Abstract
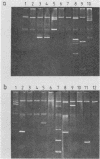
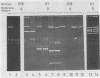
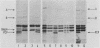
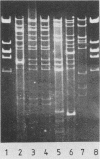
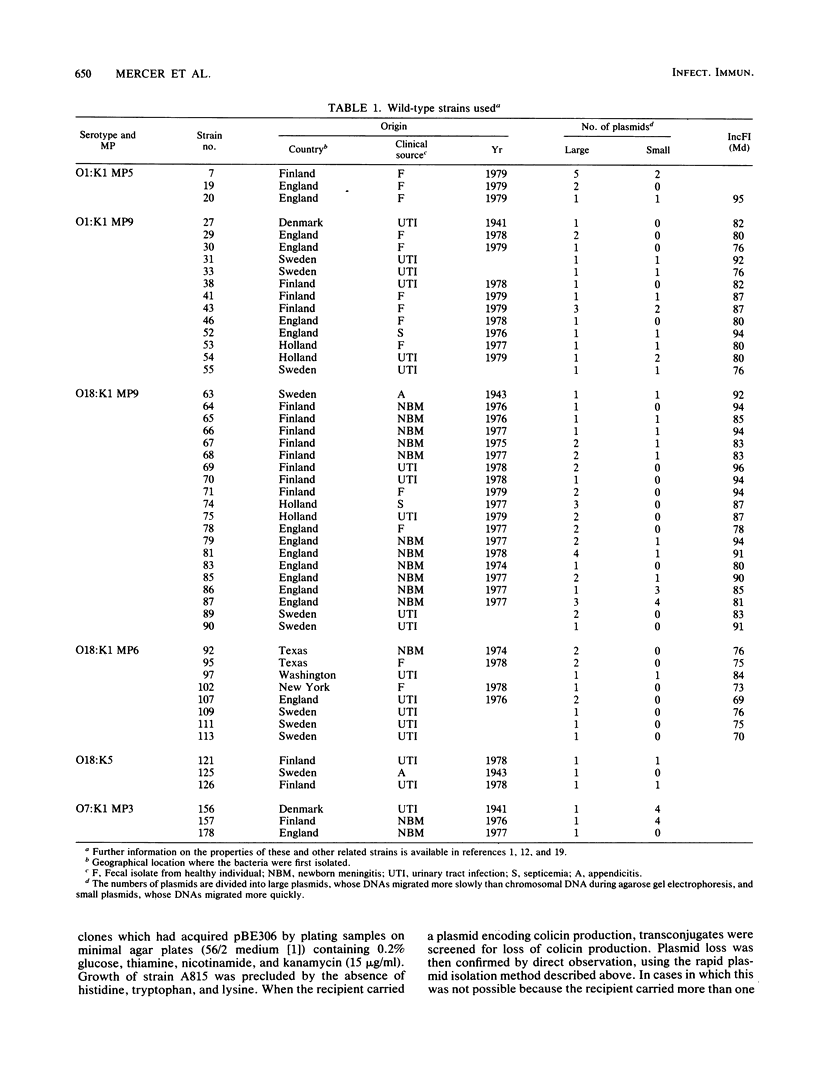
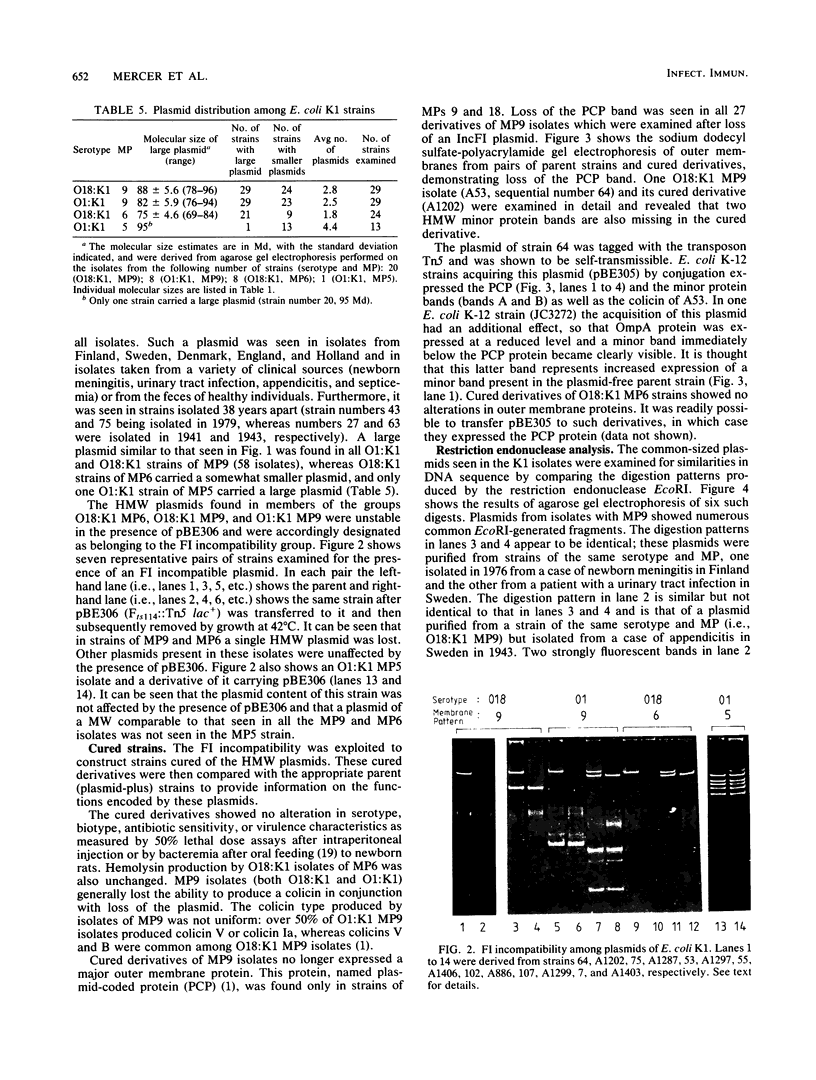
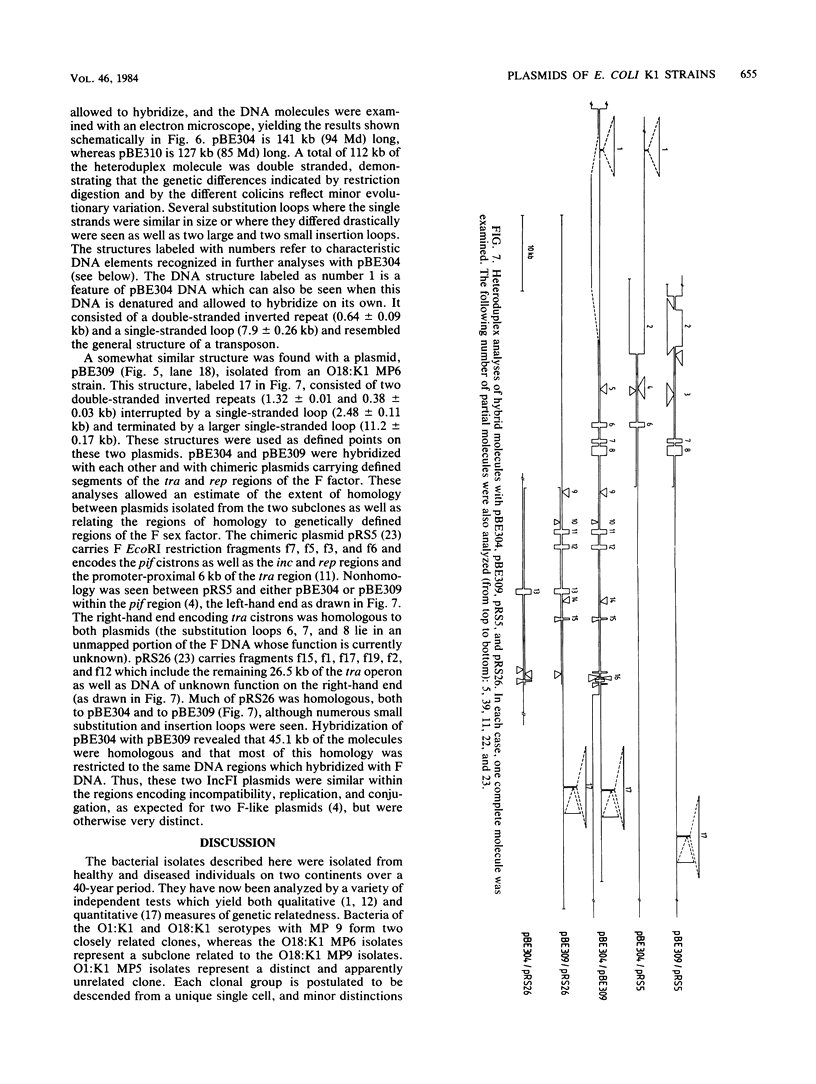
Escherichia coli K1 isolates of various O types were previously assigned to different clonal groups. Members of the two clones defined by membrane pattern 9 (MP9) and serotypes O18:K1 and O1:K1 had been found to be very similar to each other. The plasmid contents of these bacteria confirmed this conclusion. Both groups carried a self-transmissible plasmid of the FI incompatibility group that coded for colicin production and a major outer membrane protein called the plasmid-coded protein (PCP). The size of this plasmid varied from 76 to 96 megadaltons, but restriction endonuclease digestion and DNA heteroduplex analysis revealed that these plasmids were highly related. O18:K1 bacteria of MP6 had previously been determined to represent a subclone, related to but different from O18:K1 MP9 bacteria. These MP6 bacteria carried a different, smaller IncFI plasmid which did not code for colicin production or the PCP protein. This smaller plasmid was primarily related to the larger plasmid within the regions of DNA encoding incompatibility, replication, and conjugation. O1:K1 bacteria of MP5 contained other unrelated plasmids in agreement with the previous conclusion that they are unrelated to O1:K1 bacteria of MP9. The bacteria examined had been isolated from two continents over a time span of 38 years, and the results attest to conservative inheritance of plasmids within bacteria of common descent.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist P. L., Lane H. E., Malcolm L., Downard R. A. Molecular homology and incompatibility in the IncFI plasmid Group. J Gen Microbiol. 1982 Feb;128(2):223–238. doi: 10.1099/00221287-128-2-223. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy N., Beutin L., Achtman M., Skurray R., Rahmsdorf U., Herrlich P. Conjugation proteins encoded by the F sex factor. Nature. 1977 Dec 15;270(5638):580–585. doi: 10.1038/270580a0. [DOI] [PubMed] [Google Scholar]
- Kusecek B., Wloch H., Mercer A., Vaisänen V., Pluschke G., Korhonen T., Achtman M. Lipopolysaccharide, capsule, and fimbriae as virulence factors among O1, O7, O16, O18, or O75 and K1, K5, or K100 Escherichia coli. Infect Immun. 1984 Jan;43(1):368–379. doi: 10.1128/iai.43.1.368-379.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A., Witchitz J., Courvalin P. Modular evolution of disseminated Inc 7-M plasmids encoding gentamicin resistance. Plasmid. 1982 Nov;8(3):215–231. doi: 10.1016/0147-619x(82)90060-9. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Beutin L., Achtman M. Outer membrane of Escherichia coli: properties of the F sex factor traT protein which is involved in surface exclusion. J Bacteriol. 1980 Apr;142(1):285–294. doi: 10.1128/jb.142.1.285-294.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe W. R., Kaijser B., Olling S., Uwaydah M., Hanson L. A. Escherichia coli in bacteremia: K and O antigens and serum sensitivity of strains from adults and neonates. J Infect Dis. 1978 Jul;138(1):33–41. doi: 10.1093/infdis/138.1.33. [DOI] [PubMed] [Google Scholar]
- Morelli G., Montenegro M. A., Hillenbrandt G., Scherzinger E., Trautner T. A. The genome of B. subtilis phage SPP1: assignment of 5'--3'-polarity to the complementary strands of SPP1 DNA. Mol Gen Genet. 1978 Aug 4;164(1):93–97. doi: 10.1007/BF00267603. [DOI] [PubMed] [Google Scholar]
- Ochman H., Selander R. K. Evidence for clonal population structure in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(1):198–201. doi: 10.1073/pnas.81.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchaudhuri S., Maas W. K. Physical mapping of a DNA sequence common to plasmids of incompatibility group F I. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1190–1194. doi: 10.1073/pnas.74.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Mercer A., Kusećek B., Pohl A., Achtman M. Induction of bacteremia in newborn rats by Escherichia coli K1 is correlated with only certain O (lipopolysaccharide) antigen types. Infect Immun. 1983 Feb;39(2):599–608. doi: 10.1128/iai.39.2.599-608.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Aaronson W., Sutton A., Schneerson R. Comparative analysis of plasmids and some metabolic characteristics of Escherichia coli K1 from diseased and healthy individuals. Infect Immun. 1980 Jul;29(1):200–206. doi: 10.1128/iai.29.1.200-206.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurray R. A., Nagaishi H., Clark A. J. Molecular cloning of DNA from F sex factor of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1976 Jan;73(1):64–68. doi: 10.1073/pnas.73.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So M., Crosa J. H., Falkow S. Polynucleotide sequence relationships among Ent plasmids and the relationship between Ent and other plasmids. J Bacteriol. 1975 Jan;121(1):234–238. doi: 10.1128/jb.121.1.234-238.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]