, building remodeling or chemical spill, a subset of the exposed individuals report new-onset intolerances, as well as multi-system symptoms that are triggered by previously tolerated chemicals, foods, drugs, alcoholic beverages, caffeine and other structurally unrelated substances.
These observations do not fit the tenets of traditional dose-response toxicology. In his book, The Structure of Scientific Revolutions, Kuhn (1996) wrote that new scientific theories emerge when observations appear that the old, accepted paradigms cannot explain. This leads to a crisis. Consider Darwin's theory and the Copernican theory. We need to understand TILT. As opposed to the way we think of infectious diseases and classical dose-response toxicology, TILT is non-linear—an initiating event occurs, intolerances develop to structurally unrelated substances, and thereafter everyday exposures trigger multi-system symptoms.
Right now, we are at the germ theory stage in terms of our understanding of this paradigm. In the late 1800s, the microscope became the tool that allowed us, for the first time, to "see" the germs that were causing infectious diseases. Koch's postulates assured scientific rigor. If we are to see "chemical and food intolerances, " we need a new tool: environmentally-controlled hospital units that will allow us to temporarily remove persons who are ill from their entire background of exposures, and then observe them for improvement. We need to eliminate background chemical noise. Otherwise, trying to characterize the effects of any particular exposure will be like trying to hear a pin drop in a noisy room. At the same time, we can monitor the patients' physiological responses, assess individual genetic susceptibility by measuring protein expression and markers of inflammation, and do brain scans—all in real time and in response to removal from external exposures along with reintroduction of foods and other everyday exposures, alone or in combination. What could be more scientifically rigorous or useful? It is possible that we could study gene-environment interactions firsthand while people are recovering from their illnesses.
There is evidence to suggest that TILT-related illnesses may be the most prevalent environmentally-induced diseases. What are these conditions? They may be those that have been increasing in prevalence since our personal exposures have increased—with the introduction of synthetic organic chemicals following WWII and with the decrease in fresh air entering our buildings as a result of the Oil Embargo of the mid-1970’s. Asthma, allergies, ADHD, various auto-immune disorders, and depression are conditions whose prevalence appears to have increased over the past several decades. Their relationship to low level exposures remains to be elucidated.
We need to establish hospital-based environmental medical units, modeled after those in Japan, that will allow us to rigorously and scientifically evaluate individuals—to control ALL of their exposures simultaneously and observe for improvement. This approach should work well for many chronic conditions. For example, we could study 30 people with an auto-immune disease such as lupus or 30 people with autism and start to see what role, if any, everyday exposures play in these conditions.
The availability of EMUs in teaching hospitals across the country would make toxicology relevant to physicians, the public and to legislators. Doctors and their patients would see firsthand the connection between chronic illnesses and everyday exposures. People with chronic autoimmune diseases or neurological conditions would be asking to be evaluated in such units, and foundations and the government would be anxious to support such studies of the role of environmental exposures in chronic illnesses. To quantify these risks, we must invest in the necessary facilities, infrastructure, and tools to unravel this new theory of disease. What we learn will inform our risk assessments and yield evidence-based decisions to guide clinical practice and public policy.
3. Conversely, despite the immense volume of literature demonstrating connections between environmental exposures and clinical diseases and disorders, they still are accorded a relatively minor role in diagnosis, treatment, and, especially, prevention. How do we promote awareness of these connections among health professionals and health agencies?
Elise Miller
Though I have been steeped in environmental health science for almost 15 years now, I come to this Roundtable from a different angle since I am neither a scientist nor a health professional. Instead, I am a national non-profit director working with both scientists and health professionals as well as with health-affected organizations and environmental health advocates. Primarily my efforts have been focused on working with researchers to translate the science accurately for lay audiences, including policymakers, and raising awareness among health-affected constituencies, such as those in the learning and developmental disabilities sector.
In regards to the question above, I suggest the following eight actions:
Develop and integrate robust medical and nursing school curricula on environmental health concerns;
Because of the challenges of getting new medical curricula approved and taught, continue to offer trainings around the country for health professionals on these materials and ensure participants receive continuing education credits;
Update and better promote the “Green Handbook,” originally published by the American Academy of Pediatrics, regarding diagnosis, treatment and prevention of environmentally attributable diseases and disabilities;
Publicize the availability of resources of the regional Pediatric Environmental Health Specialty Units to health professionals and concerned parents more widely;
Fund more community based research, so that health professionals can draw on findings in “real world” settings to help with prevention, diagnosis and treatment;
Use scientific consensus statements on environmental contributors to different diseases and disabilities for promoting new medical school departments focused on these issues;
Listen to the health-affected person or consumer who is asking informed questions about possible environmental factors related to health problems and take the time to learn more about these issues from respected and established materials already available on line and in peer-reviewed journals;
Stay alert to (and even engage in) initiatives regionally, nationally and internationally that are changing how health professionals need to respond to this growing understanding of the links between complex environmental factors and chronic disease and disability – namely, those focused on developing and implementing green chemistry, green engineering, cradle-to-cradle design, chemical policy reform, clean production, alternatives assessment, and so forth.
Steven G. Gilbert
There is an immense and growing body of knowledge clearly demonstrating connections between environmental exposures and clinical diseases or disorders. The newly released Scientific Consensus Statement on Environmental Agents Associated with Neurodevelopmental Disorders, developed by the Collaborative on Health and the Environment’s Learning and Developmental Disabilities Initiative (posted at http://www.iceh.org/LDDI.html) is but one example documenting this connection. The challenge is disseminating this information to health professionals and health agencies that are focused on a diagnosis and treatment model rather than one of prevention. There needs to be a paradigm shift from treatment to prevention. The enormous cost of diseases and more subtle disabilities is well documented and clearly argues that investment in prevention can produce a large savings financially and benefit the individual (Landrigan et al. 2002).
A preventive approach requires a fundamental change in policy and decision making. Garrett Hardin pointed out that most problems do not require technological solutions but rather management or policy changes (Hardin 1968). One option is to adopt a new chemical policy based on the precautionary principle (Gilbert 2005b). Currently there is only limited health effects data on the approximately 3,000 chemicals produced or used at over one million pounds per year. This is contrast to the precautionary approach that requires pharmaceutical or biotechnology companies to provide the FDA with extensive data on a drugs effectiveness and safety before marketing. Similar approach could be adopted for industrial chemicals. The European Union has adapted the REACH program (Registration, Evaluation, and Authorization of Chemicals), in part based on the precaution principle, to address the lack of health data on chemicals and protect human health and the environment (see: http://ec.europa.eu/environment/chemicals/reach/reach_intro.htm).
A fundamental change in chemical policy will require wide spread agreement amongst health professionals and health agencies as well as the general public. Achieving these basic changes will require public health researchers to adapt a new perspective their research. Scientists must examine how their data or results could be used to address broader public policy issues. Consideration must be given to who uses the results to address public policy issues? Scientist must take the time to address the ethical and social implication of their work. Translation of the scientific studies must be considered a legitimate and important role on pair with publication of results in prestigious scientific journals. Once this is done scientist can become thoughtful public health advocates and translate their work for the health professional, policy makers, media, students, and the public.
An important aspect of a scientists work must be to translate their research results for the public. This can be promoted at scientific meetings by having more sessions related to the policy implications of current research. Session could also be held on national or state efforts to address individual chemicals or broader chemical policy issues. Mentors can also encourage students to consider the ethical, legal, and social implications of their research. The ELSI specialty section of the Society of Toxicology offers prizes for students that address the ELSI aspect of their work. Below is a list, by no means complete, of the ways scientists, students and public health professionals can reach out to the public (adapted from (Gilbert 2005a)):
Speak to public interests rather than to private or special interests
Recognize the ethical, legal, and social implications of toxicological research
Testify before local, state, national government committees
Meet with local, state, or national government representatives
Meet with and develop relationship with the media
Write review papers or other papers for the lay public
Volunteer to teach in K-12 class rooms or mentor students from K-12
Encourage others to be thoughtful advocates for human and environmental health
Share your knowledge with the public - join, volunteer, or become a member of the board of directors of non-profit organizations associated with human and environmental health
Lecture or discuss sciences and toxicology issues with public and community groups, colleges or technical schools
Join a local speakers bureau
Engage your local public radio station
Contribute to public wiki based web sites such as Toxipedia (www.toxipedia.org) or the encyclopedia of earth (http://www.eoearth.org/)
Clearly these efforts take time away from the primary goals of research and writing scientific papers. Academic institutions must offer significant recognition for service work related to translating research results or helping the public to appreciate science. Business must encourage their scientists to reach out to the public. Scientists and public health professional also have an ethical responsibility to consider the social context of their science and share their knowledge to promote human health and well being. In other words, to be thoughtful public health advocates. Scientists and public health professional must take the time to share their knowledge and promote awareness among health professionals and health agencies that prevention is essential to protect human and environmental health.
4. Finally, how do we embed these new perspectives into our research, especially for those of us whose primary efforts lie in the laboratory? Who can we convince to pay for them?
M. Christopher Newland
A history of neurotoxicology might be framed around the dose response curve. This elegant relationship was implicit in the initial articulation of a quantitative approach to hazard, as captured in the claim that the "dose makes the poison," and it will continue to be important as we study environmental contaminants. Ironically, this statement may also form the basis for some skepticism about whether toxicology could possibly be an interesting thing to do. As it was expressed to me at the beginning of my own career, "Toxicology is nothing but high-dose pharmacology. " Since Paracelcius's dictum was, of course, a statement about high doses, this remark wasn't entirely off base.
Did Paracelsus make us boring? Looking back at the early literature on neurotoxicants one is sometimes struck by the high doses often used. At that time there was an element of truth to the statement about high-dose pharmacology, but just an element and it was a necessary developmental stage. To learn how to grapple with the thorny problems that lie in characterizing low-level exposure, it was necessary to hone our methods and frame our questions using approaches that we were confident in, and big effects are easier to deal with than small ones. However, things become interesting when we recognize that toxicology might better be viewed as "low-dose pharmacology. "
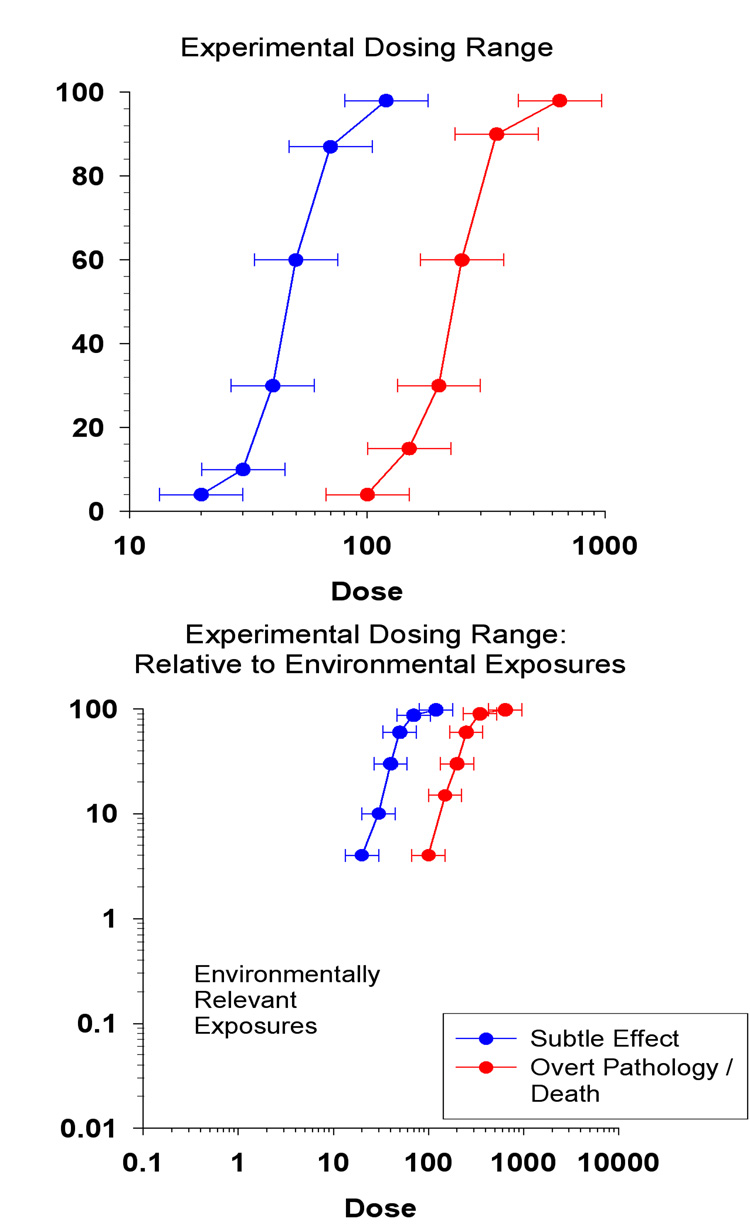
Over the course of a remarkably short span of time, about one career, we have shifted our attention to the low end of the dose response curve. Some of the challenges that await those who would examine this region can be illustrated by figure 5. These idealized does-response relationships illustrate that subtle effects appear at lower doses than more overt effects and that at low doses only very few individuals are affected.
Figure 5.
Representative dose-response curves as seen in two contexts: An experimental context (top) and a public health context (bottom). Ordinate represents percent.
The first is evident in the top panel. In studying the low-end of the dose response curve we may be examining regions in which only 5% of exposed individuals or animals are affected. Following a rule of thumb that five cases are required for statistical stability, that means that to obtain five cases, there must be 100 subjects exposed to that lowest dose, and we cannot know what that dose is or which group will be most susceptible before we design the experiment. These are big experiments that are difficult to do (Gaylor, 1979). An alternative approach is to exploit our growing ability to quantify the shape of the dose-response relationship and examine low-dose effects at the left end without abandoning the curve's empirical foundation (Glowa and MacPhail, 1995, Slikker and Gaylor, 1995).
Even so, an exposure that produces manifestly neurotoxic signs in 5% of the population would be a disaster! Even worse would be a dose that produces more silent, difficult to detect, signs (Weiss, 1996). Lead's effects on measures of cognitive function provide an example of this problem. When Needleman was conducting his initial investigations of lead in school children (Needleman, et al., 1979), national blood lead levels were about15 µg/dl. They have since dropped to 1.9 µg/dl, a reduction that has been estimated to result in a increase in a 7 point increase in IQ (Advisory Committee on Childhood Lead Poisoning and Prevention, 2007). To put this in perspective, if the 1979 lead levels were reinstated the number of children eligible for a diagnosis of mental retardation would more than double and the number of children diagnosed as gifted would drop by more than half.
This leads to the second challenge, which is illustrated in the bottom panel. Even when exploring the left end of the dose-response curve, we often still use laboratory exposures much higher than those experienced by human populations. We cannot always increase our sample size, but lower and lower exposure levels can be investigated by increasing the sensitivity of our endpoints (Weiss, 1988; Weiss and Reuhl, 1994). Here, again, lead also presents a good example. Laboratory studies using the most sensitive indicators also use exposure levels remarkably close to those actually experienced by people (Cory-Slechta, 1990). The result? We are learning that the very nature of these low-dose effects means that other factors, such as diet, age, genetic susceptibility, environmental richness, or other exposures all must play a role.
Do we abandon the LOAEL? No, we allow the concept to evolve. The LOAEL was an attempt to identify a single dose at which an effect can be determined. The thinking behind it, which is that policy should be empirically driven, is admirable and necessary and must not be abandoned. However, it may be time to leave behind the naïve idea that there is a single dose, representing a single endpoint, separating the safe from the hazardous. Did anybody really believe that? Perhaps, then, it is It is time to abandon the LOAEL and learn to identify an interval of susceptibility, where environmental contaminants not only have adverse effects of their own but, perhaps much more important, amplify hazards presented by other important influences.
To amend Paracelsus, neurotoxicology is about low-dose effects. This is good from an array of perspectives, ranging from the mechanistic to the protection of the public health. This means that to study intervals of susceptibility we need to be very good at what we are doing. We can't simply "push the dose" until an effect appears. That's for pharmacologists to do. Instead, when working with low-level exposures we need to "push the assay" so that we are working with doses and effect markers that are environmentally relevant and that address the public health issues of greatest concern.
Acknowledgements
Partial support for Bernard Weiss was provided by NIEHS grants ES013247 and ES015509-02 and Center grant ES01247.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Axtell CD, Cox C, Myers GJ, Davidson PW, Choi A, Cernichiari E, Sloan-Reeves J, Shamlaye C, Clarkson TW. Association between methylmercury from fish consumption and child development at five and a half years of age in the Seychelles child development study: An evaluation of nonlinear relationship. Environ Res Section A. 2000;84:71–80. doi: 10.1006/enrs.2000.4082. [DOI] [PubMed] [Google Scholar]
- Ashford NA, Miller CS. Chemical Exposures: Low Levels and High Stakes. 2nd ed. New York: John Wiley and Sons, Inc; 1998. [Google Scholar]
- Barnett S, Masse LN. A Benefit Cost Analysis of the Abecedarian Early Childhood Intervention. New Brunswick, New Jersey: National Institute for Early Education Research (NIEER); 2002. [Google Scholar]
- Bellinger D. Future directions for neurobehavioral studies of environmenta neurotoxicants. Neurotoxicology. 2001;22:645–656. doi: 10.1016/s0161-813x(01)00036-5. [DOI] [PubMed] [Google Scholar]
- Bowler R, Mergler D, Rauch S, Harrison R, Cone J. Affective and personality disturbance among women former microelectronic workers. J Clin Psych. 1991;47:41–52. doi: 10.1002/1097-4679(199101)47:1<41::aid-jclp2270470107>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Bowler R, Mergler D, Huel G, Harrison R, Cone J. Neuropsychological impairment among former microelectronics assembly workers. Neurotoxicology. 1991;12:87–103. [PubMed] [Google Scholar]
- Briggs D. [accessed January 3, 2008];Geneva, Switzerland: World Health Organization; Making a difference: Indicators to improve children’s environmental health. 2003 http://www.int/ceh/publications/ceh1590599/en/index.html.
- Budtz-Jørgensen E, Grandjean P, Keiding N, White RF, Weihe P. Benchmark dose calculations of methylmercury associated neurobehavioural deficits. Toxicol. Lett. 2000:112–113. 193–199. doi: 10.1016/s0378-4274(99)00283-0. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA. Behavioral measures of Neurotoxicity. Washington, D.C: National Academy Press; 1990. Bridging experimental animal and human behavioral toxicity studies; pp. 137–158. [Google Scholar]
- Cunha F, Heckman JJ, Lochner L, Masterov DV. Interpreting The Evidence On Life Cycle Skill Formation. Cambridge, MA: National Bureau Of Economic Research; 2005. [Google Scholar]
- Gaylor DW. The ED01 study: Summary and conclusions. J Environ Path Toxicol. 1979;3:179–183. [PubMed] [Google Scholar]
- Gilbert SG. Ethical, legal, and social issues: our children's future. Neurotoxicology. 2005a;26:521–530. doi: 10.1016/j.neuro.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Gilbert SG. Public Health and the Precautionary Principle. Northwest Public Health. 2005b [Google Scholar]
- Glowa JR, MacPhail RC. Quantitative approaches to risk assessment in neurotoxicology. In: Chang LW, Slikker W Jr, editors. Neurotoxicology: Approaches and Methods. San Diego: Academic Press; 1995. pp. 777–787. [Google Scholar]
- Grandjean P. Implications of the precautionary principle for primary prevention and research. Annu Rev Public Health. 2004;25:199–223. doi: 10.1146/annurev.publhealth.25.050503.153941. [DOI] [PubMed] [Google Scholar]
- Hardin G. The tragedy of the commons. The population problem has no technical solution; it requires a fundamental extension in morality. Science. 1968;162:1243–1248. [PubMed] [Google Scholar]
- Kuhn TS. The Structure of Scientific Revolutions. 3rd ed. Chicago: University of Chicago Press; 1996. [Google Scholar]
- Landrigan PJ, Schechter CB, Lipton JM, Fahs MC, chwartz J. Environmental pollutants and disease in American children: estimates of morbidity, mortality, and costs for lead poisoning, asthma, cancer, and developmental disabilities. Environ Health Perspect. 2002;110:721–728. doi: 10.1289/ehp.02110721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergler D, Huel G, Bowler R, Harrison R, Cone J. Visual dysfunction among former microelectronics assembly workers. Arch Environ Health. 1991;46:326–334. doi: 10.1080/00039896.1991.9934398. [DOI] [PubMed] [Google Scholar]
- Mergler D, Anderson HA, Chan LH, Mahaffey KR, Murray M, Sakamoto M, Stern AH. The Panel on Health Risks and Toxicological Effects of Methylmercury. Methylmercury exposure and health effects in humans: a worldwide concern. Ambio. 2007;36:3–11. doi: 10.1579/0044-7447(2007)36[3:meahei]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Messing K, Mergler D. The rat couldn’t speak, but we can: inhumanity in occupational health research. In: Hubbard R, Birke L, editors. Reinventing Biology: respect for life and the creation of knowledge. Indianapolis: Indiana University Press; 1995. pp. 21–50. [Google Scholar]
- Miller CS. Toxicant induced loss of tolerance: mechanisms of action of addictive stimuli. Addiction. 2000;96:115–139. doi: 10.1046/j.1360-0443.2001.9611159.x. [DOI] [PubMed] [Google Scholar]
- Miller CS, Mitzel HC. Chemical sensitivity attributed to pesticide exposure versus remodeling. Arch Environ Health. 1995;50:119–128. doi: 10.1080/00039896.1995.9940889. [DOI] [PubMed] [Google Scholar]
- Miller CS, Prihoda TJ. The Environmental Exposure and Sensitivity Inventory (EESI): a standardized approach for measuring chemical intolerances for research and clinical applications. Toxicol Ind Health. 1999a;15:370–385. doi: 10.1177/074823379901500311. [DOI] [PubMed] [Google Scholar]
- Miller CS, Prihoda TJ. A controlled comparison of symptoms and chemical intolerances reported by Gulf War veterans, implant recipients and persons with multiple chemical sensitivity. Toxicol Ind Health. 1999b;15:386–397. doi: 10.1177/074823379901500312. [DOI] [PubMed] [Google Scholar]
- Rice DC, de Duffard AME, Duffard R, Iregren A, Satoh H, Watanabe C. Lessons from neurotoxicology from selected model compounds: SGMOSEC joint report. Environ Health Perspect. 1996;104:205–215. doi: 10.1289/ehp.96104s2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman HL, Gunnoe C, Leviton A, Reed R, Peresie H, Maher C, et al. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. New Eng J Med. 1979;300:689–695. doi: 10.1056/NEJM197903293001301. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Gaylor DW. Concepts on quantitative risk assessment of neurotoxicants. In: Chang LW, Slikker W Jr, editors. Neurotoxicology: Approaches and Methods. San Diego: Academic Press; 1995. pp. 771–776. [Google Scholar]
- Toscano CD, Guilarte TR. Lead neurotoxicity: from exposure to molecular effects. Brain Res Brain Res Rev. 2005;49:529–554. doi: 10.1016/j.brainresrev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. An extensive new literature concerning low dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. Neurobehavioral toxicity as a basis for risk assessment. Trends in Pharmacol Sciences. 1988;9:59–62. doi: 10.1016/0165-6147(88)90118-6. [DOI] [PubMed] [Google Scholar]
- Weiss B. Long ago and far away: A retrospective on the implications of Minamata. Neurotoxicology. 1996;17:257–263. [PubMed] [Google Scholar]
- Weiss B, Reuhl K. Delayed neurotoxicity: A silent toxicity. In: Chang LW, editor. Principles of Neurotoxicology. New York: Marcel Dekker; 1994. pp. 765–784. [Google Scholar]
- Willet WC. Balancing life style and genomics research for disease prevention. Science. 2002;296:695–698. doi: 10.1126/science.1071055. [DOI] [PubMed] [Google Scholar]