Summary
Why do vertebrates use rods and cones that hyperpolarize, when in insect eyes a single depolarizing photoreceptor can function at all light levels [1, 2]? We answer this question at least in part with a comprehensive assessment of ATP consumption for mammalian rods from voltages and currents and recently published physiological and biochemical data. In darkness, rods consume 108 ATP s−1, about the same as Drosophila photoreceptors [3]. Ion fluxes associated with phototransduction and synaptic transmission dominate; as in CNS [4], the contribution of enzymes of the second-messenger cascade is surprisingly small. Suppression of rod responses in daylight closes light-gated channels and reduces total energy consumption by >75%, but in Drosophila light opens channels and increases consumption five-fold [5]. Rods therefore provide an energy-efficient mechanism for high-sensitivity vision in dim light not present in rhabdomeric photoreceptors. Rods are metabolically less “costly” than cones, since cones do not saturate in bright light [6, 7] and use more ATP s−1 for transducin activation [8] and rhodopsin phosphorylation [9]. This helps to explain why the vertebrate retina is duplex, and why some diurnal animals like primates have a small number of cones, concentrated in a region of high acuity.
Keywords: rod, photoreceptor, ATP, energy, metabolism, retina, retinal degeneration
Results and Discussion
Outer Segment
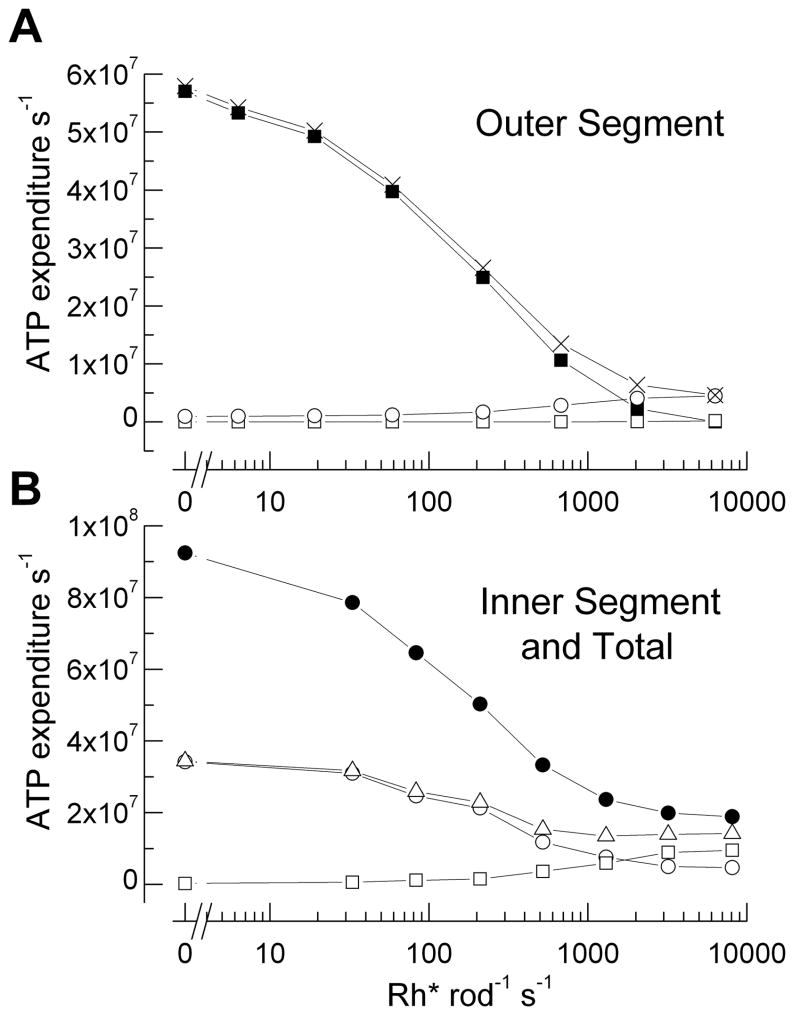
Vertebrate rods and cones have two distinct regions, an outer segment containing the enzymes and channels of the phototransduction second-messenger cascade and an inner segment with mitochondria, ion pumps, nucleus, and presynaptic terminal. In the outer segment, the principal contributors to ATP consumption are the Na+ influx through cGMP-gated channels and enzymatic processes necessary for signal transduction. To calculate the energy required to pump out Na+ entering through the channels, which must be removed to keep the cell at steady-state, we assumed a normal dark resting current of 25 pA; mouse rod responses in excess of 20 pA are routinely observed in our laboratories. Since approximately 7% of the current is from Na+/Ca2+ exchange, we could directly estimate the Na+ influx in darkness (see Supplementary Material). We then divided by 3 to calculate ATP consumption by the Na+/K+ pump, as 3 Na+ ions are pumped out of the rod for every ATP. The dependence of ATP consumption on light intensity over the physiological range was evaluated from measurements of mouse rod current responses to steady illumination [10] and are shown in Fig. 1A. ATP utilization falls by an amount equivalent to 2.3 × 106 ATP s−1 per pA decrease in inward current, from about 5.7 × 107 ATP in darkness to zero at an intensity of about 104 Rh* s−1, which closes all the cGMP-gated channels.
Figure 1.
Principal contributors to ATP consumption in mammalian rod over the physiological range of steady light intensities. (A) Outer segment. ATP required for extrusion of Na+ entering cGMP-gated channels (■), transducin GTP hydrolysis and rhodopsin phosphorylation (□), synthesis of cGMP (○), and sum of all of these processes (X). (B) Inner segment and total rod ATP consumption. ATP required for extrusion of Na+ entering through ih channels (□), extrusion of Ca2+ entering voltage-gated channels at synaptic terminal (○), sum of ATP for Na+ and Ca2+ extrusion (△), and sum of ATP turnover in whole rod (●). For the global sum, we used light intensities at which voltage responses had been recorded (Fig. 2B) and estimated photocurrents by interpolation from step response-intensity data in Woodruff et al. [10]. See text and Supplemental Material for details of calculations.
For enzymatic components, photoexcited rhodopsin (Rh*) produces the exchange of GTP for GDP on the α subunit of transducin (Tα); the GTP is hydrolyzed by the GTPase activity of Tα in conjunction with the proteins of the GAP complex [see 11]. Although it was once thought that a single Rh* could produce as many as 500 Tα-GTP molecules during its lifetime, more recent measurements indicate that, in vivo, this number is closer to 20 in a dark-adapted mouse rod [12]. ATP is also required to phosphorylate rhodopsin. Though under certain conditions as many as 6–7 phosphate groups can be attached to the rhodopsin molecule [13], under most conditions many fewer sites appear to be phosphorylated, probably no more than 3 [14, 15]. Reduction of all-trans retinal to all-trans retinol and regeneration of 11-cis retinal in the retinal pigment epithelium would require another 2–3 ATP molecules. Altogether, we therefore estimated the total number of ATP’s required for transducin activation and response termination by multiplying the number of Rh* by 25 (Fig. 1A). These are probably overestimates in bright illumination, since the rate of rhodopsin kinase is apparently accelerated in light [16], and this would reduce the number of TαGTP’s formed per Rh*.
The ATP required for cGMP synthesis as a function of light intensity was calculated (see Supplemental Material) from the dependence of the cGMP cyclase on Ca2+ concentration [17], the maximal cyclase activity of mouse retina [18], and the free-Ca2+ concentration in the mouse rod outer segment [19]. We assumed that in steady illumination the rod free-Ca2+ concentration scaled with the value of the outer segment current [20]. Fig. 1A gives the ATP required for cGMP synthesis as a function of light intensity, as well as the total ATP consumption of transduction in the outer segment, which closely follows that required for the influx of Na+ except at the brightest intensities. Thus in darkness [21, 22] and over the physiological operating range of the rod, the extrusion of Na+ dominates the consumption of ATP required for the outer segment, while the contribution from the biochemistry of signal transduction is unexpectedly small.
Inner Segment
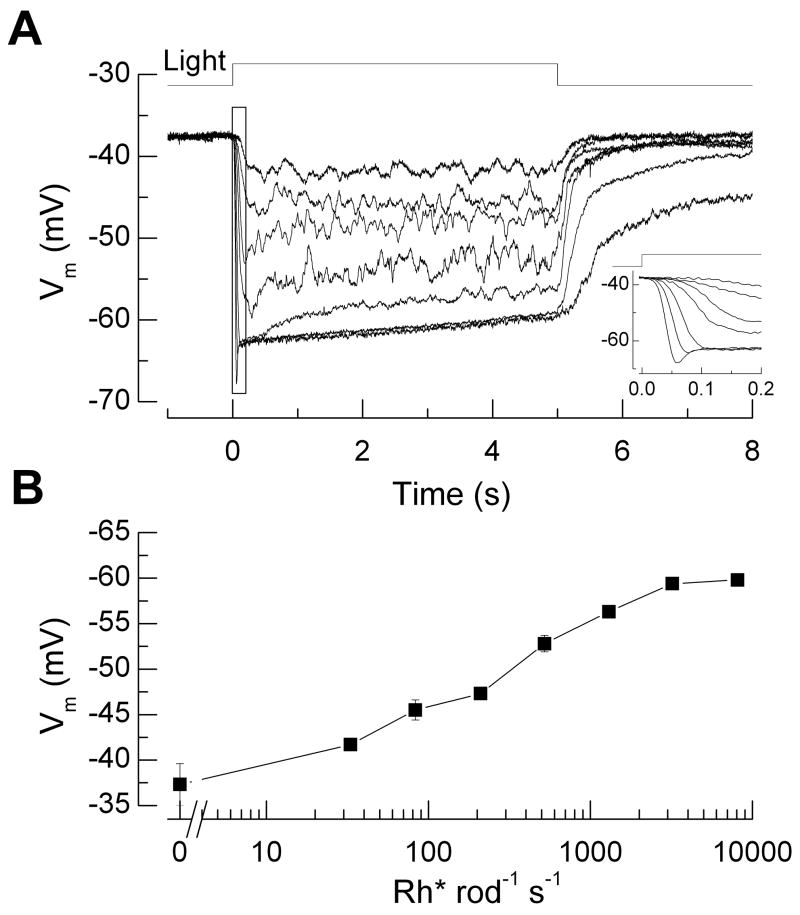
Since the major contributors to ATP consumption in the inner segment are voltage-gated influxes of Na+ and Ca2+, which must be pumped out to achieve homeostasis, we needed first to know how steady light affects the mammalian rod membrane potential. No measurements of this kind had previously been made, so we performed perforated-patch recordings to measure voltage responses to light steps from mouse rods in retinal slices (see legend to Fig. 2). We used 5 s light steps, the longest we could employ and obtain reliable measurements, since total recording time lasted only a few minutes before the pipette went whole-cell. The voltage hyperpolarized with increasing light intensity from a dark resting potential of −37.3 ± 2.3 mV (Fig. 2A), with the waveform in bright light showing a characteristic rapid relaxation or “nose”, caused by activation of a Na+/K+ current activated by hyperpolarization, usually referred to as ih (Fig. 2A, insert). These results are broadly consistent with previous measurements from amphibians [for example, 23]. In Fig. 2B we give the mean value of the membrane potential averaged from 4.5 to 5 s after the beginning of the light step. Measurements at the brightest light intensities may overestimate the amplitude of the voltage change, since the membrane potential appeared still to be gradually changing between 4.5 and 5 s. As we shall argue below, the effect of this error on the calculation of ATP utilization is likely to be small.
Figure 2.
Perforated-patch current-clamp recordings of membrane potential from mouse rod photoreceptors in dark-adapted retinal slices. (A) Response waveforms to 5 s light steps of intensities 33, 83, 210, 520, 1300, 3200, and 8100 Rh* rod−1 s−1. Data from 8 cells were averaged individually for each background light intensity and were corrected for a measured liquid junction potential of ~10 mV. Average input resistance was 5 GΩ and average access resistance, 300 MΩ. Inset: Same responses at higher temporal resolution showing rapid relaxation or “nose” in voltage waveform at high light intensities caused by activation of ih. (B) Response-intensity curve of voltage response, averaged during the interval 4.5–5 s from the responses in A, as function of steady light intensity. Methods: Experiments were conducted in accordance with protocols approved by institutional IACUC committees. Light-evoked membrane potential changes during current-clamp recordings were measured in response to background light steps of 5 sec duration delivered from an LED (λmax ~ 470 nm). To estimate the number of rhodopsin molecules activated per flash, we measured the light intensity of a 520 μm spot focused on the slice preparation by the 20X 0.75NA (Nikon) condenser objective using a calibrated photodiode (United Detector Technologies, San Diego, CA). Light intensities were converted to equivalent 501 nm photons by convolving the power-scaled spectral output of the LED with the normalized spectral sensitivity curve for mouse rhodopsin. These were then converted to Rh* s−1 by estimating the collecting area of rod photoreceptors in the experimental setup. Dim flashes were delivered during suction electrode recordings from rod outer segments in clusters [42], and the mean Rh* per rod was determined from the scaling of the time-dependent variance to the mean response [43]. Based on these factors we estimated the rod collecting area in the experimental setup to be 0.18 μm2 (n = 6 rods).
We combined these measurements of voltage with previous determinations of mean conductance and voltage-dependence of ih in guinea-pig rods [24] to calculate the current through the ih channels (see Supplementary Material). To calculate the value of the Na+ influx, we used the GHK current equation with PNa/PK of 0.3 [24] and assumed values of [Na+]i of 30 mM and [K+]i of 100 mM. The voltages in Fig. 2B were then used to calculate the Na+ influx through ih channels as a function of light intensity, which again was divided by three from the stoichiometry of the pump to give ATP consumption (Fig. 1B). A similar strategy was used to calculate the value of the Ca2+ influx through voltage-gated Ca2+ channels at the synaptic terminal from measurements of mouse rod Ca2+ currents [25]. We assumed that the currents inactivate as in salamander [26], but since Ca2+ currents decrease with hyperpolarization, the exact assumptions we made about activation or inactivation made very little difference to the ratio of total rod ATP consumption in darkness and in light. Currents were divided by 2 since Ca2+ is divalent, and since one Ca2+ is pumped by the Ca2+ ATPase per ATP expended (Fig. 1B). Fig. 1B also gives the sum of the contributions from ih and the Ca2+ and the total ATP consumption summed from processes in inner and outer segments.
In addition to ih and the Ca2+ conductance, mammalian photoreceptors have an outwardly rectifying K+ current [24, 27, 28] and a Ca2+-dependent Cl− current [27, 28], which we have not considered in our calculations. Both are likely to be small over the physiological range, and since both are activated by depolarization and therefore decreased with hyperpolarization, they would only increase the light-dark difference in rod ATP consumption. ATP required for the synthesis of glutamate and recycling into synaptic vesicles would also be larger in darkness, when the vesicles are being released, than in the light. Even in darkness each rod releases only a few hundred vesicles per second [29] requiring a total of no more than 2–4 × 106 ATP s−1 [4], a small fraction of the ATP needed for ion pumping. ATP required for protein synthesis and macromolecular turnover is similarly small [4, 21] and is unlikely to be much different in light and dark.
ATP Consumption in Darkness and Saturating Illumination
The results in Fig. 1 show that light produces a large decrease in ATP consumption due mostly to the decrease in ion influx through the cGMP-gated and Ca2+ channels, which is not compensated by the ATP required for the biochemistry of transduction. This conclusion is little affected by small errors in our measurements of membrane potential in Fig. 2B, since depolarization opens Ca2+ channels but reduces ih, producing compensating effects on ATP consumption. Our conclusion is also robust even to large changes in our assumptions: the gain of transduction or the mean value of conductance of ih could be increased by a factor of 3 with only a modest change in the light-dark difference.
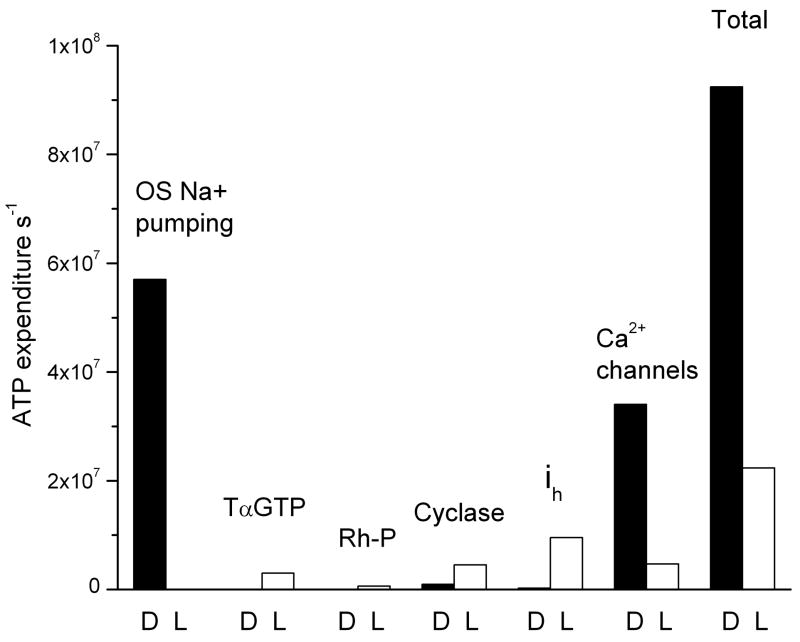
If the light were made sufficiently bright, would further activation of transducin and phosphorylation of rhodopsin alter our conclusion? We calculated maximum and minimum rates of ATP consumption in darkness and bright light for all of the major components of rod energy utilization (Fig. 3). We could take values for outer segment Na+ influx, voltage-dependent currents, and cGMP cyclase directly from Fig. 1, since at 104 Rh* s−1, the current, membrane potential, and Ca2+ concentration have all reached limiting values that do not change when the light is made even brighter. In the phototransduction cascade, however, ATP consumption will continue to increase for light levels that exceed those required to close all the cGMP-gated channels. We therefore calculated the maximum possible rate of ATP consumption in the following way. We took the turnover number of the GTPase activity as 10 s−1 based the rod limiting time constant of about 100 ms in bright backgrounds [10]. The concentration of transducin is of the order of one-twelfth that of rhodopsin [30] or about 0.25 mM. ATP utilization could therefore not exceed 2.5 mM s−1 or 1.7 × 107 ATP s−1. But since above a light intensity of about 5000 Rh* s−1, up to 80% of the transducin migrates to the inner segment [31], the ATP consumption is unlikely to exceed 3 × 106 ATP s−1. To this would be added the ATP used to phosphorylate rhodopsin. Since the kinase concentration of an outer segment is about 30 μM and its turnover number 3 ATP s−1, the maximum rate of ATP consumption for phosphorylation is only about 100 μM s−1 or 6 × 105 ATP s−1 [15]. When all of these values are summed, the total energy consumption of the rod declines from about 108 ATP s−1 in darkness to less than a quarter of this value in bright light (Fig. 3).
Figure 3.
Maximal and minimal ATP expenditure required for the biochemistry of the transduction cascade and by ion extrusion in a mouse rod in darkness and in bright illumination. The letters D and L indicate darkness and bright light. See text.
Rods and retinal oxygen consumption
Rods use most of their energy to pump ions and maintain ion gradients with relatively little used for second-messenger cascades, in agreement with previous inferences for retina [22] and central nervous system [4]. Photoreceptors are normally dependent on oxidative metabolism [22], and since one O2 is required for 6 ATP’s, and rods represent about half of the wet weight of an adult mouse retina of about 10 mg (A. Ruiz and D. Bok, personal communication), our value of 108 ATP s−1 is equivalent to 5.5 ml O2 min-1 per 100 g wt tissue weight at 37°C, in good agreement with measurements from a variety of mammalian retinas in darkness [32]. The rod specific metabolic rate of 13 μmole ATP min−1 g−1 in darkness is less than half that of a cortical neuron [4]. In bright light, retinal O2 consumption decreases by 40%–60%[21, 32, 33]; our calculations indicate that this is mostly due to the drop in ATP consumption by the rods but is not as large as Fig. 3 would predict, probably because the O2 measurements are contaminated by cones and other retinal cells.
It is unlikely that this modest increase in PO2 is harmful to the cell even when the illumination is maintained for long periods. The value of 20 PO2 mmHg found in the vicinity of the rod mitochondria in bright light is similar to the value measured by Linsenmeier and Braun [33] in both darkness and light in the inner retina of the cat. Since the cells of the inner retina are exposed to an oxygen tension of about 20 mm Hg continuously during the life of the organism and remain unaffected, it is unlikely that the increase in oxygen tension caused by exposing the rods to steady light would be deleterious. This would argue against the notion [34] that continuous stimulation of the rods by real or equivalent light can produce deleteriously elevated O2 in the outer retina and lead to cell death. Larger levels of PO2 could however be produced by the death of a significant percentage of the rods during inherited retinal degeneration, since much of the ATP and O2 consumption in the outer retina would be lost. It is possible that this would accelerate the rate of degeneration of the remaining photoreceptors [34].
Rods versus cones in the duplex retina
The large light-dependent decrease in energy consumption for the rod is unlikely to occur also in a cone. In darkness, a cone’s ATP expenditure should be similar to a rod’s, since mammalian rods and cones have similar dark currents [35] and a similar amplitude and voltage-dependence of inner segment Ca2+ current [27, 36]. However, in bright light, a cone will use much more ATP than a rod. In cones, the influx of Na+ through the cGMP-gated channels never falls further than about half that in darkness even in the brightest bleaching intensity [see for example 6, 7]; moreover, the turnover number of transducin is at least 2X greater [8], and the activity of rhodopsin kinase is significantly higher [9]. Though we cannot give a complete accounting, the ATP required in bright light in a cone is likely to be at least as large as that required in darkness and much higher than in a rod in the light.
Cones therefore impose a higher metabolic “cost” than rods, which the animal would do well to reduce. In many mammals, rods greatly outnumber cones, so that exposure to bright steady light produces a net decrease in retinal ATP and O2 utilization [21, 32, 33]. Thus, for nocturnal animals energy utilization by the retina is highest in darkness when the animal is active, but it is considerably less during the day when the animal is quiescent. Many animals that are mostly diurnal, such as primates, also have rod-dominated retinas, and this places little extra metabolic demand on the organism, since rods and cones expend nearly the same amount of ATP in darkness, but in the light the rods are “cheaper”. As a rule, it should be more economical to limit the number of cones and to place them in a specialized region of high acuity like the fovea or visual streak.
Energy consumption by ciliary and rhabdomeric photoreceptors
Since rods and cones hyperpolarize in response to light by closing channels but invertebrate rhabdomeric photoreceptors depolarize by opening them, rods might be expected to consume more energy in darkness. Our calculations indicate, however, that both rods and Drosophila melanogaster photoreceptors have nearly the same rate of about 108 ATP consumed s−1 [3, 5] and apparently for the same reason: both have a large resting membrane conductance in part to bias synaptic transmission to its most sensitive operating range [37, 38]. In bright light ATP consumption declines in the rod but increases in Drosophila because light opens channels, and the total light-gated conductance is much higher than in a rod [1]. Drosophila and other insects do this to resolve rapid changes in bright light [5]; vertebrates have two kinds of photoreceptors and detect high frequencies with cones.
Rods enable a vertebrate retina to support good night vision by collecting light with a large area of highly sensitive photopigment membrane, and they then economize by closing the rods down when, with more plentiful photons, the large photoreceptive area is unnecessary. The nocturnal spider Dinopis, the horseshoe crab Limulus, and the locust all reduce the area of transducing membrane at dawn and rebuild it at dusk [39], and many compound eyes use elaborate mechanical movements to constrict the photoreceptor entrance aperture and attenuate the incoming light [40]. Many nocturnal species also use behavioral strategies to avoid light exposure during the day. This indicates that nocturnal arthropods have to go to considerable lengths to reduce energy usage during the day because light opens photoreceptor channels. These observations strengthen suggestions that photoreceptor energy consumption can play a significant role in eye evolution [41].
Supplementary Material
Acknowledgments
We thank Mike Woodruff for help in measuring Na+/Ca2+ exchange currents and Jim Hurley, Alex Dizhoor, and Jeremy Niven for reading an earlier draft of the manuscript. This work was supported by NIH EY01844 to GLF, and NIH EY17606 and a Karl Kirschgessner New Investigator Grant to APS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 2001;413:186–193. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- 2.Fain GL. Sensory Transduction. Sunderland, MA: Sinauer; 2003. [Google Scholar]
- 3.Niven JE, Vahasoyrinki M, Juusola M. Shaker K(+)-channels are predicted to reduce the metabolic cost of neural information in Drosophila photoreceptors. Proc Biol Sci . 2003;270(Suppl 1):S58–61. doi: 10.1098/rsbl.2003.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. Journal of Cerebral Blood Flow and Metabolism. 2001;21 doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Niven JE, Anderson JC, Laughlin SB. Fly photoreceptors demonstrate energy-information trade-offs in neural coding. PLoS Biology. 2007;5:828–840. doi: 10.1371/journal.pbio.0050116. (e116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthews HR, Fain GL, Murphy RL, Lamb TD. Light adaptation in cone photoreceptors of the salamander: a role for cytoplasmic calcium. J Physiol (Lond) 1990;420:447–469. doi: 10.1113/jphysiol.1990.sp017922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burkhardt DA. Light adaptation and photopigment bleaching in cone photoreceptors in situ in the retina of the turtle. J Neurosci. 1994;14:1091–1105. doi: 10.1523/JNEUROSCI.14-03-01091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikonov SS, Brown BM, Davis JA, Zuniga FI, Bragin A, Pugh EN, Jr, Craft CM. Mouse cones require an arrestin for normal inactivation of phototransduction. Neuron. 2008;59:462–474. doi: 10.1016/j.neuron.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawamura S, Tachibanaki S. Rod and cone photoreceptors: molecular basis of the difference in their physiology. Comp Biochem Physiol A Mol Integr Physiol. 2008;150:369–377. doi: 10.1016/j.cbpa.2008.04.600. [DOI] [PubMed] [Google Scholar]
- 10.Woodruff ML, Janisch KM, Peshenko IV, Dizhoor AM, Tsang SH, Fain GL. Modulation of phosphodiesterase6 turnoff during background illumination in mouse rod photoreceptors. Journal of Neuroscience. 2008;28:2064–2074. doi: 10.1523/JNEUROSCI.2973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns M, Arshavsky V. Beyond Counting Photons: Trials and Trends in Vertebrate Visual Transduction. Neuron. 2005;48:387–401. doi: 10.1016/j.neuron.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Krispel CM, Chen D, Melling N, Chen YJ, Martemyanov KA, Quillinan N, Arshavsky VY, Wensel TG, Chen CK, Burns ME. RGS Expression Rate-Limits Recovery of Rod Photoresponses. Neuron. 2006;51:409–416. doi: 10.1016/j.neuron.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Wilden U, Kuhn H. Light-dependent phosphorylation of rhodopsin: number of phosphorylation sites. Biochemistry. 1982;21:3014–3022. doi: 10.1021/bi00541a032. [DOI] [PubMed] [Google Scholar]
- 14.Papac DI, Oatis JE, Jr, Crouch RK, Knapp DR. Mass spectrometric identification of phosphorylation sites in bleached bovine rhodopsin. Biochemistry. 1993;32:5930–5934. doi: 10.1021/bi00074a002. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy MJ, Lee KA, Niemi GA, Craven KB, Garwin GG, Saari JC, Hurley JB. Multiple phosphorylation of rhodopsin and the in vivo chemistry underlying rod photoreceptor dark adaptation. Neuron. 2001;31:87–101. doi: 10.1016/s0896-6273(01)00340-3. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura S. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature. 1993;362:855–857. doi: 10.1038/362855a0. [DOI] [PubMed] [Google Scholar]
- 17.Olshevskaya EV, Calvert PD, Woodruff ML, Peshenko IV, Savchenko AB, Makino CL, Ho YS, Fain GL, Dizhoor AM. The Y99C mutation in guanylyl cyclase-activating protein 1 increases intracellular Ca2+ and causes photoreceptor degeneration in transgenic mice. J Neurosci. 2004;24:6078–6085. doi: 10.1523/JNEUROSCI.0963-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makino CL, Peshenko IV, Wen X-H, Olshevskaya EV, Barrett R, Dizhoor AM. A role for GCAP2 in regulating the photoresponse: guanylyl cyclase activation and rod electrophysiology. J Biol Chem. 2008 doi: 10.1074/jbc.M804445200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodruff ML, Sampath AP, Matthews HR, Krasnoperova NV, Lem J, Fain GL. Measurement of cytoplasmic calcium concentration in the rods of wild- type and transducin knock-out mice. J Physiol. 2002;542:843–854. doi: 10.1113/jphysiol.2001.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews HR, Fain GL. The effect of light on outer segment calcium in salamander rods. J Physiol. 2003;552:763–776. doi: 10.1113/jphysiol.2003.050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ames A, III, Li Y-y, Heher EC, Kimble CR. Energy metabolism of rabbit retina as related to function: high cost of Na+ transport. Journal of Neuroscience. 1992;12:840–853. doi: 10.1523/JNEUROSCI.12-03-00840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ames A., III CNS energy metabolism as related to function. Brain Research Reviews. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- 23.Baylor DA, Matthews G, Nunn BJ. Location and function of voltage-sensitive conductances in retinal rods of the salamander, Ambystoma tigrinum. J Physiol (Lond) 1984;354:203–223. doi: 10.1113/jphysiol.1984.sp015372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demontis GC, Longoni B, Barcaro U, Cervetto L. Properties and functional roles of hyperpolarization-gated currents in guinea-pig retinal rods. Journal of Physiology. 1999;515:813–828. doi: 10.1111/j.1469-7793.1999.813ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgans CW, Bayley PR, Oesch NW, Ren G, Akileswaran L, Taylor WR. Photoreceptor calcium channels: insight from night blindness. Visual Neuroscience. 2005;22:561–568. doi: 10.1017/S0952523805225038. [DOI] [PubMed] [Google Scholar]
- 26.Rabl K, Thoreson WB. Calcium-dependent inactivation and depletion of synaptic cleft calcium ions combine to regulate rod calcium currents under physiological conditions. European Journal of Neuroscience. 2002;16:2070–2077. doi: 10.1046/j.1460-9568.2002.02277.x. [DOI] [PubMed] [Google Scholar]
- 27.Yagi T, MacLeish PR. Ionic conductances of monkey solitary cone inner segments. Journal of Neurophysiology. 1994;71 doi: 10.1152/jn.1994.71.2.656. [DOI] [PubMed] [Google Scholar]
- 28.Cia D, Bordais A, Varela C, Forster V, Sahel JA, Rendon A, Picaud S. Voltage-gated channels and calcium homeostasis in mammalian rod photoreceptors. Journal of Neurophysiology. 2005;93:1468–1475. doi: 10.1152/jn.00874.2004. [DOI] [PubMed] [Google Scholar]
- 29.Rieke F, Schwartz EA. Asynchronous transmitter release: control of exocytosis and endocytosis at the salamander rod synapse. J Physiol. 1996;493(Pt 1):1–8. doi: 10.1113/jphysiol.1996.sp021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsang SH, Burns ME, Calvert PD, Gouras P, Baylor DA, Goff SP, Arshavsky VY. Role of the Target Enzyme in Deactivation of Photoreceptor G Protein in Vivo. Science. 1998;282:117–121. doi: 10.1126/science.282.5386.117. [DOI] [PubMed] [Google Scholar]
- 31.Lobanova ES, Finkelstein S, Song H, Tsang SH, Chen CK, Sokolov M, Skiba NP, Arshavsky VY. Transducin translocation in rods is triggered by saturation of the GTPase-activating complex. J Neurosci. 2007;27:1151–1160. doi: 10.1523/JNEUROSCI.5010-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121:547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- 33.Linsenmeier RA, Braun RD. Oxygen distribution and consumption in the cat retina during normoxia and hyposemia. Journal of General Physiology. 1992;99:177–197. doi: 10.1085/jgp.99.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Travis GH. Mechanisms of cell death in the inherited retinal degenerations. American Journal of Human Genetics. 1998;62:503–508. doi: 10.1086/301772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikonov SS, Kholodenko R, Lem J, Pugh EN., Jr Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol. 2006;127:359–374. doi: 10.1085/jgp.200609490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thoreson WB, Tranchina D, Witkovsky P. Kinetics of synaptic transfer from rods and cones to horizontal cells in the salamander retina. Neuroscience. 2003;122:785–798. doi: 10.1016/j.neuroscience.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Fain GL, Granda AM, Maxwell JM. Voltage signal of photoreceptors at visual threshold. Nature. 1977;265:181–183. doi: 10.1038/265181a0. [DOI] [PubMed] [Google Scholar]
- 38.Laughlin SB. Matching coding, circuits, cells, and molecules to signals: general principles of retinal design in the fly’s eye. Prog Retinal Eye Res. 1994;13:165–196. [Google Scholar]
- 39.Blest AD. The turnover of phototransductive membrane in compound eyes and ocelli. Adv Insect Physiol. 1988;20:1–53. [Google Scholar]
- 40.Walcott B. Anatomical changes during light-adaptation in insect compound eyes. In: Horridge GA, editor. The Compound Eye and Vision of Insects. Oxford: Clarendon; 1975. pp. 20–33. [Google Scholar]
- 41.Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol. 2008;211:1792–1804. doi: 10.1242/jeb.017574. [DOI] [PubMed] [Google Scholar]
- 42.Sampath AP, Strissel KJ, Elias R, Arshavsky VY, McGinnis JF, Chen J, Kawamura S, Rieke F, Hurley JB. Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron. 2005;46:413–420. doi: 10.1016/j.neuron.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron. 2002;34:773–785. doi: 10.1016/s0896-6273(02)00700-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.