Abstract
Chronic abuse of cocaine is known to cause neuroadaptive changes in the nucleus accumbens (NAc) and ventral tegmental area (VTA). In addition, altered expression of the myelin-related genes MBP, MOBP, PLP1 as well as of MAL2 in NAc was recently reported by gene array analysis in brains from cocaine abusers. In the present study we used in situ hybridization to quantify transcript expression of these four genes, as well as for the myelin-related transcripts encoding quaking, EDG2, claudin-11, transferrin, CNP, and MAG in caudate, putamen, internal capsule, and NAc in postmortem brain from cocaine abusers and matched comparison subjects. Most transcripts were not different between these groups in these striatal regions, and contrary to previous reports, we did not detect any changes in the NAc. However, expression of the transcript encoding PLP1 was significantly decreased in ventral and dorsal regions of the caudate, putamen, and in the internal capsule. Additionally, expression of claudin-11 and transferrin was decreased in the caudate and internal capsule, respectively. PLP1 is expressed at very high levels in oligodendrocytes and is essential in maintaining stability of myelin sheets. Based on these findings, altered expression of PLP1 in most areas of the striatum suggests that widespread changes to the myelin structure could be associated with the adaptive changes following chronic cocaine abuse.
Keywords: Oligodendrocyte, In situ hybridization, Striatum, Glia, Caudate, Putamen, Nucleus accumbens
Introduction
Cocaine and its derivatives are among the most frequently abused drugs in the world, and are second only to alcohol as the leading cause of drug abuse treatment admissions in the US [1]. The underlying neurobiology of drug abuse and addiction has been at the center of intense research, resulting in identification of multiple cellular and neurological adaptations. However, the complex pathophysiology of drug abuse in terms of identifying molecular pathways and differences related to specific drug types, frequency, and duration of abuse (acute versus long-term effects) have only in recent years started to emerge [2].
The brain areas critically involved in cocaine-induced modulation of neurotransmission and behavioral sensitization include the striatum, and in particular the mesolimbic dopamine system, consisting of the ventral tegmental area (VTA), nucleus accumbens (NAc) and their afferent and efferent connections [3]. Chronic administration of cocaine to animals causes structural and functional adaptations to dopaminergic and glutamatergic neurotransmission in both the VTA and NAc, as well as in cortical areas with projections to these areas [4-7]. Interestingly, the changes of mesolimbic neurocircuitry appear similar to those associated with experience-dependent plasticity. Accordingly, chronic drug addiction including sensitization has been suggested to represent an extreme form of learning, and has been proposed as a model to study cellular mechanisms of plasticity [4, 8]. In addition to the biochemical changes in NAc and VTA, extended periods of drug use likely progress to include changes in other brain areas, including prefrontal cortex and hippocampus, as well as additional involvement of the basal ganglia including dorsal areas of the striatum [9].
Studies in postmortem tissue have identified multiple structural and neurochemical changes in the human brain following extended cocaine abuse [10-19]. These include changes in gene expression, which indicate involvement of similar pathways for addiction [20]. Altered expression involve gene products such as the CART, BDNF, and alpha-synuclein molecules [16, 21-23] and involve, in addition to the dopamine system, the GABA and glutamate neurotransmitter systems [3, 7, 24].
A recent unbiased gene-array study with subsequent target validation by real-time quantitative polymerase chain reaction (Q-PCR) led to the identification of cocaine-induced altered expression in the NAc of multiple genes, including a group of myelin-related proteins that is involved in establishment and maintenance of myelin sheaths [25]. Altered myelin-associated gene expression has also been associated with excessive alcohol consumption in human alcoholic brain [26-28] and in animals treated chronically with ethanol [29]. Several such molecular changes have been noted for different drugs of abuse and therefore might represent more general adaptations to addiction [30]. However, in a subsequent study, Albertson et al. [31] found that altered expression of myelin related transcripts was selectively associated with cocaine abuse and not changed in subjects abusing heroin. These findings of drug-selective neurochemical adaptations to white matter gene expression by cocaine and not heroin suggests that the pathophysiology of cocaine addiction may involve, in addition to neuronal adaptations, selective changes in white matter [18, 31].
In the present study, we used in situ hybridization to investigate expression of ten myelin related genes in the striatum of postmortem brain from cocaine abusing subjects. All of these genes, except MAL2 are specifically expressed by oligodendrocytes in the CNS where they are involved in different aspects of myelin function and maintenance [32-37]. Myelin in the CNS, which exists as compact and non-compact forms, is essential for nervous system function. We have included molecules that were previously identified as abnormally expressed in NAc [18, 25], as well as six additional white matter-related transcripts that have previously been found to be abnormally regulated in other neuropsychiatric illnesses, including schizophrenia [38-42]. Although these myelin proteins presently are not well characterized, they can be divided into two groups; a group of transmembrane glycoproteins, which include the MOBP, PLP1, CLDN-11, EDG2 and MAG molecules and a group of cytosolic proteins consisting of the MBP, CNP, QKI and transferrin proteins [43]. These proteins are expressed either in compact (PLP1, MBP, CLDN-11, MOBP, QKI) or non-compact myelin sheats (CNP, MAG) and several studies have associated these proteins with functions such as lipid binding, structural compaction, transmembrane transport, binding of inorganic molecules, and oligodendrocyte differentiation [43, 44].
Experimental Procedure
Postmortem Tissue
Brain specimens were collected during routine autopsy according to a protocol approved by Wayne State University's Human Investigation Committee, as previously described [16, 31]. All brains were tested for common drugs of abuse by quantitative analysis of samples from brain, blood, or urine. Chronicity of cocaine abuse was determined after medicolegal examination based on documented history of cocaine abuse, cardiovascular findings, as well as positive toxicology for cocaine or its metabolites. Cocaine abusing subjects (COC) had positive cocaine-related toxicology, and all subjects (CON and COC) tested negative for other drugs of abuse including opiates, barbiturates, benzodiazepines, phencyclidine and other medication except for the presence of subintoxicating levels of alcohol in two controls and two cocaine users (ethanol levels less or equal to 0.07 g/dl). None of the subjects showed evidence of chronic alcohol abuse. Postmortem interval (PMI) for all subjects was less than 20 h. However, exact PMI and length of cocaine abuse could not be determined. Tissue from cocaine abusers and controls was matched pair-wise for age, gender, and pH (Table 1).
Table 1.
Subject characteristics of postmortem brains for matched cocaine abusers and controls
| Pair | Drug | Age | Race | Gender | pH | Cause of death |
|---|---|---|---|---|---|---|
| CON, control; COC, cocaine; W, white; B, black; ASCVD, atherosclerotic cardiovascular disease; GSW, gun shot wound. For all subjects, postmortem interval <20 h | ||||||
| 1 | CON | 52 | W | M | 6.52 | ASCVD |
| COC | 52 | B | M | 6.47 | Cocaine | |
| 2 | CON | 50 | W | M | 6.42 | ASCVD |
| COC | 53 | W | M | 6.32 | Cocaine | |
| 3 | CON | 32 | B | F | 6.49 | GSW |
| COC | 34 | B | F | 6.42 | Cocaine | |
| 4 | CON | 29 | B | M | 6.52 | GSW |
| COC | 30 | B | M | 6.5 | GSW | |
| 5 | CON | 34 | B | M | 6.68 | GSW |
| COC | 34 | W | M | 6.8 | Cocaine | |
| 6 | CON | 34 | B | M | 6.63 | GSW |
| COC | 34 | B | M | 6.54 | GSW | |
| 7 | CON | 24 | B | M | 6.67 | GSW |
| COC | 30 | B | M | 6.69 | GSW | |
| 8 | CON | 48 | B | F | 6.67 | Unknown |
| COC | 49 | B | F | 6.63 | Cocaine | |
| 9 | CON | 22 | B | M | 6.46 | GSW |
| COC | 25 | B | M | 6.51 | GSW | |
| 10 | CON | 45 | B | M | 6.32 | GSW |
| COC | 38 | B | M | 6.32 | GSW | |
Following dissection of the brain into coronal slabs, a block of striatum was dissected containing nucleus accumbens, putamen and caudate. Tissue was flash frozen in isopentane cooled in liquid nitrogen, and stored at -80°C until further processing. For in situ hybridization, striatal tissue was thawed to -20°C, sectioned in a microtome at 12 μm, and thaw-mounted on polylysine-precoated 75 × 50 mm microscope slides and stored at -80°C until use.
Preparation of cDNA Clones
cDNA clones were generated by PCR amplification of regions specific for each gene product from a human brain cDNA library (Clontech). For genes with multiple splice variants, PCR amplicons were selected that recognize all known isoforms (Table 2). Following amplification, cDNA fragments were subcloned into the pCR-Blunt II-TOPO vector (Invitrogen). Sequence of inserts was confirmed by nucleotide sequencing.
Table 2.
Probes used in study
| Name | Accession # | Isoforms | Region | Size |
|---|---|---|---|---|
| Gene name, gene bank accession number, and number of isoforms recognized by probe (known isoforms in parenthesis) are shown. Region and size indicate probe location and size in basepairs (bp) | ||||
| Proteolipid protein (PLP1) | NM_000533 | 1, 2 (2) | 208-463 | 256 |
| Myelin basic protein (MBP) | NM_001025081 | 1-7 (7) | 525-802 | 278 |
| Myelin T-cell differentiation protein 2 (MAL2) | NM_052886 | 1 (1) | 116-497 | 382 |
| 2,3-cyclic nucleotide 3-phosphodiesterase (CNP) | NM_033133 | 1 (1) | 535-1,098 | 564 |
| Quaking | NM_006775 | 1-8 (8) | 770-975 | 206 |
| Claudin-11 (CLDN-11) | NM_005602 | 1 (1) | 487-798 | 312 |
| Transferrin | NM_001063 | 1 (1) | 1179-1947 | 769 |
| Myelin-associated oligodendrocyte basic protein (MOBP) | NM_182935 | 1-4a (4) | 165-381 | 217 |
| Endothelial differentiation gene 2 (EDG-2) | NM_001401 | 1, 2 (2) | 831-1294 | 464 |
| Myelin associated glycoprotein (MAG) | NM_002361 | 1, 2 (2) | 335-865 | 531 |
Probe is identical to isoform 4 and first 193 nucleotides overlap with remaining three isoforms (89%)
Probe Labeling and In-Situ Hybridization
[S35]-labeled sense and antisense RNA probes were synthesized using SP6 and T7 RNA polymerase as previously described [45]. Briefly, 100 μCi dried [S35]UTP was mixed with 1.5 μg linearized plasmid, 2 μl transcription buffer (5×; Promega), 3 mM NTPs (CTP, ATP, GTP), 1 μl 0.1 M dithiothreitol (DTT), 0.5 μl RNAse inhibitor and 25 units SP6 or T7 (Promega) in a total volume of 10 μl and incubated at 37°C for 2 h. Following incubation, probes were DNAse treated (1 μl DNAse1 for 15 min at room temperature (RT)), diluted in G50-50 buffer (100 mM Tris-HCl, 12.5 mM EDTA, 150 mM NaCl, and 0.2% sodium dodecyl sulfate, pH 7.5) and purified using micro Bio-Spin P-30 chromatography columns (Bio-Rad). Following addition of 1 μl 1 M DTT, probe activity was determined by scintillation counter analysis.
In situ hybridization was performed according to our previously published protocol [46]. Briefly, for each probe 2 slides per subject were fixed for 1 h in 4% (W/V) formaldehyde. Following several washes in SSC buffer (300 mM NaCl, 30 mM sodium citrate, pH 7.2) and dehydration in graded ethanol solutions, slides were incubated with probe corresponding to approximately 1.5 × 106 cpm in 150 μl 50% hybridization buffer overnight at 55°C. On the next day, unhybridized riboprobe was removed by consecutive SSC washes and RNAase treatment. Following dehydration in graded ethanol washes, slides were dried for 1 h at RT and apposed to a BIOMAXMR film (Kodak) for 1 to 3 weeks. Sense and antisense [S35]-labeled RNA probes were run in parallel in separate experiments to test for antisense probe specificity.
Image and Data Analysis
Images of each slide were captured with a CCD based imaging system using Scion Image 4.0.2 software (Scion Corporation). In order to accurately measure transcript expression in caudate and putamen, which for most probes were localized to isolated darker stained islands of white matter (Fig. 1), we used a density slice tool to specifically target and measure gray scale values (GSV) above tissue background levels. This approach excluded the non-labeled surrounding grey matter from analysis. GSVs were measured in dorsal and ventral compartments of caudate, putamen, and internal capsule as well as in the NAc (Fig. 1). Tissue background, which was measured by density slice analysis in non-labeled gray matter areas, was subtracted from specific GSV. Observations were averaged between the two slides to generate one value for each analyzed region and converted to amount of bound radioactivity (nCi/gram tissue) by comparison to [14C] standards (Amersham Biosciences [47]). Finally, the amount of bound probe was expressed as concentration of target mRNA per gram of tissue (fmol/gram) by taking into account the number of [35S] labeled uridine residues in each probe.
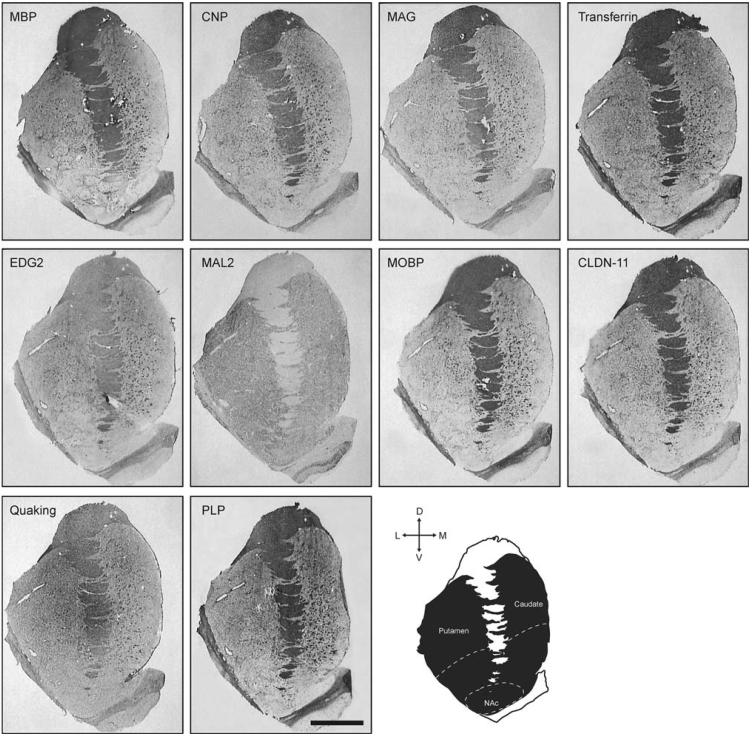
Fig. 1.
Expression of transcripts included in this study. For comparison, all images shown are from the comparison subject in pair #3 (Table 1). Diagram illustrates the striatum with indication of dorsal and ventral regions, as well as the approximate limits of nucleus accumbens. Bar = 10 mm
After confirming that data were normally distributed (Kolmogorov-Smirnov test), statistical analysis comparing COC versus CON for each region was performed using two-tailed paired t-tests. Comparison of dorsal and ventral expression levels was done using analysis of variance (ANOVA) with region as an independent measure followed by posthoc analysis by Tukey's multiple comparison test. For all analyses α = 0.05.
Results
We analyzed transcript expression in striate subregions from 10 matched pairs of cocaine abusing individuals and comparison subjects for molecules that are known to be involved in maintenance and function of myelin in the CNS, including myelin basic protein (MBP); 2,3-cyclic nucleotide 3-phosphodiesterase (CNP); myelin associated glycoprotein (MAG); transferring (TFN); endothelial differentiation gene 2 (EDG2); myelin T-cell differentiation protein 2 (MAL2); myelin-associated oligodendrocyte basic protein (MOBP); claudin-11 (CLDN11); quaking (QKI); and proteolipid protein (PLP1) (Table 2)
We detected expression of all transcripts in the striatum in all subjects. For all genes except MAL2, expression was principally associated with white matter, with intense labeling of the internal capsule (IC) and in a punctuate pattern within the caudate and putamen (Fig. 1). Generally, very few positively labeled puncta were present for any of these genes in NAc. MAL2 transcript expression was not detected in IC. and was uniformly expressed in the gray matter masses of the striatum, including NAc. MAL2, unlike the MAL2-related myelin and lymphocyte protein (MAL) [48, 49], which is involved in myelin-related functions, likely is not expressed by oligodendrocytes but is rather expressed in polarized cells including neurons where it is involved in apical cell transport [50]
We found highest expression of transcripts for MBP and PLP1, with average expression levels across all regions in excess of 3 fmol/g (Fig. 2). This is in agreement with the predominant expression of these molecules among the myelin-specific genes expressed by oligodendrocytes [44]. The other transcripts were expressed at low levels. We did not find significantly different expression levels between dorsal and ventral regions for any of the molecules analyzed
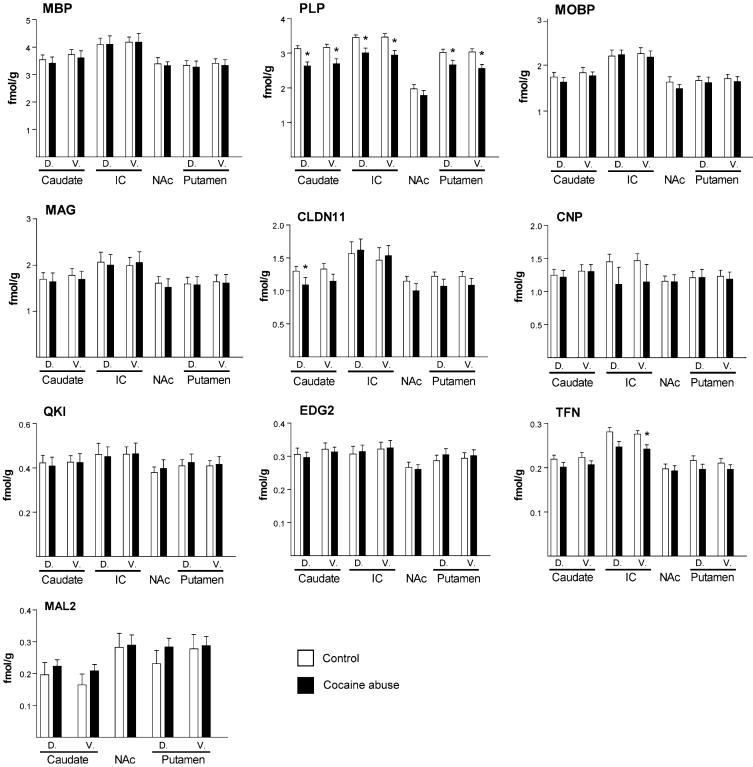
Fig. 2.
Quantification of expression of transcripts for PLP1, MAL2, MOBP, QKI, EDG2, CLDN11, TFN, CNP, MAG, and MBP (White and black represent control and cocaine groups respectively). D: dorsal; V: ventral. Expression is indicated as femtomoles per gram of tissue (fmol/g). * = significant findings; P < 0.05
In all striatal regions except NAc, expression of PLP1 was significantly decreased in the cocaine abusers (Fig. 3); including changes in caudate (dorsal: t = 3.76, df = 9, P = 0.005; ventral: t = 3.82, df = 9, P = 0.004), putamen (dorsal: t = 2.67, df = 9, P = 0.026; ventral: t = 3.72, df = 9, P = 0.005), and intertal capsule (dorsal: t = 3.40, df = 9, P = 0.008; ventral: t = 5.10, df = 9, P = 0.0006). In addition, we found decreased expression of CLDN11 in dorsal caudate (t = 2.26, df = 9, P = 0.05) and of TFN in ventral internal capsule (t = 2.58, df = 9, P = 0.03). We did not find altered expression of MAL2, MOBP, QKI, EDG2, CNP, MAG, and MBP in any of the striatal subregions analyzed
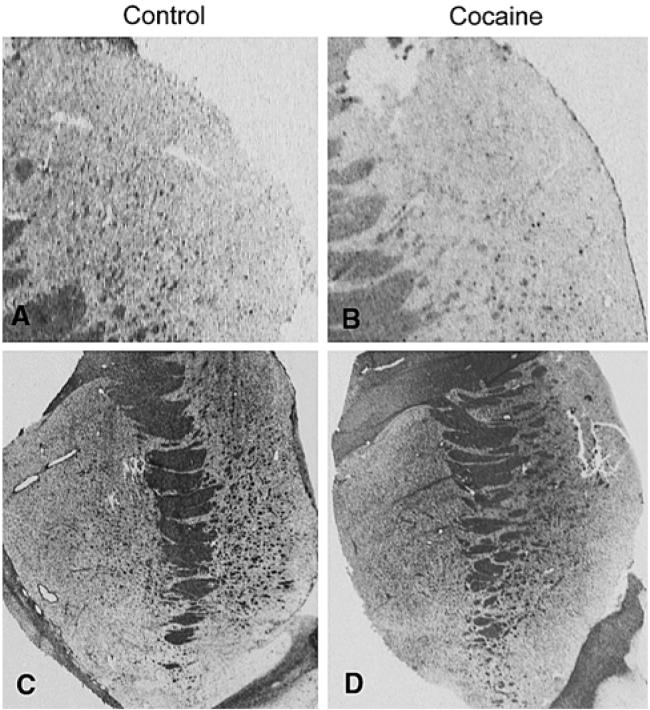
Fig. 3.
Representative autoradiograms for Claudin 11 and PLP1 expression in matched control (a, c) and cocaine abusing subjects (b, d). a, b: Claudin 11 expression in dorsal caudate nucleus. c, d: PLP1 expression in striatum
Discussion
In the present study we have measured transcript expression of MAL2 and the myelin related molecules MBP, MOBP, and PLP1 that were previously reported altered in NAc cocaine abusing subjects [25]. Furthermore, we have analyzed expression of other myelin related molecules expressed by oligodendrocytes, including transcripts encoding QKI, EDG2, CLDN11, TFN, CNP, and MAG.
The most robust finding in this study was decreased expression of PLP1 in all regions of the striatum except NAc in cocaine abusers. Altered expression of PLP1 was previously reported in two independent gene-array studies [25, 51]. PLP1, which constitutes approximately 50% of all protein in myelin sheaths in the CNS, is a complex trans-membrane alternatively spliced structural protein, involved in establishing and maintenance of compact myelin sheaths, in addition to directly participating in the interaction of oligodendrocytes with axons [35, 52, 53]. Expression of PLP1 is tightly regulated with peak expression during periods of active myelination in the developing nervous system. Increased or decreased expression of PLP1 as a result of point-mutations or duplications of the PLP1 gene cause dysmyelination of axon tracts and has been associated with pathologies such as Pelizaeus-Merzbacher disease (PMD) and X-linked spastic paraplegia type 2 (SPG2) [35, 52, 54]. In addition, expression of the PLP1 transcript was reported decreased in temporal cortex in schizophrenia [55]. The severity of the mutant PLP1 phenotypes has been studied extensively in human postmortem brain as well as in rodent experimental models. These studies have illustrated the complex nature of this molecule and have led to the realization that PMD and SPG2 are caused by both dosage effects as well as altered molecular function caused by missense mutations. In addition, these effects are highly influenced by genetic background. While the role of PLP1 in the CNS is not entirely clear, it likely involves additional functions beyond stabilizing myelin membranes, including intracellular transport and signaling [35, 52, 53]. The finding of decreased expression of transcripts for PLP1 in the present study is therefore consistent with a general dysmyelination phenotype as a result of severe long-term cocaine abuse that might not form part of the initial neuroadaptive changes described in animal models. Addiction has been proposed to gradually involve additional areas of the basal ganglia, cortex, and hippocampus, including more dorsal striatal regions [9]. Decreased expression of PLP1 in these regions therefore might represent more chronic effects of cocaine abuse on myelin structure. Interestingly, recent studies using diffusion tensor imaging, which measures diffusion of water in the CNS and can be used as a marker for myelination, have reported evidence for reduced integrity of myelin sheets in cocaine dependent subjects [56, 57].
We speculate that the long-term adaptive changes of myelination in the striatum reflect specific cocaine-mediated changes of the biochemical neurocircuitry rather than a nonspecific toxic effect on oligodendrocytes. This is based on several observations, including that changes to PLP1 expression were not associated with all areas of the striatum, changes were previously reported to be associated specifically with cocaine abuse (not heroin) [31], and expression of transcripts for other myelin-related molecules was not altered. In addition, unaffected expression of the other main structural myelin protein, MBP, which is largely coexpressed with PLP1, suggests that decreased PLP1 expression is not due to a general reduction of myelin membranes or oligodendrocytes, but represents a specific neuroadaptive regulation of gene expression in response to cocaine abuse.
In addition to PLP1, we identified decreased expression of CLDN11 and TFN. Consistent with its requirement for myelin production, iron is primarily associated with oligodendrocytes in the CNS [58]. TFN, which acts as mobile carrier of iron, is essential for myelination, and may regulate expression of other myelin genes including MBP [59-62]. Interestingly, altered TFN expression has been associated with schizophrenia and expression of TFN and MBP during development has been reported delayed in animals following chronic ethanol treatment [29, 38, 63]. CLDN11 is a developmentally regulated protein that is expressed in interlamellar strands of myelin sheaths [64, 65], and is involved in establishment of tight-junctions in myelinating cells and has overlapping functions with PLP1 in maintaining myelin's structural integrity [66-69]. Based on these functions, decreased CLDN11 and TFN expression could compromise myelin function and structural integrity.
Unlike previous work by Albertson et al. [25], we found relatively few changes in oligodendrocyte-related transcript expression in the striatum of cocaine abusing subjects, and none associated with the NAc. This inconsistency might be explained by several methodological factors that are different between this and the previous study. First, unlike Albertson et al. where homogenized tissue corresponding to medial parts of NAc was used, in the present study gene expression was quantified selectively within white matter puncta throughout the entire NAc although we saw no obvious medial to lateral gradient in expression of these transcripts. Second, despite advances in the use of gene array technology in human postmortem research, this method is inherently associated with significant problems, including identification of false positive findings [70]. These problems have, to a certain extent, been accommodated through secondary target validation, which often involves Q-PCR of positively identified target transcripts. Of note, all isoforms for the 10 transcripts in this study are detected by the Affymetrix GeneChip Array set (HG-U133) used by Albertson et al. [25], and the probes used in the present study also hybridized to all known isoforms. Hence, methodological differences between gene array, PCR, in situ hybridization in addition to divergence in cohort characteristics might account for the dissimilarities observed between the present findings and those reported by Albertson et al. [25].
In summary, we examined striatal expression of myelin related genes in postmortem brain from cocaine abusers. Decreased expression of PLP1, TFN, and CLDN11 suggest that altered myelin structure and integrity may be part of the phenotypic changes induced by chronic cocaine abuse.
Acknowledgements
The authors would like to acknowledge technical assistance from Eduardo Reyes and Charlotte Hammond. This work was supported by a Civitan Emerging Scholar Award (LVK), Department of Psychiatry at the University of Alabama at Birmingham, and DA06470 (MB).
References
- 1.Community Epidemiology Work Group . Proceedings of the community epidemiology work group. Vol I/II. U.S. Department of Health and Human Services, NIH; Philadelphia, PA: 2006. pp. 11–20. [Google Scholar]
- 2.Adinoff B. Neurobiologic processes in drug reward and addiction. Harv Rev Psychiatry. 2004;12:305–320. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Thomas MJ, Malenka RC. Synaptic plasticity in the mesolimbic dopamine system. Philos Trans R Soc Lond B Biol Sci. 2003;358:815–819. doi: 10.1098/rstb.2002.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortiz J, Fitzgerald LW, Lane S, et al. Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology. 1996;14:443–452. doi: 10.1016/0893-133X(95)00152-4. [DOI] [PubMed] [Google Scholar]
- 6.Nestler EJ. Cellular responses to chronic treatment with drugs of abuse. Crit Rev Neurobiol. 1993;7:23–39. [PubMed] [Google Scholar]
- 7.Steketee JD. Cortical mechanisms of cocaine sensitization. Crit Rev Neurobiol. 2005;17:69–86. doi: 10.1615/critrevneurobiol.v17.i2.20. [DOI] [PubMed] [Google Scholar]
- 8.Beninger RJ, Gerdjikov T. The role of signaling molecules in reward-related incentive learning. Neurotox Res. 2004;6:91–104. doi: 10.1007/BF03033301. [DOI] [PubMed] [Google Scholar]
- 9.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 10.De Luca V, Likhodi O, Van Tol HH, et al. Regulation of alpha7-nicotinic receptor subunit and alpha7-like gene expression in the prefrontal cortex of patients with bipolar disorder and schizophrenia. Acta Psychiatr Scand. 2006;114:211–215. doi: 10.1111/j.1600-0447.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Lu G, Yao H, et al. Postmortem changes in the central nervous system and adrenal medulla of the heroin addicts. Int J Neurosci. 2005;115:1443–1449. doi: 10.1080/00207450590956549. [DOI] [PubMed] [Google Scholar]
- 12.Ferrer-Alcon M, Garcia-Sevilla JA, Jaquet PE, et al. Regulation of nonphosphorylated and phosphorylated forms of neurofilament proteins in the prefrontal cortex of human opioid addicts. J Neurosci Res. 2000;61:338–349. doi: 10.1002/1097-4547(20000801)61:3<338::AID-JNR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Sevilla JA, Ventayol P, Busquets X, et al. Regulation of immunolabelled mu-opioid receptors and protein kinase C-alpha and zeta isoforms in the frontal cortex of human opiate addicts. Neurosci Lett. 1997;226:29–32. doi: 10.1016/s0304-3940(97)00227-9. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto E, Frolich L, Ozawa H, et al. Alteration of glutamyltranspeptidase binding proteins in postmortem brains of heroin addicts. Alcohol Clin Exp Res. 1996;20:301A–304A. [PubMed] [Google Scholar]
- 15.Meador-Woodruff JH, Little KY, Damask SP, et al. Effects of cocaine on dopamine receptor gene expression: a study in the postmortem human brain. Biol Psychiatry. 1993;34:348–355. doi: 10.1016/0006-3223(93)90178-g. [DOI] [PubMed] [Google Scholar]
- 16.Bannon MJ, Pruetz B, Manning-Bog AB, et al. Decreased expression of the transcription factor NURR1 in dopamine neurons of cocaine abusers. Proc Natl Acad Sci USA. 2002;99:6382–6385. doi: 10.1073/pnas.092654299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mash DC, Ouyang O, Qin Y, et al. Norepinephrine transporter immunoblotting and radioligand binding in cocaine abusers. J Neurosci Methods. 2005;143:6. doi: 10.1016/j.jneumeth.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Bannon M, Kapatos G, Albertson D. Gene expression profiling in the brains of human cocaine abusers. Addict Biol. 2005;10:119–126. doi: 10.1080/13556210412331308921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez D, Kim JH, Krystal J, et al. Imaging the neurochemistry of alcohol and substance abuse. Neuroimaging Clin N Am. 2007;17:6. doi: 10.1016/j.nic.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann E, Colantuoni C, Deep-Soboslay A, et al. Transcriptional changes common to human cocaine, cannabis and phencyclidine abuse. PLoS ONE. 2006;1(1):e114. doi: 10.1371/journal.pone.0000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fagergren P, Hurd Y. CART mRNA expression in rat monkey and human brain: relevance to cocaine abuse. Physiol Behav. 2007;92:7. doi: 10.1016/j.physbeh.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 22.Corominas M, Roncero C, Ribases M, et al. Brain-derived neurotrophic factor and its intracellular signaling pathways in cocaine addiction. Neuropsychobiology. 2007;55:11. doi: 10.1159/000103570. [DOI] [PubMed] [Google Scholar]
- 23.Mash DC, Ouyang O, Pablo J, et al. Cocaine abusers have an overexpression of alpha-synuclein in dopamine neurons. J Neurosci. 2003;23:7. doi: 10.1523/JNEUROSCI.23-07-02564.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rockhold RW. Glutamatergic involvement in psychomotor stimulant action. Prog Drug Res. 1998;50:155–192. doi: 10.1007/978-3-0348-8833-2_4. [DOI] [PubMed] [Google Scholar]
- 25.Albertson DN, Pruetz B, Schmidt CJ, et al. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Lewohl JM, Dodd PR, et al. Gene expression profiling of individual cases reveals consistent transcriptional changes in alcoholic human brain. J Neurochem. 2004;90:1050–1058. doi: 10.1111/j.1471-4159.2004.02570.x. [DOI] [PubMed] [Google Scholar]
- 27.Iwamoto K, Bundo M, Yamamoto M, et al. Decreased expression of NEFH and PCP4/PEP19 in the prefrontal cortex of alcoholics. Neurosci Res. 2004;49:379–385. doi: 10.1016/j.neures.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Lewohl JM, Wang L, Miles MF, et al. Gene expression in human alcoholism: microarray analysis of frontal cortex. Alcohol Clin Exp Res. 2000;24:1873–1882. [PubMed] [Google Scholar]
- 29.Chiappelli F, Taylor AN, Espinosa de los Monteros A, et al. Fetal alcohol delays the developmental expression of myelin basic protein and transferrin in rat primary oligodendrocyte cultures. Int J Dev Neurosci. 1991;9:67–75. doi: 10.1016/0736-5748(91)90074-v. [DOI] [PubMed] [Google Scholar]
- 30.Lehrmann E, Colantuoni C, Deep-Soboslay A, et al. Transcriptional changes common to human cocaine, cannabis and phencyclidine abuse. PLoS ONE. 2006;1:e114. doi: 10.1371/journal.pone.0000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albertson DN, Schmidt CJ, Kapatos G, et al. Distinctive profiles of gene expression in the human nucleus accumbens associated with cocaine and heroin abuse. Neuropsychopharmacology. 2006;31:2304–2312. doi: 10.1038/sj.npp.1301089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 33.Briese M, Ulfig N. Expression of the G-protein-coupled receptor endothelial differentialtion gene-2 in the developing human forebrain with reference to myelination. Neuroembryology. 2003;2:114–122. [Google Scholar]
- 34.Yan Y, Lagenaur C, Narayanan V. Molecular cloning of M6: identification of a PLP/DM20 gene family. Neuron. 1993;11:423–431. doi: 10.1016/0896-6273(93)90147-j. [DOI] [PubMed] [Google Scholar]
- 35.Griffiths I, Klugmann M, Anderson T, et al. Current concepts of PLP and its role in the nervous system. Microsc Res Tech. 1998;41:344–358. doi: 10.1002/(SICI)1097-0029(19980601)41:5<344::AID-JEMT2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 36.Holz A, Schwab ME. Developmental expression of the myelin gene MOBP in the rat nervous system. J Neurocytol. 1997;26:467–477. doi: 10.1023/a:1018529323734. [DOI] [PubMed] [Google Scholar]
- 37.Hardy RJ. Molecular defects in the dysmyelinating mutant quaking. J Neurosci Res. 1998;51:417–422. doi: 10.1002/(SICI)1097-4547(19980215)51:4<417::AID-JNR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 38.McCullumsmith RE, Gupta D, Beneyto M, et al. Expression of transcripts for myelination-related genes in the anterior cingulate cortex in schizophrenia. Schizophr Res. 2007;90:15–27. doi: 10.1016/j.schres.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dracheva S, Davis KL, Chin B, et al. Myelin-associated mRNA and protein expression deficits in the anterior cingulate cortex and hippocampus in elderly schizophrenia patients. Neurobiol Dis. 2006;21:531–540. doi: 10.1016/j.nbd.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Dracheva S, Chin B, Woo DA, et al. Differential expression of myelin related genes in schizophrenic brain. Biol Psychiatry. 2005;57:121S. [Google Scholar]
- 41.Davis KL, Stewart DG, Friedman JI, et al. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 42.Tkachev D, Mimmack ML, Ryan MM, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 43.Kursula P. Structural properties of proteins specific to the myelin sheath. Amino Acids. 2008;34:175–185. doi: 10.1007/s00726-006-0479-7. [DOI] [PubMed] [Google Scholar]
- 44.Campagnoni AT. Molecular biology of myelin proteins from the central nervous system. J Neurochem. 1988;51:1–14. doi: 10.1111/j.1471-4159.1988.tb04827.x. [DOI] [PubMed] [Google Scholar]
- 45.Beneyto M, Kristiansen LV, Oni-Orisan A, et al. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- 46.Clinton SM, Meador-Woodruff JH. Abnormalities of the NMDA receptor and associated intracellular molecules in the thalamus in schizophrenia and bipolar disorder. Neuropsycho-pharmacology. 2004;29:1353–1362. doi: 10.1038/sj.npp.1300451. [DOI] [PubMed] [Google Scholar]
- 47.Geary WA, 2nd, Toga AW, Wooten GF. Quantitative film autoradiography for tritium: methodological considerations. Brain Res. 1985;337:99–108. doi: 10.1016/0006-8993(85)91613-0. [DOI] [PubMed] [Google Scholar]
- 48.Frank M, Atanasoski S, Sancho S, et al. Progressive segregation of unmyelinated axons in peripheral nerves, myelin alterations in the CNS, and cyst formation in the kidneys of myelin and lymphocyte protein-overexpressing mice. J Neurochem. 2000;75:1927–1939. doi: 10.1046/j.1471-4159.2000.0751927.x. [DOI] [PubMed] [Google Scholar]
- 49.Frank M. MAL, a proteolipid in glycosphingolipid enriched domains: functional implications in myelin and beyond. Prog Neurobiol. 2000;60:531–544. doi: 10.1016/s0301-0082(99)00039-8. [DOI] [PubMed] [Google Scholar]
- 50.Marazuela M, Alonso MA. Expression of MAL and MAL2, two elements of the protein machinery for raft-mediated transport, in normal and neoplastic human tissue. Histol Histopathol. 2004;19:925–933. doi: 10.14670/HH-19.925. [DOI] [PubMed] [Google Scholar]
- 51.Lehrmann E, Oyler J, Vawter MP, et al. Transcriptional profiling in the human prefrontal cortex: evidence for two activational states associated with cocaine abuse. Pharmacogenomics J. 2003;3:27–40. doi: 10.1038/sj.tpj.6500146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campagnoni AT, Skoff RP. The pathobiology of myelin mutants reveal novel biological functions of the MBP and PLP genes. Brain Pathol. 2001;11:74–91. doi: 10.1111/j.1750-3639.2001.tb00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wight PA, Dobretsova A. Where, when and how much: regulation of myelin proteolipid protein gene expression. Cell Mol Life Sci. 2004;61:810–821. doi: 10.1007/s00018-003-3309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson TJ, Klugmann M, Thomson CE, et al. Distinct phenotypes associated with increasing dosage of the PLP gene: implications for CMT1A due to PMP22 gene duplication. Ann NY Acad Sci. 1999;883:234–246. [PubMed] [Google Scholar]
- 55.Aston C, Jiang L, Sokolov BP. Microarray analysis of postmortem temporal cortex from patients with schizophrenia. J Neurosci Res. 2004;77:858–866. doi: 10.1002/jnr.20208. [DOI] [PubMed] [Google Scholar]
- 56.Moeller FG, Hasan KM, Steinberg JL, et al. Diffusion tensor imaging eigenvalues: preliminary evidence for altered myelin in cocaine dependence. Psychiatry Res. 2007;154:253–258. doi: 10.1016/j.pscychresns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Moeller FG, Hasan KM, Steinberg JL, et al. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- 58.Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 59.Paez PM, Garcia CI, Pasquini JM. Expression of myelin basic protein in two oligodendroglial cell lines is modulated by apotransferrin through different transcription factors. J Neurosci Res. 2006;83:606–618. doi: 10.1002/jnr.20750. [DOI] [PubMed] [Google Scholar]
- 60.Ortiz E, Pasquini JM, Thompson K, et al. Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J Neurosci Res. 2004;77:681–689. doi: 10.1002/jnr.20207. [DOI] [PubMed] [Google Scholar]
- 61.Espinosa-Jeffrey A, Kumar S, Zhao PM, et al. Transferrin regulates transcription of the MBP gene and its action synergizes with IGF-1 to enhance myelinogenesis in the md rat. Dev Neurosci. 2002;24:227–241. doi: 10.1159/000065698. [DOI] [PubMed] [Google Scholar]
- 62.Espinosa de los Monteros A, Kumar S, Zhao P, et al. Transferrin is an essential factor for myelination. Neurochem Res. 1999;24:235–248. doi: 10.1007/s11064-004-1826-2. [DOI] [PubMed] [Google Scholar]
- 63.Stewart DG, Davis KL. Possible contributions of myelin and oligodendrocyte dysfunction to schizophrenia. Int Rev Neurobiol. 2004;59:381–424. doi: 10.1016/S0074-7742(04)59015-3. [DOI] [PubMed] [Google Scholar]
- 64.Bronstein JM, Chen K, Tiwari-Woodruff S, et al. Developmental expression of OSP/claudin-11. J Neurosci Res. 2000;60:284–290. doi: 10.1002/(SICI)1097-4547(20000501)60:3<284::AID-JNR2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 65.Arroyo EJ, Scherer SS. On the molecular architecture of myelinated fibers. Histochem Cell Biol. 2000;113:1–18. doi: 10.1007/s004180050001. [DOI] [PubMed] [Google Scholar]
- 66.Chow E, Mottahedeh J, Prins M, et al. Disrupted compaction of CNS myelin in an OSP/Claudin-11 and PLP/DM20 double knockout mouse. Mol Cell Neurosci. 2005;29:405–413. doi: 10.1016/j.mcn.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 67.Wolburg H, Wolburg-Buchholz K, Liebner S, et al. Claudin-1, claudin-2 and claudin-11 are present in tight junctions of choroid plexus epithelium of the mouse. Neurosci Lett. 2001;307:77–80. doi: 10.1016/s0304-3940(01)01927-9. [DOI] [PubMed] [Google Scholar]
- 68.Tiwari-Woodruff SK, Buznikov AG, Vu TQ, et al. OSP/claudin-11 forms a complex with a novel member of the tetraspanin super family and beta1 integrin and regulates proliferation and migration of oligodendrocytes. J Cell Biol. 2001;153:295–305. doi: 10.1083/jcb.153.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morita K, Sasaki H, Fujimoto K, et al. Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis. J Cell Biol. 1999;145:579–588. doi: 10.1083/jcb.145.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blalock EM, Chen KC, Stromberg AJ, et al. Harnessing the power of gene microarrays for the study of brain aging and Alzheimer's disease: statistical reliability and functional correlation. Ageing Res Rev. 2005;4:481–512. doi: 10.1016/j.arr.2005.06.006. [DOI] [PubMed] [Google Scholar]