Abstract
Hypothesis
Toll-like receptor 4 (TLR4) is a receptor for saturated fatty acids (SFAs) and global deficiency of TLR4 has been shown to protect against inflammation, insulin resistance (IR), and atherosclerotic lesion formation. Because macrophages (Mθs) express TLR4 and are important in IR and atherosclerotic lesion formation due to their infiltration of white adipose tissue (WAT) and the artery wall, respectively, we hypothesized that deficiency of Mθ TLR4 could protect against these disorders.
Methods
Bone marrow transplantation of agouti, LDL receptor deficient (Ay/a;LDLR-/-) mice with marrow from either C57BL/6 or TLR4-/- mice was performed. Recipient mice with the presence (MθTLR4+/+) or absence (MθTLR4-/-) of Mθ TLR4 were then placed on one of four diets: 1) low fat (LF); 2) high fat (HF); 3) high fat rich in SFAs (HFSFA); and 4) the HFSFA diet supplemented with fish oil (HFSFA+FO).
Results
There were no differences in body composition or plasma lipids between MθTLR4+/+ and MθTLR4-/- mice on any of the diets. However, there was a decrease in some macrophage and inflammatory markers in WAT of female LF-fed MθTLR4-/- mice compared to MθTLR4+/+ mice. MθTLR4-/- mice fed LF diet also displayed decreased atherosclerotic lesion area. There were no differences in Mθ accrual in WAT or atherosclerosis between MθTLR4+/+ and MθTLR4-/- mice fed any of the high fat diets. Finally, there was no difference in insulin sensitivity between MθTLR4+/+ and MθTLR4-/- mice fed the HFSFA diet.
Conclusions
These data suggest that under certain dietary conditions, Mθ expression of TLR4 can be an important mediator of Mθ accumulation in both WAT and the artery wall.
Keywords: macrophage, TLR4, adipose tissue, atherosclerosis, fatty acids, insulin resistance, diet
Introduction
Obesity is a prevailing issue in today’s society, with a recent epidemiological study projecting that 41% of the US will be obese by 2015 [1]. Obesity is a critical component of the metabolic syndrome, along with hypertension, dyslipidemia, impaired glucose tolerance, and insulin resistance (IR) [2-4]. Obese individuals have a two to three fold increased risk of death from all causes [5], with the majority of risk due to cardiovascular disease [6]. Because obesity is characterized by adipose tissue expansion, much effort is being placed into researching the contribution of adipose tissue to development of metabolic diseases associated with obesity.
One key cell type that may link obesity with metabolic disorders is the macrophage (Mθ). Mθs are cells of the innate immune system that have been implicated to play a pivotal role in development of diseases such as cancer, atherosclerosis, and most recently, IR [7-11]. Although it has long been known that Mθs infiltrate the artery wall and are integral to the formation of atherosclerotic lesions [12, 13], recently, it has been demonstrated that Mθs infiltrate white adipose tissue (WAT) and may contribute to inflammation and IR in obesity [8, 14]. Studies have shown that these adipose tissue macrophages (ATMs) contribute the majority of pro-inflammatory cytokine production in adipose tissue, and the presence of ATMs precedes the occurrence of hyperinsulinemia [8, 14]. Important for our studies, ATMs have been shown to be derived from bone marrow [14].
Because of the close link between obesity, macrophages, and inflammation, the activation of Mθs via toll like receptor 4 (TLR4) has become an important area of research. TLR4 is a pathogen associated molecular pattern recognition receptor that is activated by LPS. The lipid A moiety of LPS contains acylated hydroxyl saturated fatty acids (SFAs), which, when removed entirely or replaced with polyunsaturated fatty acids (PUFAs), results in a loss of LPS-induced inflammation [15]. This attribute of LPS led to the hypothesis that SFAs could be natural ligands for TLR4; a theory that has been confirmed by several different groups who have shown that the activation of TLR4 by SFAs leads to the induction of JNK, ERK1/2, and PI3K pathways, culminating in inflammatory gene expression [16-19]. TLR4 is able to sense and respond to different types of fatty acids, as SFAs result in activation, while PUFAs block the activation of TLR4 [17-22].
The responsiveness of TLR4 to fatty acids makes TLR4 an appealing intermediary between obesity and the recruitment of Mθs to WAT and the artery wall. Recently, global TLR4 deletion studies have demonstrated conflicting results regarding the role of TLR4 in body composition and Mθ infiltration of WAT in mice challenged with high fat diets. However, all of the studies shared the common finding that TLR4-/- mice were protected against inflammation and insulin resistance [18, 23-25]. Our current studies expand upon these findings by investigating the effect of macrophage TLR4 on insulin resistance, the influx of Mθs to WAT, local inflammation, and atherosclerosis.
METHODS
Mice
All animal care and experimental procedures were performed with approval from the Institutional Animal Care and Usage Committee of Vanderbilt University. TLR4-/-mice on a C57BL/6 background were kindly provided by Drs. Satoshi Uematsu and Shizuo Akira, University of Osaka, Japan. The TLR4-/- mice were subsequently crossed with C57BL/6 mice from our colony. The heterozygous offspring were intercrossed to produce the TLR4-/- and TLR4+/+ littermates used as bone marrow donors. The recipient, Ay/a;LDLR-/- mice were produced by intercrossing Ay/a mice with low density lipoprotein receptor deficient (LDLR-/-) mice, both of which were on a C57BL/6 background [26, 27]. These studies were performed in male and female mice; however, data shown are from female mice only.
Bone Marrow Transplantation (BMT)
Bone marrow cells collected from TLR4-/- and TLR4+/+ donors were injected into the retro-orbital venous plexus of lethally irradiated recipient Ay/a;LDLR-/- mice. Reconstitution was confirmed by performing PCR for TLR4 genotype of DNA isolated from blood of recipient mice (data not shown).
Diets
Mice were fed ad libitum and given free access to water throughout the study. Mice were placed on experimental diets 4 wks post-BMT and were maintained on the respective diets for 12 wks. All diets (Table 1) were purchased from Research Diets Inc. (New Brunswick, NJ). Cocoa butter was selected as the source for SFAs for the HFSFA diet to allow for the highest concentration of SFAs in the diet while still providing essential fatty acids.
Table 1.
Composition of experimental diets
| Dietary Treatment | Diet Name | Fat Source | % kcal from Fat | Fatty Acid Composition (% of total fat) | Cholesterol (g/kg diet) | Sucrose (g/kg diet) | ||
|---|---|---|---|---|---|---|---|---|
| SFA | MUFA | PUFA | ||||||
| Low total fat | LF | Olive Oil | 12 | 14.2 | 72.1 | 13.6 | 1.5 | 341 |
| Low SFA | HF | Olive Oil | 41 | 14.2 | 72.1 | 13.6 | 1.5 | 341 |
| High SFA | HFSFA | Cocoa Butter | 41 | 62.5 | 34.4 | 3.0 | 1.5 | 341 |
| High SFA + n-3 PUFA (fish oil) | HFSFA+FO | Cocoa Butter + Menhaden Oil | 41 | 56.0 | 30.4 | 13.6 | 1.5 | 341 |
Data represent the source, amount, and fatty acid composition of fat used in respective diets. Data were provided by Research Diets, New Brunswick, NJ.
Total Body Fat
Total body fat was determined by NMR using the Bruker Minispec (Woodlands, TX) in the Mouse Metabolic Phenotyping Center (MMPC) at Vanderbilt University.
Hyperinsulinemic-euglycaemic Clamps
Mice were catheterized at least 5 d before experimentation and hyperinsulinemic-euglycaemic clamps were performed on conscious mice after a 5 h fast as previously described [28]. Briefly, a 5 μCi bolus of [3-3H] glucose was given, followed by a constant 0.05 μCi infusion for 90 min (t= -90 to 0min), which served as the tracer equilibration period. At 0 min the insulin clamp began with a continuous infusion of human insulin at 4 mU • kg-1 • min-1 (Humulin R, Eli Lilly, Indianapolis, IN). The [3-3H] glucose infusion was increased to 0.2 μCi/min for the clamp and euglycemia (~150-160 mg/dl) was maintained by measuring arterial blood glucose every 10 min and infusing 50% dextrose as necessary. Following the 120 min insulin clamp a 16 μCi bolus of 2[14C] deoxyglucose (2[14C]DG) was given to determine the tissue specific glucose metabolic index (Rg) as previously described [28]. At t=150 min mice were anesthetized with an overdose of sodium pentobarbital and the soleus, gastrocnemius, superficial vastus lateralis, WAT, liver, heart, and brain were excised
Blood Collection and Plasma Parameters
After 12 wks of feeding on their respective diets, mice were fasted overnight before blood was collected. Plasma total cholesterol (TC) and triacylglyerol (TG) levels were measured using kits from Raichem (San Diego, CA). Non-esterified fatty acid (NEFA) measurements were performed using the NEFA-C kit by Wako (Neuss, Germany). Glucose levels were determined on whole blood using Johnson & Johnson’s Lifescan OneTouch glucometer (Northridge, CA). Insulin and leptin measurements were performed by the Vanderbilt Hormone Assay and Analytical Services Core using RIAs.
F4/80 Staining of Adipose Tissue
Perigonadal fat pads were extracted upon sacrifice, fixed in 10% formalin, and embedded in paraffin. Sections were stained for F4/80 using an antibody from Serotec (Raleigh, NC) at a 1:100 dilution. Secondary antibody (Dako, Denmark) was used at a 1:100 dilution.
Gene Expression
RNA was isolated from perigonadal WAT using the RNeasy Mini kit by Qiagen (Valencia, CA) according to the manufacturer’s instructions. Realtime RT-PCR was performed using the Applied Biosystem 7700 sequence detection system (Foster City, CA) or the iQ5 cycler from BioRad. Primer-probe sets were purchased from Applied Biosystem’s “Assays-on-demand” (Foster City, CA). Gene expression was normalized to 18S and the delta delta CT method was used to calculate relative gene expression [29].
Fatty Acid Composition of Adipose Tissue
Gas chromatography (GC) was used to analyze fatty acid composition of the triacylglyerol portion of perigonadal fat pads as described previously [27, 30].
Atherosclerotic Lesion Area
Hearts were excised upon sacrifice, frozen, and were sectioned at the aortic root. Neutral lipids were stained using oil-red-O (ORO). Lesion area was quantified using Histometrix version 6 imaging and analysis software by Kinetic Imaging, Ltd. (Durham, NC).
Statistics
Statistical analyses were performed using SPSS for 2-way ANOVA to detect main effects of diet and MθTLR4 genotype on specific phenotypic measures as well as the interactions between these effects. The Bonferroni procedure was used for post-hoc analyses. GraphPad Prism 4 software was used to perform Student’s t-test (comparing mice with TLR4+/+ versus TLR4-/- bone marrow for each diet). Data are expressed as mean ± SEM and were considered significant at P<0.05.
RESULTS
Use of BMT to develop a model with Mθ-specific deletion of TLR4
Ay/a;LDLR-/- mice were used as bone marrow recipients because of their susceptibility to hyperlipidemia, IR, obesity and atherosclerosis [26, 27]. These mice were lethally irradiated followed by transplantation with marrow from C57BL/6 or TLR4-/- donors (Supplemental Fig. 1). Recipient mice are hereafter referred to as MθTLR4+/+ and MθTLR4-/- respectively. At 4 weeks post-BMT, mice were placed on one of four experimental diets (Table 1) for 12 wks. All diets were high in cholesterol (0.15%) and sucrose (341 g/kg diet). The LF diet was low in total fat (12%) and calories (3.94 kcal/g) and used olive oil as the fat source, providing 14.2% of fat from SFAs, 72.1% of fat from monounsaturated fatty acids (MUFAs), and 13.6% of fat from PUFAs. The other three diets were high in total fat (41%) and calories (4.69 kcal/g). The HF diet also used olive oil as the fat source; thus the fatty acid composition was the same as the LF diet but the total fat content was higher. The HFSFA diet contained cocoa butter as the fat source resulting in 62.5% SFAs, 34.4% MUFAs, and 3% PUFAs. Finally, the HFSFA+FO diet also used cocoa butter as the fat source, but was supplemented with menhaden oil providing, 56% SFAs, 30.4% MUFAS, and 13.6% PUFAs (Table 1).
Body composition of recipient mice
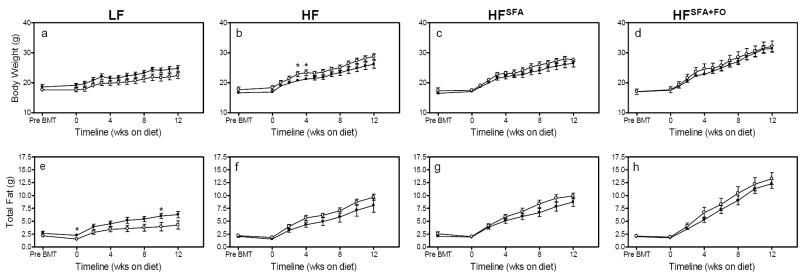
Body weights were measured weekly and total body fat was analyzed biweekly (Fig. 1A-H). There were diet effects on weight gain and adiposity, such that the HFSFA+FO mice gained the most weight, had the greatest total adipose tissue, as well as the largest perigonadal, perirenal and subscapular fat pads (P<0.005, Table 2). MθTLR4-/- mice fed LF diets tended to weigh less and have less total fat than MθTLR4+/+ mice; however, there were no significant differences between recipients of TLR4+/+ and TLR4-/- marrow at sacrifice (Fig. 1A & E). In contrast, the MθTLR4-/- mice fed any of the three high fat diets demonstrated modest but non-significant increases in body weight (Fig. 1B-D) and total body fat (Fig. 1F-H) compared to MθTLR4+/+ mice. Upon sacrifice, there were no significant differences in total body weight, total fat mass, perigonadal, perirenal, or subscapular fat pad weight between MθTLR4+/+ and MθTLR4-/- mice on any of the diets (Table 2).
Figure 1. Total body weight and fat over 12 wks of diet.
Body weight was measured every week and total body fat was measured every other week using NMR in mice that had received either TLR4+/+ (black bars) or TLR4-/- (white bars) marrow and had been fed LF diet (A & E), HF (B & F), HFSFA diet (C & G), and HFSFA+FO diet (D & H). Data are expressed as the mean ± SEM from 5-11 mice per group. *P<0.05.
Table 2.
Body composition of recipient mice after 12 wks on respective diets
| n | Body Wt (g) | Total Fat (g) | Perigonadal Fat Pad (g) | Perirenal Fat Pad (g) | Subscapular Fat Pad (g) | |
|---|---|---|---|---|---|---|
| LF Diet | ||||||
| MθTLR4+/+ | 8 | 25 ± 1 | 6.3 ± 0.6 | 0.81 ± 0.10 | 0.31 ± 0.04 | 0.07 ± 0.01 |
| MθTLR4-/- | 6 | 22 ± 1 | 4.3 ± 0.8 | 0.52 ± 0.08 | 0.23 ± 0.07 | 0.06 ± 0.01 |
| HF Diet | ||||||
| MθTLR4+/+ | 8 | 26 ± 1 | 8.1 ± 1.3* | 1.01 ± 0.17** | 0.50 ± 0.11* | 0.08 ± 0.01 |
| MθTLR4-/- | 5 | 29 ± 1 | 9.7 ± 0.6* | 1.30 ± 0.08** | 0.66 ± 0.20* | 0.09 ± 0.01 |
| HFSFA Diet | ||||||
| MθTLR4+/+ | 10 | 26 ± 1 | 8.7 ± 0.9** | 1.04 ± 0.10* | 0.59 ± 0.07* | 0.10 ± 0.01 |
| MθTLR4-/- | 6 | 28 ± 1 | 9.9 ± 0.5** | 1.15 ± 0.08* | 0.57 ± 0.06* | 0.09 ± 0.02 |
| HFSFA+FO Diet | ||||||
| MθTLR4+/+ | 9 | 31 ± 1‡‡ | 12.3 ± 0.9‡‡ | 1.61 ± 0.12‡‡‡ | 0.79 ± 0.09*** | 0.14 ± 0.02‡ |
| MθTLR4-/- | 6 | 32 ± 1‡‡ | 13.3 ± 1.2‡‡ | 1.74 ± 0.12‡‡‡ | 0.81 ± 0.06*** | 0.13 ± 0.01‡ |
Data are mean ± SEM from 5-11 mice per group. Body composition was measured after mice were fed their respective diets for 12 wks. Student’s t-test revealed there were no significant differences between MθTLR4+/+ and MθTLR4-/-mice. Two-way ANOVA revealed a main effect for diets for all weights listed (P<0.00001). There were no significant main effects for macrophage TLR4 genotype. Significant findings of post-hoc tests as indicated.
P<0.05 for diet compared to LF
P<0.005 for diet compared to LF
P<0.0001 for diet compared to LF
P<0.05 for diet compared to all other diets
P<0.005 for diet compared to all other diets
P<0.0005 for diet compared to all other diets
Insulin sensitivity in HFSFA fed mice
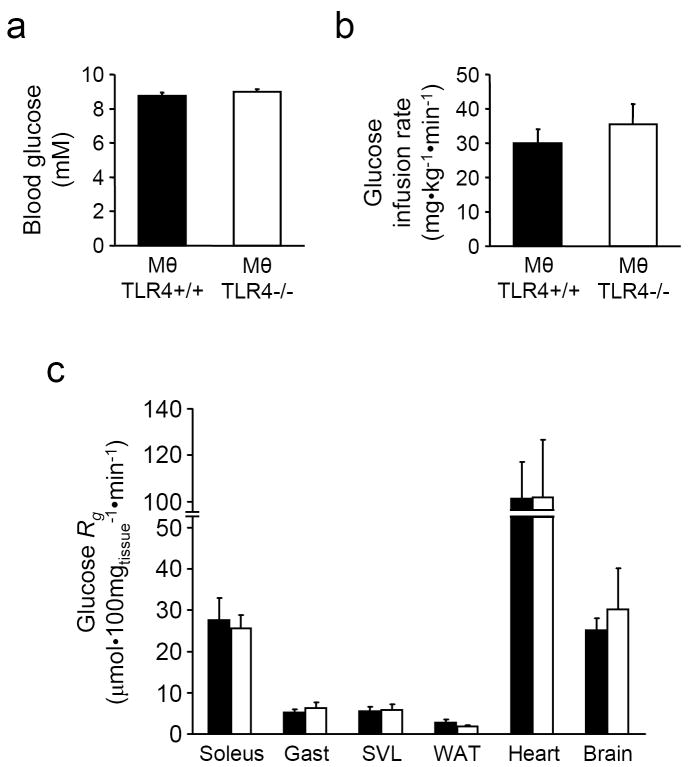
Hyperinsulinemic-euglycaemic clamps were performed on mice with or without Mθ TLR4 fed the HFSFA diet. Arterial glucose levels were clamped at similar levels in both groups (Fig. 2A). Glucose infusion rates required to maintain euglycemia were not significantly different between the two groups, suggesting similar levels of insulin sensitivity (Fig. 2B). A bolus infusion of 2[14C]DG was given during the clamp to determine the glucose metabolism index (Rg), a measure of tissue glucose uptake. There were no significant differences in the uptake of glucose in skeletal muscle from mice in the presence or absence of Mθ TLR4 (Fig. 2C). Rg was also not different in adipose tissue or brain retrieved from MθTLR4+/+ or MθTLR4-/- mice fed HFSFA diet (Fig. 2C).
Figure 2. Hyperinsulinemic-euglycaemic clamps in HFSFA fed mice.
Blood glucose levels (A), average glucose infusion rate during the steady state period (80 to 120 min) (B), and tissue specific glucose metabolic index (Rg; C) during a 120min hyperinsulinemic-euglycaemic clamp in chronically catheterized, conscious MθTLR4+/+ (black) and MθTLR4-/- (white) mice fed HFSFA diet. Mice were fasted for 5h before the experiment. Data are mean ± SEM for 4-5 mice per group. Gastroc = gastrocnemius muscle; SVL = superficial vastus lateralis; WAT = white adipose tissue
Fatty acid composition of WAT
GC analyses of WAT revealed the fatty acid composition of TGs reflected the dietary fat sources, but were not influenced by the absence of Mθ TLR4 (Supplemental Fig. 2). The TG portion of WAT from mice fed the LF diet consisted of 21% SFAs (14:0, 16:0, and 18:0), 73% MUFAs (16:1 and 18:1), and 6% PUFAs (18:2). Similarly, WAT from mice fed the HF diet contained 16% SFAs, 77% MUFAs, and 7% PUFAs. WAT from the HFSFA fed mice had a slight increase in SFAs and decrease in PUFAs (25% SFAs, 72% MUFAs, and 3% PUFAs). As expected, WAT from mice fed HFSFA+FO diet were the only ones to contain the long chain PUFAs, EPA (20:5) and DHA (22:6), while displaying 33% SFAs, 60% MUFAs, and 7% PUFAs (18:2, 20:4, 20:5, 22:5, and 22:6).
Plasma parameters of recipient mice
After 12 wks on their respective diets, plasma was collected from overnight fasted mice and used to analyze TC, TG, NEFA, glucose, insulin, and leptin levels (Table 3). Because of the high cholesterol content of all the diets and the LDLR deficiency of the recipient mice, plasma lipids were elevated by all four diets. There were dietary effects for the degree of hyperlipidemia (P<0.001), with the HFSFA+FO mice having the lowest TC and NEFA levels. NEFA levels were also significantly lower in the HFSFA group than the HF and LF groups. Blood glucose levels were highest in the HFSFA and HFSFA+FO fed mice. Likewise, plasma insulin levels were significantly higher in these groups compared to the LF and HF fed groups (P<0.01). Finally, in agreement with the increased body weight and adiposity, leptin levels were highest in the HFSFA+FO groups (P<0.00001). Despite these main effects of the different diets, there were no significant differences in any of the plasma parameters between MθTLR4+/+ and MθTLR4-/- mice on any of the diets.
Table 3.
Plasma parameters of recipient mice upon sacrifice
| n | Cholesterol (mmol/L) | Triacylglyerols (mmol/L) | NEFAs (mEq/L) | Glucose (mmol/L) | Insulin (ng/ml) | Leptin (ng/ml) | |
|---|---|---|---|---|---|---|---|
| LF | |||||||
| MθTLR4+/+ | 8 | 23.0 ± 1.2 | 2.5 ± 0.4 | 1.42 ± 0.18 | 3.5 ± 0.3 | 0.26 ± 0.05 | 10 ± 2 |
| MθTLR4-/- | 6 | 21.0 ± 1.2 | 2.1 ± 0.4 | 1.42 ± 0.18 | 3.9 ± 0.4 | 0.21 ± 0.04 | 7 ± 1 |
| HF | |||||||
| MθTLR4+/+ | 8 | 17.9 ± 2.6 | 2.5 ± 0.3 | 1.36 ± 0.13 | 3.6 ± 0.3 | 0.25 ± 0.04 | 23 ± 5 |
| MθTLR4-/- | 5 | 20.0 ± 2.1 | 2.3 ± 0.1 | 1.47 ± 0.17 | 4.2 ± 0.3 | 0.21 ± 0.03 | 24 ± 1 |
| HFSFA | |||||||
| MθTLR4+/+ | 10 | 18.6 ± 1.1 | 2.7 ± 0.2 | 1.00 ± 0.05† | 5.2 ± 0,3† | 0.55 ± 0.10†† | 26 ± 3* |
| MθTLR4-/- | 6 | 18.7 ± 1.2 | 2.5 ± 0.2 | 1.08 ± 0.03† | 5.9 ± 1.2† | 0.51 ± 0.10†† | 30 ± 3* |
| HFSFA+FO | |||||||
| MθTLR4+/+ | 9 | 13.6 ± 1.4‡ | 2.0 ± 0.2 | 1.04 ± 0.04† | 5.5 ± 0.4†† | 0.68 ± 0.11†† | 54 ± 11‡ |
| MθTLR4-/- | 6 | 15.4 ± 1.9‡ | 2.1 ± 0.3 | 1.01 ± 0.09† | 6.5 ± 0.4†† | 0.56 ± 0.08†† | 64 ± 6‡ |
Data are mean ± SEM from 5-11 mice per group. Plasma parameters were measured after recipient mice were fed their respective diets for 12 wks. Mice were fasted overnight before sacrifice and plasma collection. Student’s t-tests performed using Prism Graphpad revealed no significant differences among MθTLR4+/+ and MθTLR4-/- groups for each diet. Two-way ANOVA revealed main effect for diet for cholesterol (P<0.001), NEFA (P<0.001), glucose (P<0.0001), insulin (P<0.0001), and leptin (P<0.00001). Main effects for macrophage TLR4 genotype reached near significance for glucose (P=0.07) Significant findings of post-hoc tests as indicated.
P<0.05 for diet compared to LF
P<0.05 for diet compared to LF and HF
P<0.01 for diet compared to LF and HF
P<0.00001 for diet compared to all other diets
Mθ accrual in adipose tissue of recipient mice
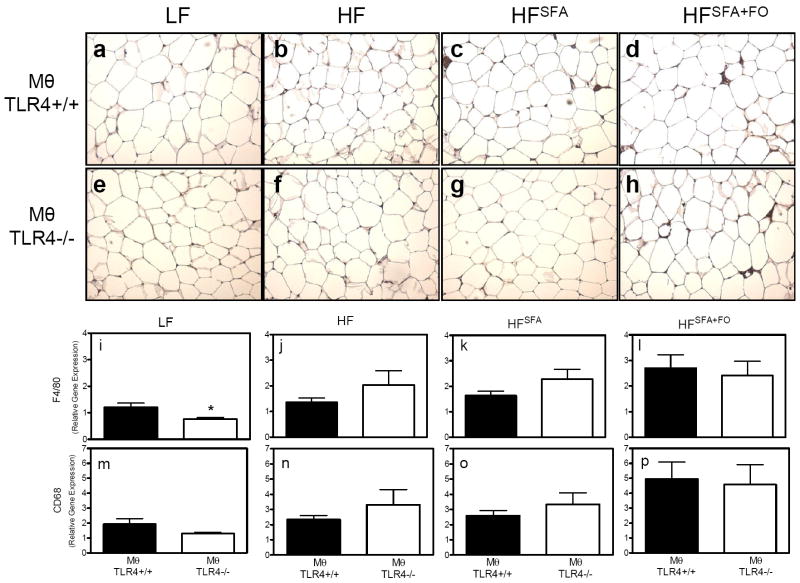
Paraffin sections of perigonadal WAT from MθTLR4+/+ and MθTLR4-/- mice stained with an antibody for the Mθ surface marker F4/80 demonstrated no overt differences between MθTLR4+/+ and MθTLR4-/-mice (Fig. 3A-H). However, quantitative analysis of mRNA expression of F4/80 in adipose tissue revealed that when on the LF diet, MθTLR4-/- mice had significantly less F4/80 gene expression compared to MθTLR4+/+ mice (Fig. 3I; P<0.05). The MθTLR4-/- mice also demonstrated a trend toward a reduction in CD68 expression (Fig. 3M). This difference was not seen in mice challenged with any of the three high fat diets (Fig. 3J-L & N-P).
Figure 3. F4/80 staining and Mθ expression in adipose tissue.
WAT sections from mice fed LF (A & E), HF (B & F), HFSFA (C & G), and HFSFA+FO diets were immunostained with an antibody for F4/80, a macrophage surface marker. Images are shown at 10x magnification for mice receiving TLR4+/+ (A-D) and TLR4-/- (E-H) marrow. RNA was isolated from the perigondal fat pads and real-time RT-PCR was used to determine F4/80 expression (I-L) and CD68 expression (M-P) from mice on their respective diets. Mice receiving TLR4+/+ marrow are shown in black bars and those receiving TLR4-/- marrow are shown in white bars. Data are expressed as mean ± SEM from 5-11 mice per group. Two-way ANOVA revealed a main effect for diets for F4/80 (P<0.001) and CD68 (P<0.005). There were no significant main effects for macrophage TLR4 genotype. Significant findings of post-hoc tests are as indicated. *P<0.05.
Adipose tissue inflammation of recipient mice
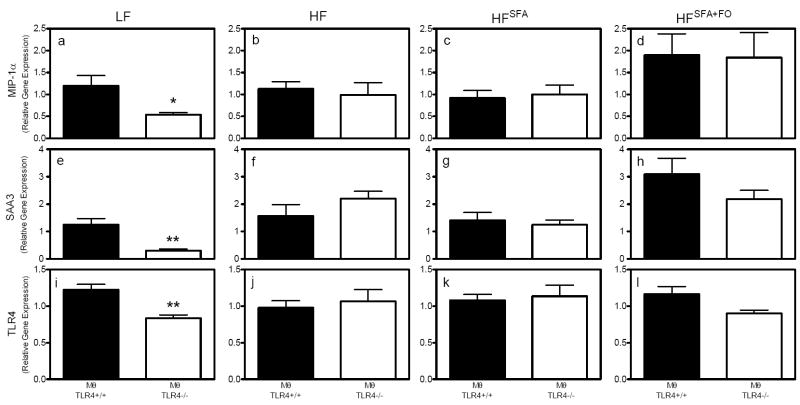
To determine the inflammatory status of adipose tissue in response to the presence or absence of Mθ TLR4, real-time RT-PCR was used to analyze gene expression of several inflammatory genes. There were dietary effects for both macrophage inflammatory protein-1α (MIP-1α) and serum amyloid A (SAA), whereby the HFSFA+FO mice had significantly greater expression of these inflammatory genes compared to the LF and HFSFA groups (P<0.05). Comparisons between MθTLR4-/- mice and MθTLR4+/+ mice demonstrated that MIP-1α mRNA levels were significantly lower in LF-fed MθTLR4-/- mice compared to MθTLR4+/+ mice (P<0.05; Fig. 4A). Similarly, SAA3 and TLR4 expression levels were reduced in MθTLR4-/- mice (P<0.01; Fig. 4E & I). When mice were challenged with any of the three high fat diets, there were no significant differences in MIP-1α (Fig. 4B-D), SAA3 (Fig. 4F-H), or TLR4 (Fig. 4J-L) mRNA levels among MθTLR4-/- mice and MθTLR4+/+ mice.
Figure 4. Inflammation in WAT.
Local inflammation was determined in WAT by analyzing gene expression using realtime RT-PCR. MIP-1α (A-D), SAA3 (E-H), and TLR4 (I-L) mRNA expression were measured in mice fed their respective diets. Data are expressed as mean ± SEM from 5-11 mice per group. Macrophage genotypes are indicated below graphs. Two-way ANOVA revealed a main effect for diets for MIP-1α (P<0.05) and SAA3 (P<0.00001). Main effects for macrophage TLR4 genotype reached near significance for TLR4 expression (P=0.08). Significant findings of post-hoc tests are as indicated. *P<0.05, **P<0.01.
M1 versus M2 phenotype of adipose tissue from LF mice
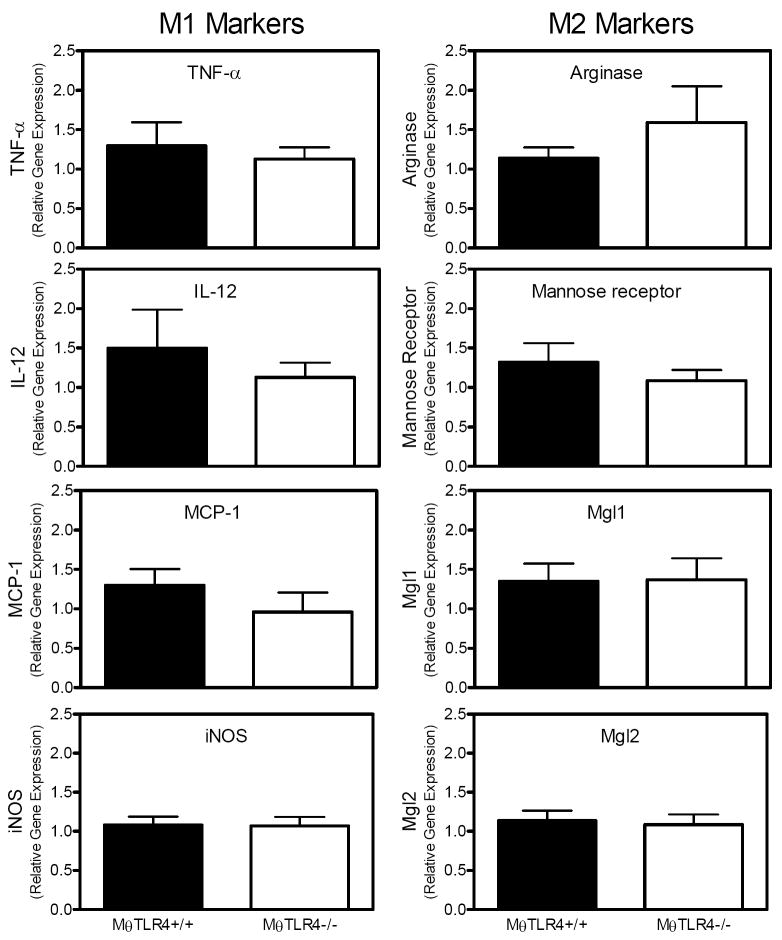
To further characterize the Mθs present in adipose tissue, the gene expression of classical (M1) versus alternative activation (M2) macrophage markers was assessed by real-time RT-PCR in perigonadal fat pads. The HFSFA+FO mice demonstrated a significant dietary effect by displaying elevated levels of both M1 and M2 markers compared to mice fed the other diets (data not shown). There were no significant differences in expression of M1 markers such as TNF-α, interleukin (IL)-12, monocyte chemoattractant protein (MCP)-1, and inducible nitric oxide synthase (iNOS) or M2 markers such as arginase, mannose receptor, macrophage galactose N-acetyl-galactosamine specific lectin (Mgl) 1, and Mgl2 in the LF-fed mice with or without MθTLR4 (Figure 5).
Figure 5. M1 and M2 classification of ATMs.
Markers of M1 and M2 macrophages were determined in WAT by analyzing gene expression for TNF-α, IL-12, MCP-1, iNOS, arginase, mannose receptor, Mgl1, and Mgl2 (M1 versus M2 as indicated on figure). Mice receiving TLR4+/+ marrow are shown in black bars and those receiving TLR4-/-marrow are shown in white bars. Data are shown only from mice fed the LF diet and are expressed as mean ± SEM from 5-11 mice per group.
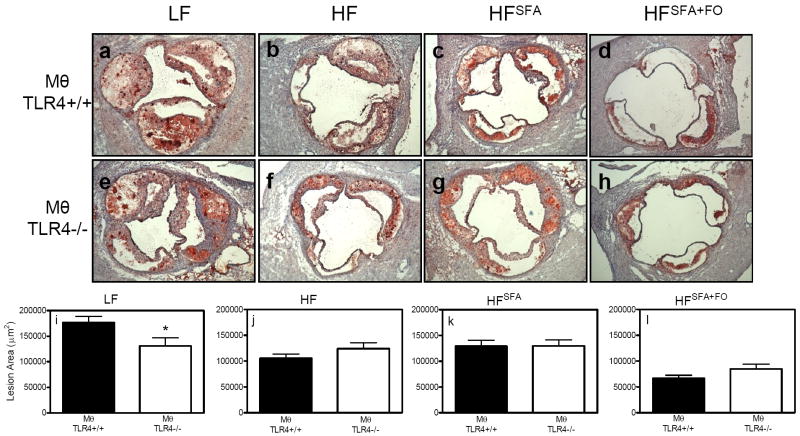
Atherosclerotic lesion area of recipient mice
To quantify atherosclerotic lesion area, hearts were sectioned at the aortic root and neutral lipids were stained with ORO (Fig. 6A-H). The mice fed the LF diet displayed the largest lesion areas (Fig. 6I) compared to mice fed the high fat diets (Fig. 6J-L). In accordance with their reduced plasma lipid levels, mice fed the HFSFA+FO diet were protected from atherosclerotic lesion development (Fig. 6L). In LF-fed mice, MθTLR4-/- mice demonstrated smaller lesions (130,998 ± 15,719 μm2) compared to MθTLR4+/+ mice (176,856 ± 11,671 μm2; P<0.05; Fig. 6I). There were no significant differences in lesion areas between mice with or without Mθ TLR4 fed the high fat diets (Fig. 6J-L).
Figure 6. Quantification of lesion area.
Hearts were sectioned at the aortic root and atherosclerotic lesions were stained using ORO. Images are shown at 10x magnification (A-H). Lesion areas were quantified using Kinetic Histometrix software in 5-11 mice per group (I-L). Data are presented as mean ± SEM and macrophage genotypes are listed below graphs. *P<0.05.
DISCUSSION
Our results support the possibility of a role for Mθ TLR4 in regulating ATM content and inflammation in the presence of LF diet feeding. Despite an absence of differences in body weight, total fat, or perigonadal fat pad weight, MθTLR4-/- mice fed a diet low in total fat displayed decreased ATM accumulation as evidenced by F4/80 mRNA expression (Fig. 3). MθTLR4-/- mice also exhibited decreased local inflammation with significantly lower MIP-1α and SAA3 expression levels in WAT (Fig. 4), although significant differences were not detected for other inflammatory markers tested (Fig. 5). These data support a capacity for Mθ TLR4 in regulating Mθ accumulation and inflammation in WAT, even in the absence of differences in body weight and adipose tissue mass. In addition, our studies are the first to demonstrate that deficiency of Mθ TLR4 can protect against atherosclerotic lesion formation.
Several studies have investigated the effects of global TLR4 deficiency on body composition, adipose tissue physiology, inflammation, and IR in vivo [18, 23-25, 31]. These investigators found that the absence of whole body TLR4 results in a significant decrease in inflammation and IR; however, conflicting results with regards to body weight and fat gain, as well as ATM accumulation, were reported [18, 23-25, 31]. Similar to studies involving global TLR4 deletions [18], our results involving Mθ TLR4 deletion, were specific to female mice, suggesting a sexual dimorphism in the effects of Mθ TLR4. Our studies also demonstrate that dietary fat can modulate metabolic responses to Mθ TLR4 deficiency.
Previous reports have established that in the absence of whole body TLR4, mice fed high fat diets or subjected to lipid infusion are protected against insulin resistance as evidenced by glucose tolerance tests, insulin signalling, and hyperinsulinemic-euglycaemic clamps [18, 23-25]. Because SFAs are the key ligands for TLR4, our current studies investigate the more specific question of the role of Mθ TLR4 on insulin sensitivity in the presence of a high fat diet rich in SFAs. However, using highly sensitive hyperinsulinemic-euglycaemic clamp techniques, we demonstrate that Mθ TLR4 exerts little to no effect on insulin sensitivity when this mouse model is challenged with a HFSFA diet (Fig. 2).
While our results provide evidence that supports a role for Mθ TLR4 in ATM content and inflammation, these effects were apparent only in the context of LF diet feeding and were only moderate. There are several reasons why the impact of high fat diet feeding may not have been affected by Mθ TLR4 deficiency in this model system. 1) It is possible that in the presence of a high fat diet, the system is challenged to a point that subtle differences in ATM content are no longer detected when only macrophage TLR4 expression is different between groups. Furthermore, dietary cholesterol itself can contribute to macrophage accumulation in WAT [32], and the interaction between dietary cholesterol and fatty acids is unexplored. 2) Our studies utilized bone marrow transplantation, which results in suppression of body weight gain and fat expansion and could have influenced the results in our high fat diet groups. 3) The source and type of SFAs are also important to consider. In our studies, we used cocoa butter as a fat source rich in SFAs. Cocoa butter is almost solely composed of SFAs; however, they are medium-chain SFAs that are absorbed and metabolized differently than long-chain SFAs, which are more prominent in animal fat sources. 4) Because we were also interested in atherosclerotic lesion development, we performed these studies in LDLR-/- mice. It is possible that the LDLR may also be important for dietary fatty acid interactions and may influence macrophage recruitment. 5) It should also be noted that the Ay/a mice develop adult onset obesity due to hyperphagia resulting from ectopic expression of the agouti protein that acts as an antagonist of the melanocortin 4 receptor [33, 34]. Thus, it cannot be ruled out that the effects of ectopic expression of the agouti protein might influence results seen in this mouse model. Although more work is required to understand the role of Mθ TLR4 in macrophage recruitment to WAT and the artery wall, our data demonstrate that the absence of Mθ TLR4 exerts effects on macrophage accrual under certain dietary conditions.
Not only is Mθ content in WAT important, the classification of Mθs provides an indication of their activation status. M1 polarized Mθs are pro-inflammatory while M2 polarized Mθs are anti-inflammatory. We had anticipated that absence of Mθ TLR4 might cause WAT from MθTLR4-/- mice to have lower expression of M1 markers and higher expression of M2 markers; however, our results demonstrated no differences between MθTLR4+/+ and MθTLR4-/- mice (Figure 5). Thus, in this mouse model, expression of TLR4 by ATMs does not appear to influence the recruitment of one type of Mθ over another (M1 vs. M2), although inflammatory markers such as MIP-1α and SAA were reduced in WAT of MθTLR4-/- mice. While our data does not suggest a role for Mθ TLR4 in M1 versus M2 phenotype in WAT in this model, a role of TLR4 in general in M1/M2 responses cannot be ruled out considering the importance of both TLR4 and M1/M2 classification in the initiation of an inflammatory response.
We have previously demonstrated that fish oil supplementation of an olive oil-based diet increases body fat mass and decreases plasma lipids, atherosclerosis, ATMs and inflammation in LDLR-/- mice [35]. In the current study, feeding of the BMT recipient Ay/a;LDLR-/- mice with the HFSFA+FO diet resulted in many of the same outcomes, with the exception of beneficial effects in WAT. In fact, HFSFA+FO feeding appeared to induce the greatest amount of inflammatory Mθ accumulation in WAT (Figure 4). The differences between these two studies could be explained by the base fat source of the diet, the effect of BMT, or the extent of obesity between the two models. Taken together our studies demonstrate that fish oil supplementation has beneficial effects on plasma lipids and atherogenesis regardless of the other components of the diet, but that it cannot ameliorate metabolic disorders related to adipose tissue when the diet is also rich in SFAs. This is an important distinction when considering supplementation with fish oil for the treatment of metabolic diseases.
Several studies have suggested a role for TLR4 in atherosclerotic lesion area development in mice [36-39]. In addition, patients heterozygous or homozygous for two single nucleotide polymorphisms (Asp299Gly and Thr399Ile) are hyporesponsive to TLR4 activation, and as a result, are less susceptible to atherosclerosis [40-42]. Our studies show the novel finding that Ay/a;LDLR-/- mice fed a diet low in total fat supplemented with cholesterol develop smaller lesions in the absence of Mθ TLR4. These effects were lost when the mice were challenged with any of the three high fat diets. Previous work from our laboratory showed that while hyperlipidemia was critical in Mθ influx into the artery wall, obesity was a better predictor of Mθ entrenchment in WAT [26, 27]. The current studies suggest that Mθ TLR4 plays a definitive role in Mθ entry into both the artery wall and WAT, providing a commonality in mechanisms of macrophage recruitment to these two tissues.
The LF diet was low in total fat, yet mice fed this diet displayed the highest plasma lipid levels (Table 3) and greatest atherosclerotic lesion areas (Fig. 6). While the LF diet was low in total fat, all of the diets contained equal amounts of added cholesterol and sucrose. The added cholesterol and high carbohydrates likely contributed to the resulting hyperlipidemia and large atherosclerotic lesions seen in animals fed the LF diet. A similar effect was recently noted by Li and colleagues who showed that a diet high in cholesterol but low in fat caused hyperlipidemia and atherosclerotic lesion formation in LDLR-/- mice [43]. Together, our data suggest that complex interactions exist between dietary levels of total fat, fatty acid composition, cholesterol and sucrose, resulting in differences in plasma lipids and atherosclerosis (Table 3 and Fig. 6).
Our current studies establish a potential role for Mθ TLR4 in metabolic diseases such as obesity and cardiovascular disease. Mθ TLR4 deletion protects against Mθ accumulation in WAT and the resulting pro-inflammatory response that follows, as well as Mθ accumulation in the artery wall and the resulting formation of atherosclerotic lesions. These results suggest a specific capacity for Mθ TLR4 to influence Mθ accrual and inflammation in pathological conditions related to obesity.
Supplementary Material
Acknowledgments
This work was supported by a Pilot & Feasibility Award from the Vanderbilt Digestive Diseases Research Centre (VDDRC, DK058404) to AH Hasty and by NIH grants R01HL089466 and DK54902. This work was also supported by a predoctoral fellowship awarded to KR Coenen by the AHA (0715090B) and an American Diabetes Association Mentor-Based Post-doctoral Fellowship grant to R Lee-Young. MJ Puglisi and KR Coenen were supported by the Vanderbilt Molecular Endocrinology Training Program (NIH T32DK07563). Insulin, leptin, and fatty acid analysis were performed in the Analytical Services Core of the Diabetes Research and Training Centre (DK20593)/Vanderbilt Mouse Metabolic Phenotyping Centre (DK59637). We received assistance from the Cell Imaging Core of the VDDRC (DK058404) for the immunohistochemical analyses. In addition, we would like to thank the members of our laboratory for their careful reading and critique of our manuscript.
Abbreviations
- IR
insulin resistance
- Mθ
macrophage
- WAT
white adipose tissue
- ATMs
adipose tissue macrophages
- TLR4
toll like receptor 4
- SFAs
saturated fatty acids
- PUFAs
polyunsaturated fatty acids
- LDLR-/-
low density lipoprotein receptor deficient
- MMPC
Mouse Metabolic Phenotyping Centre
- TC
total cholesterol
- TG
triacylglyerol
- NEFA
non-esterified fatty acid
- GC
gas chromatography
- ORO
oil-red-O
- MθTLR4+/+
recipients of TLR4+/+ marrow
- MθTLR4-/-
recipients of TLR4-/- marrow
- LF
low fat diet
- HF
high fat diet
- HFSFA
high fat diet with SFA source
- HFSFA+FO
HFSFA with added fish oil
- MIP-1α
macrophage inflammatory protein-1α
- SAA
serum amyloid A
- IL
interleukin
- MCP
monocyte chemoattractant protein
- iNOS
inducible nitric oxide synthase
- Mgl
macrophage galactose N-acetyl-galactosamine specific lectin
Footnotes
Duality of Interest The authors have no conflicts to disclose.
References
- 1.Wang Y, Beydoun MA. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 2.Reilly MP, Rader DJ. The metabolic syndrome: more than the sum of its parts? Circulation. 2003;108:1546–1551. doi: 10.1161/01.CIR.0000088846.10655.E0. [DOI] [PubMed] [Google Scholar]
- 3.Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- 4.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 5.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. Jama. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 7.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 8.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Gregorio GB, Yao-Borengasser A, Rasouli N, et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54:2305–2313. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 10.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 13.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 14.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munford RS, Hall CL. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science. 1986;234:203–205. doi: 10.1126/science.3529396. [DOI] [PubMed] [Google Scholar]
- 16.Hwang D. Modulation of the expression of cyclooxygenase-2 by fatty acids mediated through toll-like receptor 4-derived signaling pathways. Faseb J. 2001;15:2556–2564. doi: 10.1096/fj.01-0432com. [DOI] [PubMed] [Google Scholar]
- 17.Lee JY, Ye J, Gao Z, et al. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 18.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suganami T, Tanimoto-Koyama K, Nishida J, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 20.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 21.Lee JY, Plakidas A, Lee WH, et al. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol. 2005;174:5390–5397. doi: 10.4049/jimmunol.174.9.5390. [DOI] [PubMed] [Google Scholar]
- 23.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 24.Poggi M, Bastelica D, Gual P, et al. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia. 2007;50:1267–1276. doi: 10.1007/s00125-007-0654-8. [DOI] [PubMed] [Google Scholar]
- 25.Suganami T, Mieda T, Itoh M, Shimoda Y, Kamei Y, Ogawa Y. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem Biophys Res Commun. 2007;354:45–49. doi: 10.1016/j.bbrc.2006.12.190. [DOI] [PubMed] [Google Scholar]
- 26.Coenen KR, Gruen ML, Chait A, Hasty AH. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007;56:564–573. doi: 10.2337/db06-1375. [DOI] [PubMed] [Google Scholar]
- 27.Coenen KR, Hasty AH. Obesity Potentiates Development of Fatty Liver and Insulin Resistance, but Not Atherosclerosis in High Fat Diet-Fed Agouti LDLR Deficient Mice. Am J Physiol Endocrinol Metab. 2007 doi: 10.1152/ajpendo.00171.2007. [DOI] [PubMed] [Google Scholar]
- 28.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycaemic clamps in the conscious mouse. Diabetes. 2006;55:390–397. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Saraswathi V, Hasty AH. The role of lipolysis in mediating the proinflammatory effects of very low density lipoproteins in mouse peritoneal macrophages. J Lipid Res. 2006;47:1406–1415. doi: 10.1194/jlr.M600159-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 Deficiency Selectively Protects Against Obesity Induced by Diets High in Saturated Fat. Obesity (Silver Spring) 2008 doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- 32.Subramanian S, Han CY, Chiba T, et al. Dietary Cholesterol Worsens Adipose Tissue Macrophage Accumulation and Atherosclerosis in Obese LDL Receptor-Deficient Mice. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.107.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 34.Lu D, Willard D, Patel IR, et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 35.Saraswathi V, Gao L, Morrow JD, Chait A, Niswender KD, Hasty AH. Fish oil increases cholesterol storage in white adipose tissue with concomitant decreases in inflammation, hepatic steatosis, and atherosclerosis in mice. J Nutr. 2007;137:1776–1782. doi: 10.1093/jn/137.7.1776. [DOI] [PubMed] [Google Scholar]
- 36.Michelsen KS, Arditi M. Toll-like receptor signaling and atherosclerosis. Curr Opin Hematol. 2006;13:163–168. doi: 10.1097/01.moh.0000219662.88409.7c. [DOI] [PubMed] [Google Scholar]
- 37.Pasterkamp G, Van Keulen JK, De Kleijn DP. Role of Toll-like receptor 4 in the initiation and progression of atherosclerotic disease. Eur J Clin Invest. 2004;34:328–334. doi: 10.1111/j.1365-2362.2004.01338.x. [DOI] [PubMed] [Google Scholar]
- 38.Michelsen KS, Wong MH, Shah PK, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michelsen KS, Doherty TM, Shah PK, Arditi M. TLR signaling: an emerging bridge from innate immunity to atherogenesis. J Immunol. 2004;173:5901–5907. doi: 10.4049/jimmunol.173.10.5901. [DOI] [PubMed] [Google Scholar]
- 40.Arbour NC, Lorenz E, Schutte BC, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 41.Kiechl S, Lorenz E, Reindl M, et al. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 42.Ameziane N, Beillat T, Verpillat P, et al. Association of the Toll-like receptor 4 gene Asp299Gly polymorphism with acute coronary events. Arterioscler Thromb Vasc Biol. 2003;23:e61–64. doi: 10.1161/01.ATV.0000101191.92392.1D. [DOI] [PubMed] [Google Scholar]
- 43.Hartvigsen K, Binder CJ, Hansen LF, et al. A diet-induced hypercholesterolemic murine model to study atherogenesis without obesity and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2007;27:878–885. doi: 10.1161/01.ATV.0000258790.35810.02. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.