Abstract
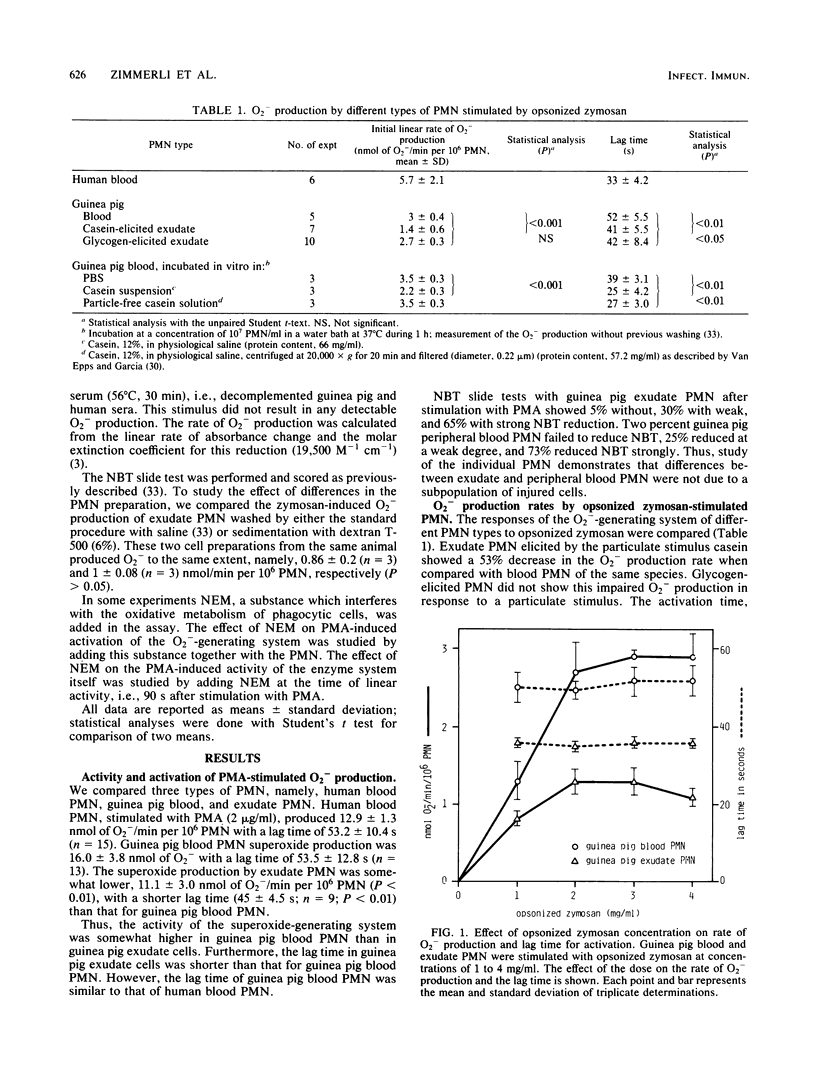
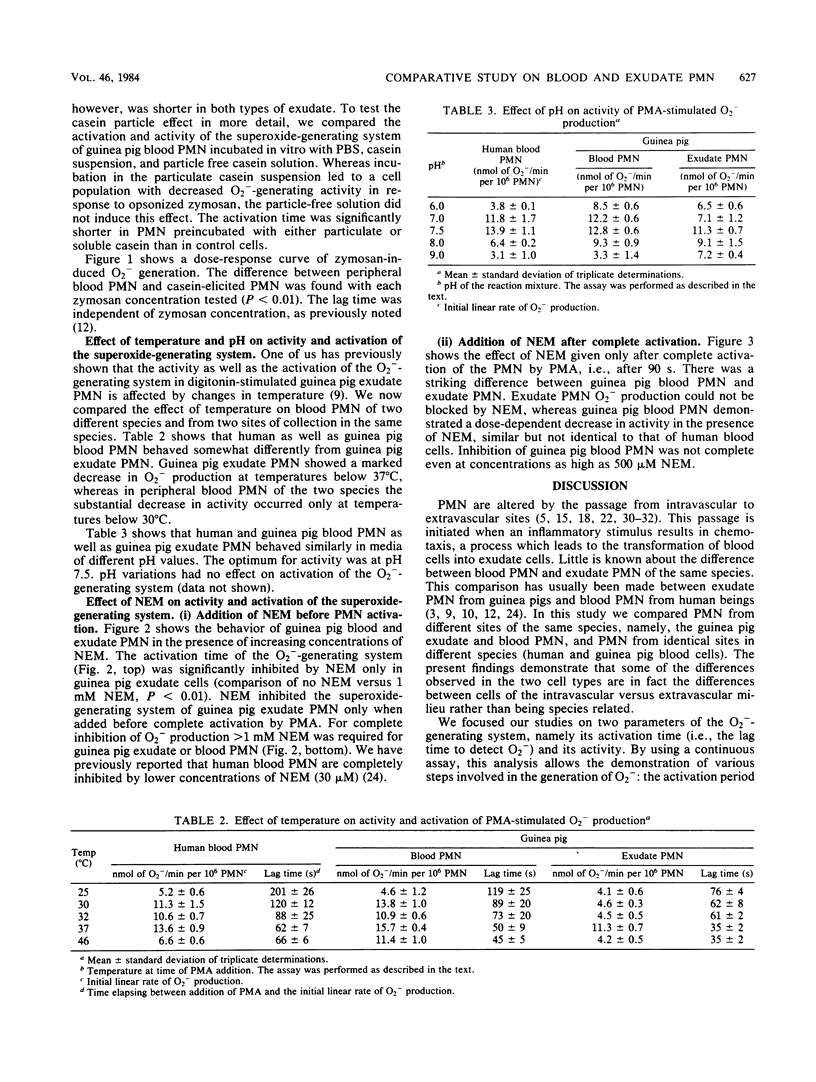
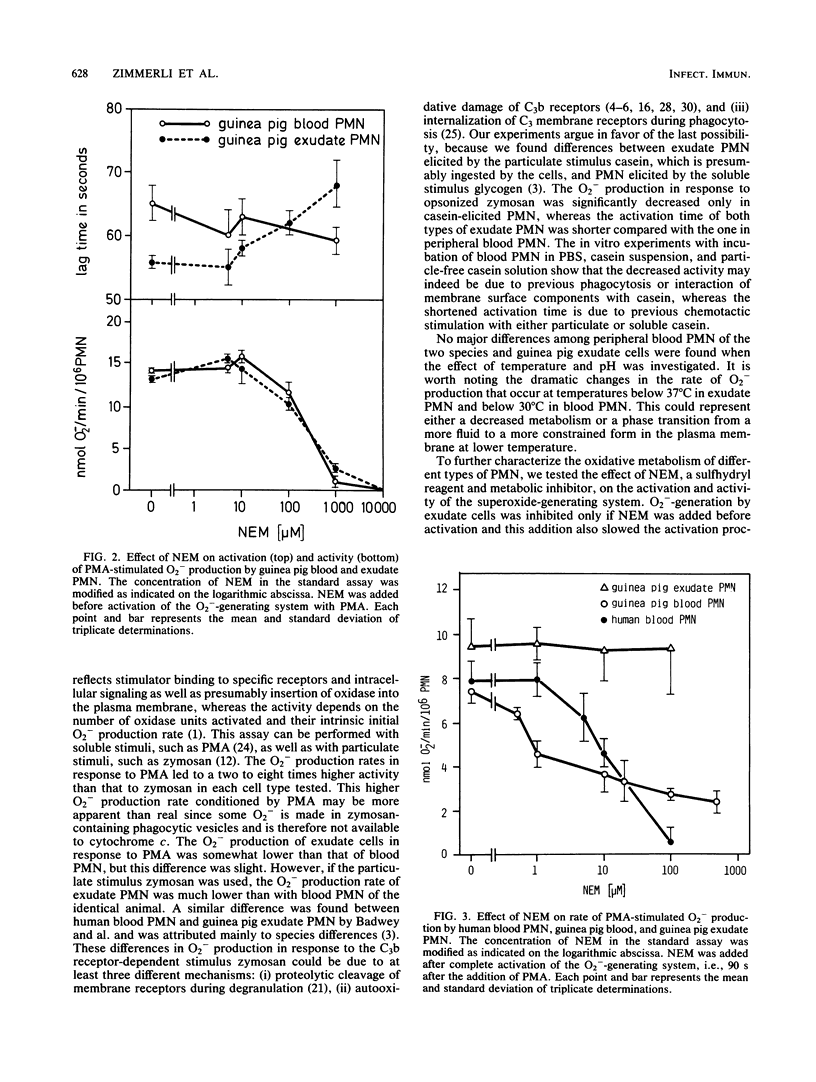
Blood polymorphonuclear leukocytes (PMN), upon interaction with specific chemotactic stimuli, leave the blood stream and migrate to tissues. At such a location, and upon contact with invading microorganisms, they generate superoxide (O2-) as a part of the respiratory burst of phagocytosis. We have compared the O2(-)-generating system of guinea pig peritoneal exudate PMN with that of peripheral blood PMN from the same species or of human origin. The rate of O2- production by casein-induced guinea pig exudate cells in response to a particulate stimulus (opsonized zymosan) was significantly decreased when compared with peripheral blood PMN. Furthermore, the activation time of the O2(-)-generating system was shorter in exudate than in peripheral blood PMN. Important differences in sensitivity to a metabolic inhibitor were found: (i) N-ethylmaleimide increased the activation time of the O2(-)-generating system only in guinea pig exudate cells and not in blood cells; (ii) when N-ethylmaleimide was added after complete activation of the PMN, the O2- production rate was inhibited in guinea pig as well as human blood PMN, but not in guinea pig exudate PMN. We conclude that exudation markedly alters one of the most important antibacterial mechanisms of PMN, the superoxide-generating system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badwey J. A., Curnutte J. T., Robinson J. M., Lazdins J. K., Briggs R. T., Karnovsky M. J., Karnovsky M. L. Comparative aspects of oxidative metabolism of neutrophils from human blood and guinea pig peritonea: magnitude of the respiratory burst, dependence upon stimulating agents, and localization of the oxidases. J Cell Physiol. 1980 Dec;105(3):541–545. doi: 10.1002/jcp.1041050319. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Boxer L. A., Allen J. M., Davis J. Autooxidation as a basis for altered function by polymorphonuclear leukocytes. Blood. 1977 Aug;50(2):327–335. [PubMed] [Google Scholar]
- Baehner R. L., Gilman N., Karnovsky M. L. Respiration and glucose oxidation in human and guinea pig leukocytes: comparative studies. J Clin Invest. 1970 Apr;49(4):692–700. doi: 10.1172/JCI106281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Baehner R. L., Davis J. The effect of 2-deoxyglucose on guinea pig polymorphonuclear leukocyte phagocytosis. J Cell Physiol. 1977 Apr;91(1):89–102. doi: 10.1002/jcp.1040910110. [DOI] [PubMed] [Google Scholar]
- CARTWRIGHT G. E., ATHENS J. W., WINTROBE M. M. THE KINETICS OF GRANULOPOIESIS IN NORMAL MAN. Blood. 1964 Dec;24:780–803. [PubMed] [Google Scholar]
- Chenoweth D. E., Lane T. A., Rowe J. G., Hugli T. E. Quantitative comparisons of neutrophil chemotaxis in four animal species. Clin Immunol Immunopathol. 1980 Mar;15(3):525–535. doi: 10.1016/0090-1229(80)90064-1. [DOI] [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide generation by digitonin-stimulated guinea pig granulocytes. A basis for a continuous assay for monitoring superoxide production and for the study of the activation of the generating system. J Clin Invest. 1978 Apr;61(4):1081–1087. doi: 10.1172/JCI109007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide production by digitonin-stimulated guinea pig granulocytes. The effects of N-ethyl maleimide, divalent cations; and glycolytic and mitochondrial inhibitors on the activation of the superoxide generating system. J Clin Invest. 1978 Apr;61(4):1088–1096. doi: 10.1172/JCI109008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E., Wilson M. K., Newburger P. E. Con-A-stimulated superoxide production by granulocytes: reversible activation of NADPH oxidase. Blood. 1982 Nov;60(5):1188–1194. [PubMed] [Google Scholar]
- Cohen H. J., Newburger P. E., Chovaniec M. E., Whitin J. C., Simons E. R. Opsonized zymosan-stimulated granulocytes-activation and activity of the superoxide-generating system and membrane potential changes. Blood. 1981 Nov;58(5):975–982. [PubMed] [Google Scholar]
- Cohen H. J., Whitin J. C., Chovaniec M. E., Tape E. H., Simons E. R. Is activation of the granulocyte by concanavalin-A a reversible process? Blood. 1984 Jan;63(1):114–120. [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Biological defense mechanisms. The effect of bacteria and serum on superoxide production by granulocytes. J Clin Invest. 1974 Jun;53(6):1662–1672. doi: 10.1172/JCI107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English D., Roloff J. S., Lukens J. N. Regulation of human polymorphonuclear leukocyte superoxide release by cellular responses to chemotactic peptides. J Immunol. 1981 Jan;126(1):165–171. [PubMed] [Google Scholar]
- Fearon D. T., Kaneko I., Thomson G. G. Membrane distribution and adsorptive endocytosis by C3b receptors on human polymorphonuclear leukocytes. J Exp Med. 1981 Jun 1;153(6):1615–1628. doi: 10.1084/jem.153.6.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I. Abnormal phagocyte chemotaxis: pathophysiology, clinical manifestations, and management of patients. Rev Infect Dis. 1981 Nov-Dec;3(6):1196–1220. doi: 10.1093/clinids/3.6.1196. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Durocher J. R., Kaplan A. P. Interaction of leukocyte chemotactic factors with the cell surface. I. Chemotactic factor-induced changes in human granulocyte surface charge. J Clin Invest. 1975 May;55(5):967–974. doi: 10.1172/JCI108026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Wright D. G., Malech H. L., Davis J. M., Klempner M. S., Kirkpatrick C. H. Disorders of phagocyte chemotaxis. Ann Intern Med. 1980 Apr;92(4):520–538. doi: 10.7326/0003-4819-92-4-520. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Gallin J. I. Separation and functional characterization of human neutrophil subpopulations. Blood. 1978 Apr;51(4):659–669. [PubMed] [Google Scholar]
- Lane T. A., Lamkin G. C., Windle B. E. Phagocytosis-induced modulation of human neutrophil chemotaxis receptors. Blood. 1981 Aug;58(2):228–236. [PubMed] [Google Scholar]
- Lehmeyer J. E., Snyderman R., Johnston R. B., Jr Stimulation of neutrophil oxidative metabolism by chemotactic peptides: influence of calcium ion concentration and cytochalasin B and comparison with stimulation by phorbol myristate acetate. Blood. 1979 Jul;54(1):35–45. [PubMed] [Google Scholar]
- McPhail L. C., Henson P. M., Johnston R. B., Jr Respiratory burst enzyme in human neutrophils. Evidence for multiple mechanisms of activation. J Clin Invest. 1981 Mar;67(3):710–716. doi: 10.1172/JCI110087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburger P. E., Chovaniec M. E., Cohen H. J. Activity and activation of the granulocyte superoxide-generating system. Blood. 1980 Jan;55(1):85–92. [PubMed] [Google Scholar]
- Niedel J. E., Kahane I., Cuatrecasas P. Receptor-mediated internalization of fluorescent chemotactic peptide by human neutrophils. Science. 1979 Sep 28;205(4413):1412–1414. doi: 10.1126/science.472759. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Ward P. A. Chemotactic factors and the neutrophil. Semin Hematol. 1979 Apr;16(2):163–174. [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Scott R. B., Cooper L. W. Leucocyte glycogen response in inflammatory exudates. Br J Haematol. 1974 Mar;26(3):485–496. doi: 10.1111/j.1365-2141.1974.tb00490.x. [DOI] [PubMed] [Google Scholar]
- Van Epps D. E., Garcia M. L. Enhancement of neutrophils function as a result of prior exposure to chemotactic factor. J Clin Invest. 1980 Aug;66(2):167–175. doi: 10.1172/JCI109841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade B. H., Mandell G. L. Polymorphonuclear leukocytes: dedicated professional phagocytes. Am J Med. 1983 Apr;74(4):686–693. doi: 10.1016/0002-9343(83)91028-8. [DOI] [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. Secretory responses of human neutrophils: exocytosis of specific (secondary) granules by human neutrophils during adherence in vitro and during exudation in vivo. J Immunol. 1979 Jul;123(1):285–294. [PubMed] [Google Scholar]
- Zimmerli W., Lew P. D., Waldvogel F. A. Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. J Clin Invest. 1984 Apr;73(4):1191–1200. doi: 10.1172/JCI111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W., Waldvogel F. A., Vaudaux P., Nydegger U. E. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982 Oct;146(4):487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]


