Abstract
Eosinophil-associated Gastrointestinal Disorders (EGID) are characterized by an inappropriate accumulation of eosinophils within the gastrointestinal tract. The underlying etiology and pathophysiology that lead to development of EGID are far from being elucidated. However, there is growing evidence to support the role of aeroallergens and food allergens in the pathogenesis of these disorders. Recent advances have highlighted the role of Th2 driven cytokines in the development of EGID, and clinical studies have verified that children and adults with EGID often have positive skin testing to food allergens. The most common form of EGID, Eosinophilic Esophagitis (EE), has garnered intense investigation following an increased recognition over the past decade. Recently, there have been several important studies providing insight into both the cellular mechanisms governing EE and in clinical therapies directed toward the treatment of EE. In the article herein, we will review the most recent scientific advances influencing our understanding of EGID with special emphasis on the role of allergens in the pathogenesis of EGID.
Keywords: Eosinophilic Esophagitis, Eosinophil, Allegy, Aeroallergens, Eosinophil-associated gastrointestinal disorders (EGID)
Background
Eosinophils are present throughout the healthy gastrointestinal tract, except for the esophagus, which is typically devoid of eosinophils.1,2 Eosinophil-associated Gastrointestinal disorders (EGID) are characterized by an inappropriate accumulation of eosinophils within the gastrointestinal tract. Eosinophil levels may be elevated in one or multiple segments of the GI tract in these disorders. Over the past decade there has been an increasing incidence of primary EGID,3,4 as well as a robust increase in the data linking the development of primary EGID to atopy. Accordingly, the focus of our review will be on role of allergens in the development of EGID.
Diagnosis and Clinical Presentation
The most common form of EGID is known as Eosinophilic Esophagitis (EE), which is characterized by an infiltration of eosinophils within the esophagus. Other forms of EGID include: Eosinophilic gastritis, eosinophilic enteritis, eosinophilic colitis and eosinophilic gastroenteritis. While these disorders are less frequent than EE, their pathogenesis and treatment are probably similar.
Clinically, patients with EGID often present with dysphagia, vomiting or abdominal pain.3 Specifically, patients with EE often present with symptoms that mimic gastroesophageal reflux disease (GERD) or food impaction and pediatric patients may also present with failure to thrive.5,6 Data from 2 separate cohorts of patients with EE suggest that EGID is a chronic condition with a waxing and waning clinical course.7,8 Approximately 70% of patients with EGID are males and there is often a history of other atopic disorders.7,8 The natural history of untreated EGID has not been fully delineated; however, significant complications such as esophageal stricture can occur.9,10,11
The number of eosinophils per high power field required to definitively diagnose EGID has not been uniformly agreed upon, which can make the diagnosis of EGID quite challenging. For this reason, an experienced pathologist familiar with the number of eosinophils typically found in the GI tract at their medical canter is essential for interpreting biopsy specimen in patients with suspected EGID. Efforts have been made to standardize the diagnosis of EGID. The diagnostic criteria for eosinophilic esophagitis were largely established at the First International Gastrointestinal Eosinophilic Research Symposium (>15 eosinophils/HPF noted in at least one of 4 biopsy specimen)12. Additionally, the quantity and distribution of eosinophils in the gastrointestinal tract of pediatric patients without apparent pathologic disease has been reported (table 1),1 though the application of this data to the diagnosis of EGID has not been rigorously established.
Table 1. Quantity of eosinophils in the gastrointestinal tract of children.
The table above illustrates eosinophil levels in the gastrointestinal tract of children (n=28) without apparent gastrointestinal disease. Note that eosinophils are present throughout the healthy gastrointestinal tract, except for the esophagus. Irrespective of gut location, eosinophils are most abundant in the lamina propria and are typically present at much lower levels in the surface epithelium and crypt/glandular epithelium. 1
| Gastrointestinal Segment | Lamina Propria | Villous Lamina Propria | Surface Epithelium | Crypt/Glandular Epithelium | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Max | Mean | Max | Mean | Max | Mean | Max | |
| Esophagus | N/A | N/A | N/A | N/A | 0.03 +/0.10 | 1 | N/A | N/A |
| Antrum | 1.9 +/- 1.3 | 8 | N/A | N/A | 0.0 +/- 0.0 | 0 | 0.02 +/- 0.04 | 1 |
| Fundus | 2.1 +/- 2.4 | 11 | N/A | N/A | 0.0 +/- 0.0 | 0 | 0.008 +/-0.03 | 1 |
| Duodenum | 9.6 +/- 5.3 | 26 | 2.1 +/- 1.4 | 9 | 0.06 +/- 0.09 | 2 | 0.26 +/- 0.36 | 6 |
| Ileum | 12.4 +/- 5.4 | 28 | 4.8 +/- 2.8 | 15 | 0.47 +/- 0.25 | 4 | 0.80 +/- 0.51 | 4 |
| Ascending Colon | 20.3 +/- 8.2 | 50 | N/A | N/A | 0.29 +/- 0.25 | 3 | 1.4 +/- 1.2 | 11 |
| Transverse Colon | 16.3 +/- 5.6 | 42 | N/A | N/A | 0.22 +/- 0.39 | 4 | 0.77 +/- 0.61 | 4 |
| Rectum | 8.3 +/- 5.9 | 32 | N/A | N/A | 0.15 +/- 0.13 | 2 | 1.2 +/- 1.1 | 9 |
N=28
The mean number of eosinophils/hpf +/- the S.D. for each an atomical region of the gastrointestinal tract and each region of the mucosa is shown. N/A, not applicable.
This table has been adapted with permission from publisher.
In addition to elevated eosinophil numbers, the usual morphology of the gastrointestinal tract is typically disrupted in EGID. Depending on the location of the eosinophilic infiltrate, additional manifestations may include the presence of: eosinophilic microabscesses, basilar hypertrophy, cryptitis, disruption of the surface epithelium or fibrosis.13 Studies of patients with EE have also shown the presence of elevated numbers of T-lymphocytes14,15 and mast cells15,16 in the esophagus. Although the presence of elevated eosinophils and disruption of the typical GI tract morphology are critical for the diagnosis of EGID, the presence gastrointestinal eosinophilia alone is not sufficient. The differential diagnosis for eosinophilia within the gastrointestinal tract is broad and includes EGID, hypereosinophilic syndrome, inflammatory bowel disease and parasitic or fungal infection. The differential diagnosis of esophageal eosinophilia is even more expansive and includes: GERD, chronic esophagitis and scleroderma as well. In order to diagnose EGID, the patient must have a biopsy and clinical presentation consistent with EGID. Additionally, other causes of gastrointestinal eosinophilia (parasitic infection, drug hypersensitivity, etc.) must be ruled out.
The number of patients presenting with symptoms and biopsy results consistent with EGID has increased over the past decade.3,4 While the etiology of this epidemic remains unclear, there is a well documented association of atopy with EGID. Clinical studies in children and adults have demonstrated an association with food allergy.17,18 In fact, patients with EE often have positive skin test results to multiple different antigens, with milk and egg being among the most common.18 Additionally, patients with EE often have multiple positive atopy patch test results.19 This latter finding suggests a unique, non-IgE mediated mechanism for the development of eosinophilic esophagitis.
There are multiple therapeutic approaches that may be utilized to treat EE. However, recommended first line therapy for esophageal eosinophilia includes a 4-6 week trial of acid blockade with a proton pump inhibitor (PPI).20 If esophageal eosinophilia persists despite an adequate trial of a PPI then the diagnosis of EE is solidified. Initial therapy often involves removal of the suspected offending food antigen. This can be done by eliminating foods based on the results of skin prick testing (SPT), atopy patch testing (APT), or by simply eliminating the most common food allergens from the diet. This approach is sufficient for resolution of symptoms and/or histology in 30-75% of children18,19,21 and an elemental diet leads to resolution in 98% of patients.7,17 Unfortunately, elemental diets are often difficult to tolerate among older children and adults. However, recent findings indicate that empiric antigen elimination in adults is also effective.22 Other therapies directed toward alleviating allergic symptoms have been utilized as therapeutic agents for EGID. Treatment with swallowed fluticasone or budesonide has proven to be efficacious in the treatment of pediatric patients with EE23,24,25. A study of 19 adult patients with EE also demonstrated significant symptomatic and histologic improvement with fluticasone propionate.26
There have also been case reports demonstrating clinical improvement of EE with montelukast.27 However, when cysteinyl leukotriene levels were examined in biopsies from patients with EGID, the only statistically significant increase in leukotrienes was found in patients with eosinophilic gastroenteritis.28 Additional data has emerged suggesting that omalizumab (anti-IgE) may also be helpful for patients with EGID. A recent study of nine patients with EGID demonstrated symptomatic improvement after receiving omalizumab once every two weeks for 16 weeks.29 However, there was no change in the number of esophageal eosinophils in these patients. Eosinophil levels in the duodenum and gastric antrum decreased but these changes did not reach statistical significance. In summary, given that patients with EGID have positive SPT and APT results and respond to therapies directed at an underlying allergic pathology, the current clinical data strongly supports a role for food antigens in the development of EGID.
Additional clinical data supporting a role for aeroallergens in patients with EE has also begun to emerge. Spergel and his colleagues published a case report of a patient with EE whose symptoms worsened with pollen exposure.30 Subsequent studies by Fitzgerald et al. demonstrated a seasonal variation to the diagnosis of eosinophilic esophagitis, with the diagnosis being most infrequent during the winter months when aeroallergens were low.31 A recent analysis of 23 adult patients with EE also demonstrated that a significant number of adults with EE are sensitized to inhaled and environmental allergens.32 While these clinical studies seem to support a role for aeroallergens in the pathogenesis of these disorders, further investigations were needed to elucidate the molecular pathways by which allergens influence the development of EGID.
Molecular Pathogenesis
To explore the molecular pathogenesis of EGID, animal models of EE have been developed. The results of these investigations have revealed that EE is driven by Th2 cytokine pathways known to be associated with allergic disorders.33, 34 Murine models of EE have also demonstrated that IL-5 and IL-13 are important mediators of the inflammatory pathway leading to EE.35
IL-5 has a well established role in the production, survival, and activation of eosinophils. In murine models of EE, overexpression of IL-5 in CD2 Tcells of transgenic mice leads to the development of EE, and likewise, the absence of IL-5 protects mice against the development of eosinophilic esophagitis.34 Recent data has also linked IL-5 to the development of esophageal remodeling in EE.36 Mishra and his colleagues evaluated the extent of collagen deposition in a murine model of EE and demonstrated a significant increase in collagen deposition in the esophagus of CD2-IL-5 transgenic mice. Additionally, there was a significant reduction in the extent of collagen deposition among mice deficient in IL-5. They also found significant elevation of IL-5 levels in the esophagus of patients with EE and CD2-IL-5 transgenic mice. Taken together, the wealth of data regarding IL-5 and eosinophilic esophagitis suggests that IL-5 is an important mediator of esophageal eosinophilia and remodeling.
Accordingly, investigations have been conducted to determine if blockade of IL-5 by the Anti-IL-5 antibody mepolizumab is therapeutic for EE. A recent study of 4 patients with EE by Stein et. al, demonstrated that inhibition of IL-5 with the Anti-IL-5 antibody mepolizumab effectively reduced peripheral blood eosinophilia and the number of circulating CCR3+ cells.37 Mean esophageal eosinophilia was also reduced ∼9 fold in patients receiving mepolizumab, and this correlated with increased quality of life. A separate analysis of 25 patients receiving mepolizumab demonstrated that blood eosinophilia decreased nearly 20 fold while on therapy and levels remained decreased for 3 months after discontinuing the medication. Additionally, these patients developed elevated levels of IL-5, attributed to a likely long lived mepolizumab-IL-5 complex. Surprisingly, Anti-IL-5 treated patients produced elevated levels of Tcell IL-5 and had increased levels of IL-5 receptor expression on eosinophils, suggesting that IL-5 may have an endogenous autoregulatory pathway.38 The clinical implications of this finding are not yet clear. Elevated levels of IL-5 are also thought to be related to the pathology seen in Hypereosinophilic Syndrome (HES), and mepolizumab has proved to be steroid-sparing for HES.39 Taken together, these data suggest that mepolizumab may be a promising therapeutic intervention for patients with EE.
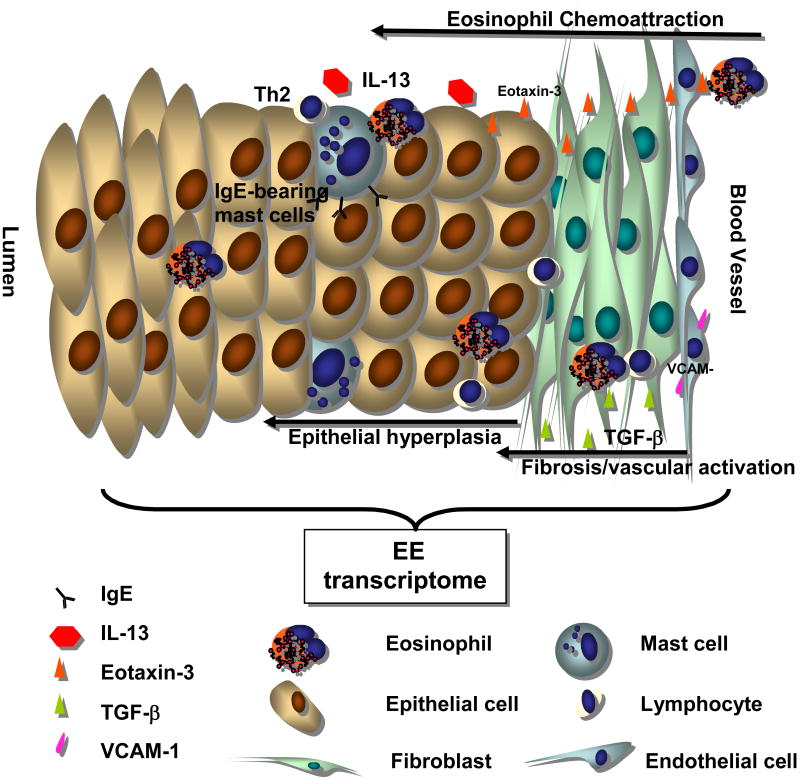
While IL-5 plays a central role in the development of EGID, eosinoiphil recruitment into the gastrointestinal tract results from the expression of multiple other cytokines, eosinophil chemoattractants and adhesion molecules (figure 1). Animal models of EE have demonstrated that IL-13 can induce EE through a STAT6 dependent mechanism.35 IL-13 appears to have in important role in the development of gastrointestinal eosinophilia as well as in the development of esophageal remodeling. It has been proposed that IL-13 can lead to eosinophilic esophagitis by increasing levels of eotaxin-3. Animal models have shown that IL-13 can also lead to the development of eosinophilic esophagitis when it is introduced into the airway.40 Given that IL-13 is known to be involved in the development of allergic airway disease, this observation supports a potential role for aeroallergens in the development of EE.
Figure 1. Molecular Pathogenesis of Eosinophilic Esophagitis.
EE arises from the interaction between genetic factors and environmental exposures. This interaction leads to the secretion of a complex array of cellular mediators. The expression of IL-13 and TGF-β likely contributes to the release of eotaxin-3, increased collagen production (fibrosis) and vascular activation (VCAM-1). Finally, IgE can be detected on the surface of mast cells and likely contributes to mast cell activation. This figure was adapted with the publisher's permission.46
To further understand the molecular mechanisms responsible for EGID, Blanchard et al examined the gene expression profile in the esophageal tissues of patients with eosinophilic esophagitis.41 This generated a unique EE signature profile involving altered expression of nearly 1% of the human genome. Further analysis of the unique EE transcription profile identified that expression of eotaxin-3 mRNA increased 53 fold compared to normal controls. This makes eotaxin-3 the most highly upregulated gene in the EE genome. Eotaxin-3 appears to be expressed primarily by the epithelial cells of the esophagus, although its role in EE has not been completely defined. Blanchard and her colleagues also identified a single nucleotide polymorphism (SNP) within eotaxin-3 that was associated with increased susceptibility to EE. Other investigations have confirmed that increased expression of eotaxin-3 and its receptor CCR3 in esophageal tissues correlates with EE disease activity.42 There is also data emerging that suggests elevated levels of eotaxin-3 in the esophagus may be helpful in separating EE from GERD.43 These are all important insights, as differentiating EE from GERD or chronic esophagitis is often difficult, and as of yet, no specific biomarkers have been identified that can reliably differentiate these clinical entities. However, blood levels of eosinophils, eosinophil-derived neurotoxin and eotaxin-3 are somewhat helpful in differentiating EE from GERD.44
Other important advances in the understanding of EE have stemmed from investigations into the development of esophageal remodeling. Aceves et al hypothesized that eosinophil derived TGF-β and its signaling molecule phosportylated SMAD2/3, which are responsible for the development of fibrosis in asthma models, may also play a role in esophageal remodeling.45 Their investigations suggest that both TGF-β and phosphorylated SMAD2/3 are responsible for increased fibrosis in the subepithelial region of the esophagus. Further analysis of their population also revealed elevated levels of Vascular Cell Adhesion Molecule-1 (VCAM-1) and increased vascular density of the esophagus. Stricture formation is a serious complication of EGID and more research into the development of esophageal remodeling is needed.
In summary, EGID are a group of disorders characterized by an inappropriate accumulation of eosinophils within the gastrointestinal tract. Their pathogenesis is most likely the result of local aberrant Th2 cytokines secreted in response to food, aeroallergens and/or as yet unidentified agents. Most patients respond to elemental diet and steroids. However, both modes of therapy are undesirable long term solutions for EGID. Mepolizumab has recently been shown to be a promising therapeutic intervention for patients with EGID and may spare patients from the long term sequalae of steroid use. The development of other antibody mediated therapies directed at cytokines and chemokines responsible for the pathologic manifestations of EGID, as well as the development of disease-specific biomarkers (especially non-invasive ones) are intriguing areas for future research.
Abbreviations used
- APT
Atopy patch testing
- EGID
Eosinophil-associated gastrointestinal disorder
- EE
Eosinophilic esophagitis
- GERD
Gastroesophageal reflux disease
- GI
Gastrointestinal
- HES
Hypereosinophilic syndrome
- Hpf
High powered field
- PPI
Proton pump inhibitor
- SNP
Single nucleotide polymorphism
- SPT
Skin prick testing
- STAT
Signal transducer and activator of transcription
- Th
T helper
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* = article of special interest
** = article of outstanding interest
- 1.DeBrosse CW, Case JW, Putnam PE, Collins MH, Rothenberg ME. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol. 2006;9:210–8. doi: 10.2350/11-05-0130.1. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg ME, Mishra A, Brandt EB, Hogan SP. Gastrointestinal Eosinophils. Immunol Rev. 2001;179:139–55. doi: 10.1034/j.1600-065x.2001.790114.x. [DOI] [PubMed] [Google Scholar]
- 3.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic Esophagitis. N Engl J Med. 2004;351:1422–30. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 4.Straumann A, Simon HU. Eosinophilic Esophagitis-escalating epidemiology? J Allergy Clin Immunol. 2005;115:418–9. doi: 10.1016/j.jaci.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Putnam PE. Eosinophilic Esophagitis in Children: Clinical Manifestations. Gastrointest Endoscopy Clin N Am. 2008;18:11–23. doi: 10.1016/j.giec.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Petinuk SP, Miller CK, Kaul A. Eosinophilic esophagitis in infants and toddlers. Dysphagia. 2007;22(1):44–8. doi: 10.1007/s00455-006-9040-9. [DOI] [PubMed] [Google Scholar]
- 7.Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic Esophagitis a 10 year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3(12):1198–206. doi: 10.1016/s1542-3565(05)00885-2. [DOI] [PubMed] [Google Scholar]
- 8.Assa'ad AH, Putnam PE, Collins MH, et al. Pediatric patients with eosinophilic Esophagitis an 8-year follow up. J Allergy Clin Immunol. 2007;119(3):731–8. doi: 10.1016/j.jaci.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 9.Khan S, Orenstein SR, Di Lorenzo C, et al. Eosinophilic Esophagitis: strictures, impactions, dysphagia. Dig Dis Sci. 2003;48:22–9. doi: 10.1023/a:1021769928180. [DOI] [PubMed] [Google Scholar]
- 10.Siaw EK, Sayed K, Jackson RJ. Eosinophilic gastroenteritis presenting as acute gastric perforation. J Pediatr Gastroenterol Nutr. 2006;43(5):691–4. doi: 10.1097/01.mpg.0000239996.66011.89. [DOI] [PubMed] [Google Scholar]
- 11.Straumann A. The natural history and complications of eosinophilic Esophagitis. Gastrointest Endoscoply Clin N Am. 2008;18(1):99–118. doi: 10.1016/j.giec.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Furuta GT, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133(4):1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Collins MH. Histopathologic Features of Eosinophilic Esophagitis. Gastrointest Endoscopy Clin N Am. 2008;18(1):59–71. doi: 10.1016/j.giec.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Lucendo AJ, Navarro M, Commas C, et al. Immunophenotypic characterization and quantification of the epithelial inflammatory infiltrate in eosinophilic esophagitis through stereology: an analysis of the cellular mechanisms of the disease and the immunologic capacity of the esophagus. Am J Surg Pathol. 2007;31:598–6006. doi: 10.1097/01.pas.0000213392.49698.8c. [DOI] [PubMed] [Google Scholar]
- 15.Straumann A, Bauer M, Fischer B, Blaser K, Simon HU. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108(6):954–61. doi: 10.1067/mai.2001.119917. [DOI] [PubMed] [Google Scholar]
- 16.Kirsch R, Bokhary R, Marcon MA, Cutz E. Activated mucosal mast cells differentiate eosinophilic (allergic) esophagitis from gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2007;44(1):20–6. doi: 10.1097/MPG.0b013e31802c0d06. [DOI] [PubMed] [Google Scholar]
- 17 *.Markowitz JE, Spergel JM, Ruchelli E, et al. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol. 2003;98:777–82. doi: 10.1111/j.1572-0241.2003.07390.x. [DOI] [PubMed] [Google Scholar]; The authors demonstrate that institution of an elemental diet is a highly effective therapy for patients with EE. This is a fundamental observation in the treatment of EE.
- 18.Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol. 2002;109(2):363–8. doi: 10.1067/mai.2002.121458. [DOI] [PubMed] [Google Scholar]
- 19 **.Spergel JM, Brown-Whitehorn T, Beausoleil JL, et al. Predictive values for skin prick test and atopy patch test for eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:509–11. doi: 10.1016/j.jaci.2006.11.016. [DOI] [PubMed] [Google Scholar]; The authors demonstrate that patients with eosinophilic esophagitis often have positive atopy patch test results. This indicates that patients with eosinophilic esophagitis develop non-IgE mediated immunologic responses to food antigens.
- 20.Liacouras CA. Pharmacologic treatment of eosinophilic esophagitis. Gastrointest Endoscopy Clin N Am. 2008;18:169–178. doi: 10.1016/j.giec.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histological outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4(9):1097–102. doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 22 *.Gonsalves N, Guang-Yu Y, Doerfler B, et al. A prospective clinical trial of six food elimination diet and reintroduction of causative agents in adults with eosinophilic esophagitis. Gastroenterology. 2008;134(4) 1:A–104. [Google Scholar]; This is the first investigation to demonstrate that elimination diet is an effective therapy for adults with EE. Similar investigations are needed to confirm these important results.
- 23.Noel RJ, Putnam PE, Collins MH, et al. Clincial and immunopathologic effects of swallowed fluticasone for eosinophilic esophagitis. Clin Gastroenterol hepatol. 2004;2(7):568–75. doi: 10.1016/s1542-3565(04)00240-x. [DOI] [PubMed] [Google Scholar]
- 24.Konikoff MR, Noel RN, Blanchard C, et al. A randomized double-blind placebo-controlled trial of fluticasone proprionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131(5):1381–91. doi: 10.1053/j.gastro.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 25.Aceves SS, Dahil R, Newbury RO, et al. Topical viscous budesonide suspension for treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2005;116(3):705–6. doi: 10.1016/j.jaci.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic Esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63(1):3–12. doi: 10.1016/j.gie.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 27.Attwood SE, Lewis CJ, Bronder CS, et al. Eosinophilic oesophagitis: a novel treatment using montelukast. Gut. 2003;52(2):181–5. doi: 10.1136/gut.52.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta SK, Peters-Golden M, Fitzgerald JF, et al. Cysteinyl Leukotriene levels in esophageal biopsies in children with eosinophilic inflammation: are they all the same? Am J Gastroenterol. 2006;101(5):1125–8. doi: 10.1111/j.1572-0241.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- 29.Foroughi S, Foster B, Kim N, et al. Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J Allergy Clin Immunol. 2007;120(3):594–601. doi: 10.1016/j.jaci.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis. J Allergy Clin Immunol. 2003;112(4):796–7. doi: 10.1016/s0091-6749(03)01715-9. [DOI] [PubMed] [Google Scholar]
- 31.Wang FT, Gupta SK, Fitzgerald JP. Is there a seasonal variation in the incidence or intensity of eosinophilic Esophagitis in newly diagnosed children? J Clin Gastroenterol. 2007;41(5):451–3. doi: 10.1097/01.mcg.0000248019.16139.67. [DOI] [PubMed] [Google Scholar]
- 32.Roy-Ghanata S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2008;6(5):531–5. doi: 10.1016/j.cgh.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 33.Mishra A, Hogan SP, Brandt EB, et al. IL-5 promotes eosinophil trafficking to the esophagus. J Immunol. 2002;168(5):2464–9. doi: 10.4049/jimmunol.168.5.2464. [DOI] [PubMed] [Google Scholar]
- 34.Mishra A, Hogan SP, Brandt EB, et al. An etiological role for aeroallergens and eosinophils in experimental eosinophilic esophagitis in mice. J Clin Invest. 2001;107(1):83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophlic esophagitis by an IL-5, eotaxin-1 and STAT6-dependent mechanism. Gastroenterology. 2003;125(5):1419–27. doi: 10.1016/j.gastro.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Mishra A, Wang M, Pemmaraaju VR, et al. Esophageal remodeling develops as a consequence of tissue specific IL-5 induced eosinophilia. Gastroenterology. 2008;134(1):204–14. doi: 10.1053/j.gastro.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein ML, Collins MH, Villanueva JM, et al. Antii-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;118(6):1312–9. doi: 10.1016/j.jaci.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 38 *.Stein ML, Villanueva JM, Buckmeier BK, et al. Anti-IL-5 (mepolizumab) therapy reduces eosinophil activation ex vivo and increases IL-5 and IL-5 receptor levels. J Allergy Clin Immunol. 2008 Apr 14; doi: 10.1016/j.jaci.2008.02.033. Epublication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrate that anti-IL-5 induces a sustained decrease in eosinophil levels, believed to be due to a long lived mepolizumab-IL-5 complex. Additionally, they demonstrate that IL-5 levels are elevated post-treatment with mepolizumab. This indicates that IL-5 may have its own autoregulatory pathway, providing a novel insight into the regulation of IL-5.
- 39.Rothenberg ME, Klion AD, Roufosse FE, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Eng J Med. 2008;358(12):1215–28. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 40.Mishra A, Rotheberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5 eotaxin-1 and STAT6-dependent mechanism. Gastroenterology. 2003;125(5):1419–27. doi: 10.1016/j.gastro.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 41 **.Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116(2):536–47. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]; These investigations lead to the development of an EE specific transcriptome Within that transcriptome, the authors identified eotaxin-3 as the most highly upregulated gene.
- 42.Bullock JZ, Villanueva JM, Blanchard C, et al. Interplay of adaptive th2 immunity with eotaxin-3/c-C chemokine receptor 3 in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45(1):22–31. doi: 10.1097/MPG.0b013e318043c097. [DOI] [PubMed] [Google Scholar]
- 43.Bhattacharya B, Caristen J, Sabo E, et al. Increased expression of eotaxin-3 distinguishes between eosinophilic esophagitis and gastroesophageal reflux disease. Hum Pathol. 2007;38(12):1744–53. doi: 10.1016/j.humpath.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 44.Konikoff MR, Blanchard C, Kirby C, et al. Potential of blood eosinophils, eosinophil deriverd neurotoxin and eotaxin-3 as biomarkers of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1328. doi: 10.1016/j.cgh.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 45.Aceves SS, Newbury RO, Dohil R, et al. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119(1):206–12. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 46.Blanchard C, Rothenberg ME. Basic pathogenesis of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18(1):133–43. doi: 10.1016/j.giec.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]