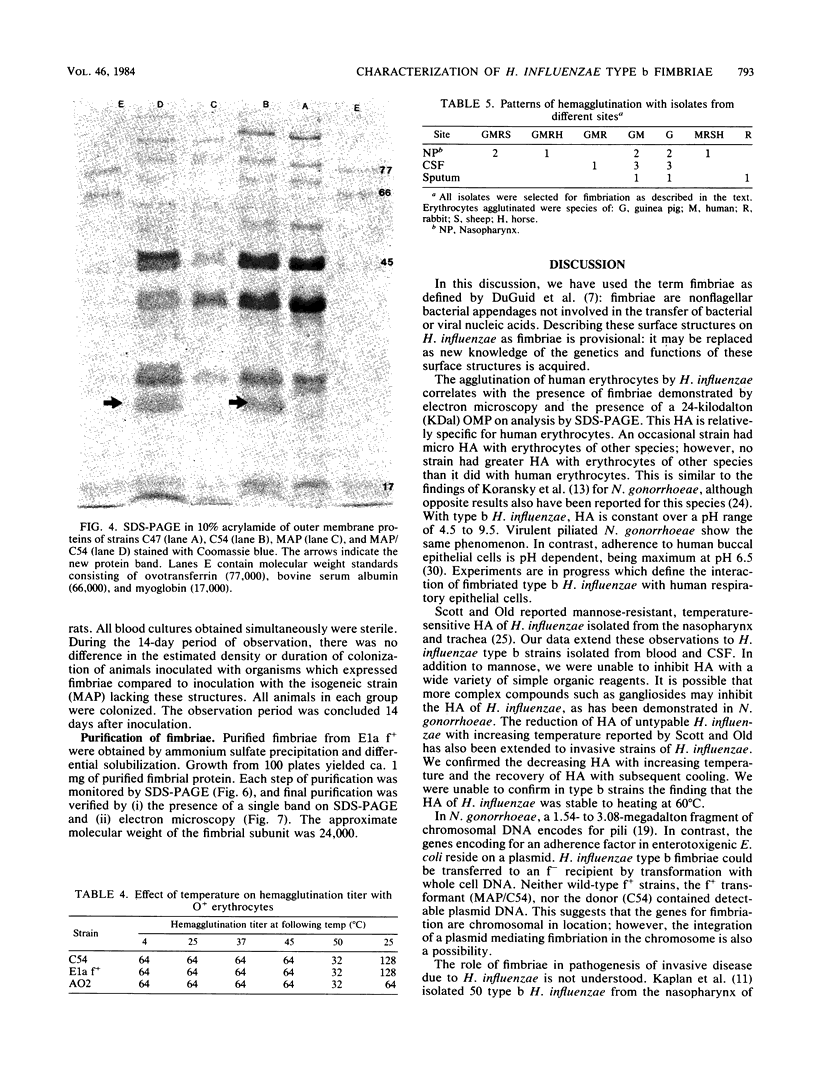
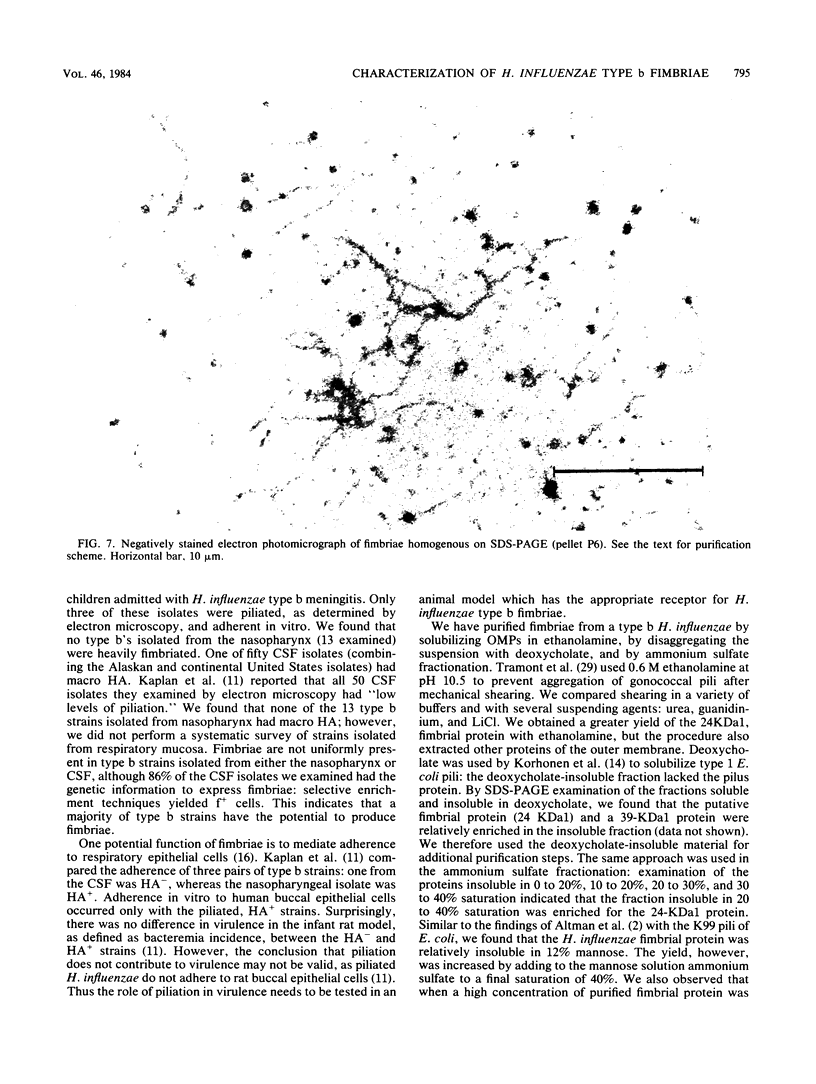
Abstract
We confirmed that the fimbriae of Haemophilus influenzae type b conferred hemagglutinating activity (HA) towards human erythrocytes, and erythrocytes of certain other species. Most (17/25) cerebrospinal fluid isolates lacked detectable HA on direct testing, but selective enrichment for fimbriation (f+) indicated that 22 of 25 strains could produce these surface structures. HA was unchanged from pH 4.5 to 9.5 and was not inhibited by mannose or certain other simple sugars. The HA titer of a suspension of three f+ strains was slightly decreased at 50 degrees C; HA was lost by heating at 60 degrees C for 3 min. Growth on a variety of solid and liquid media and under differing degrees of oxygenation did not change the HA titer of a suspension of three f+ strains. Fimbriation was not lost on repeated subculture. Wild-type fimbriated strains, and those derived by transformation, did not contain detectable plasmid DNA. Transformation of a strain lacking fimbriae to f+ was associated with the appearance of an outer membrane protein of 24 kilodaltons. This protein was purified from one strain to homogeneity on sodium dodecyl sulfate-polyacrylamide gel electrophoresis by selective detergent solubilization and ammonium sulfate fractionation. Colonization capacity was equivalent with an isogenic untypable strain lacking or possessing fimbriae. Fimbriae of type b H. influenzae possess characteristics similar to those structures on other gram-negative bacteria; their role in cell physiology or pathogenesis of invasive disease is unknown.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adegbola R. A., Old D. C. New fimbrial hemagglutinin in Serratia species. Infect Immun. 1982 Oct;38(1):306–315. doi: 10.1128/iai.38.1.306-315.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann K., Pyliotis N. A., Wetherall J. D., Mukkur T. K. A chromatographic method for the purification of K99 pili from enterotoxigenic Escherichia coli. J Gen Microbiol. 1983 Jun;129(6):1975–1982. doi: 10.1099/00221287-129-6-1975. [DOI] [PubMed] [Google Scholar]
- Back A. E., Oberhofer T. R. Use of the Minitek system for biotyping Haemophilus species. J Clin Microbiol. 1978 Mar;7(3):312–313. doi: 10.1128/jcm.7.3.312-313.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W., Bendler J. W., 3rd, Goodgal S. H. The type b capsulation locus of Haemophilus influenzae: map location and size. J Gen Microbiol. 1972 May;70(3):411–422. doi: 10.1099/00221287-70-3-411. [DOI] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Connor E. M., Loeb M. R. A hemadsorption method for detection of colonies of Haemophilus influenzae type b expressing fimbriae. J Infect Dis. 1983 Nov;148(5):855–860. doi: 10.1093/infdis/148.5.855. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Anderson E. S., Campbell I. Fimbriae and adhesive properties in Salmonellae. J Pathol Bacteriol. 1966 Jul;92(1):107–138. doi: 10.1002/path.1700920113. [DOI] [PubMed] [Google Scholar]
- Giesa F. R., Zajac I., Bartus H. F., Actor P. Isopycnic separation of Escherichia coli cultures possessing colonization factor antigen I. J Clin Microbiol. 1982 Jun;15(6):1074–1076. doi: 10.1128/jcm.15.6.1074-1076.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerina N. G., Langermann S., Clegg H. W., Kessler T. W., Goldman D. A., Gilsdorf J. R. Adherence of piliated Haemophilus influenzae type b to human oropharyngeal cells. J Infect Dis. 1982 Oct;146(4):564–564. doi: 10.1093/infdis/146.4.564. [DOI] [PubMed] [Google Scholar]
- Kahn M. E., Gromkova R. Occurrence of pili on and adhesive properties of Haemophilus parainfluenzae. J Bacteriol. 1981 Feb;145(2):1075–1078. doi: 10.1128/jb.145.2.1075-1078.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan S. L., Mason E. O., Jr, Wiedermann B. L. Role of adherence in the pathogenesis of Haemophilus influenzae type b infection in infant rats. Infect Immun. 1983 Nov;42(2):612–617. doi: 10.1128/iai.42.2.612-617.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976 Mar;93(1):9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- Koransky J. R., Scales R. W., Kraus S. J. Bacterial hemagglutination by Neisseria gonorrhoeae. Infect Immun. 1975 Sep;12(3):495–498. doi: 10.1128/iai.12.3.495-498.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Nurmiaho E. L., Ranta H., Edén C. S. New Method for isolation of immunologically pure pili from Escherichia coli. Infect Immun. 1980 Feb;27(2):569–575. doi: 10.1128/iai.27.2.569-575.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lampe R. M., Mason E. O., Jr, Kaplan S. L., Umstead C. L., Yow M. D., Feigin R. D. Adherence of Haemophilus influenzae to buccal epithelial cells. Infect Immun. 1982 Jan;35(1):166–172. doi: 10.1128/iai.35.1.166-172.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D., Clayton N. L., Setlow J. K. A plasmid cloning vehicle for Haemophilus influenzae and Escherichia coli. J Bacteriol. 1982 Sep;151(3):1605–1607. doi: 10.1128/jb.151.3.1605-1607.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Street C. H., Chappell C. L., Cousar E. S., Morris F., Horn R. G. Pili of Neisseria meningitidis: effect of media on maintenance of piliation, characteristics of Pili, and colonial morphology. Infect Immun. 1979 Apr;24(1):194–201. doi: 10.1128/iai.24.1.194-201.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce W. A., Buchanan T. M. Attachment role of gonococcal pili. Optimum conditions and quantitation of adherence of isolated pili to human cells in vitro. J Clin Invest. 1978 Apr;61(4):931–943. doi: 10.1172/JCI109018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pichichero M. E., Loeb M., Anderson, Smith D. H. Do pili play a role in pathogenicity of Haemophilus influenzae type B? Lancet. 1982 Oct 30;2(8305):960–962. doi: 10.1016/s0140-6736(82)90161-1. [DOI] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S., Smith A. L., Anderson P., Smith D. H. The paradox of Hemophilus infuenzae type B bacteremia in the presence of serum bactericidal activity. J Clin Invest. 1976 Oct;58(4):1019–1029. doi: 10.1172/JCI108525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L., Smith D. H., Averill D. R., Jr, Marino J., Moxon E. R. Production of Haemophilus influenzae b meningitis in infant rats by intraperitoneal inoculation. Infect Immun. 1973 Aug;8(2):278–290. doi: 10.1128/iai.8.2.278-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C., Sadoff J. C., Boslego J. W., Ciak J., McChesney D., Brinton C. C., Wood S., Takafuji E. Gonococcal pilus vaccine. Studies of antigenicity and inhibition of attachment. J Clin Invest. 1981 Oct;68(4):881–888. doi: 10.1172/JCI110343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trust T. J., Lambden P. R., Watt P. J. The cohesive properties of variants of Neisseria gonorrhoeae strain P9: specific pilus-mediated and non-specific interactions. J Gen Microbiol. 1980 Jul;119(1):179–187. doi: 10.1099/00221287-119-1-179. [DOI] [PubMed] [Google Scholar]
- Wong K., Roberts M. C., Smith A. L. The activity of Sch 29482 against type b Haemophilus influenzae lacking or possessing detectable beta-lactamase activity. J Antimicrob Chemother. 1982 Feb;9 (Suppl 100):163–170. doi: 10.1093/jac/9.suppl_c.163. [DOI] [PubMed] [Google Scholar]