Abstract
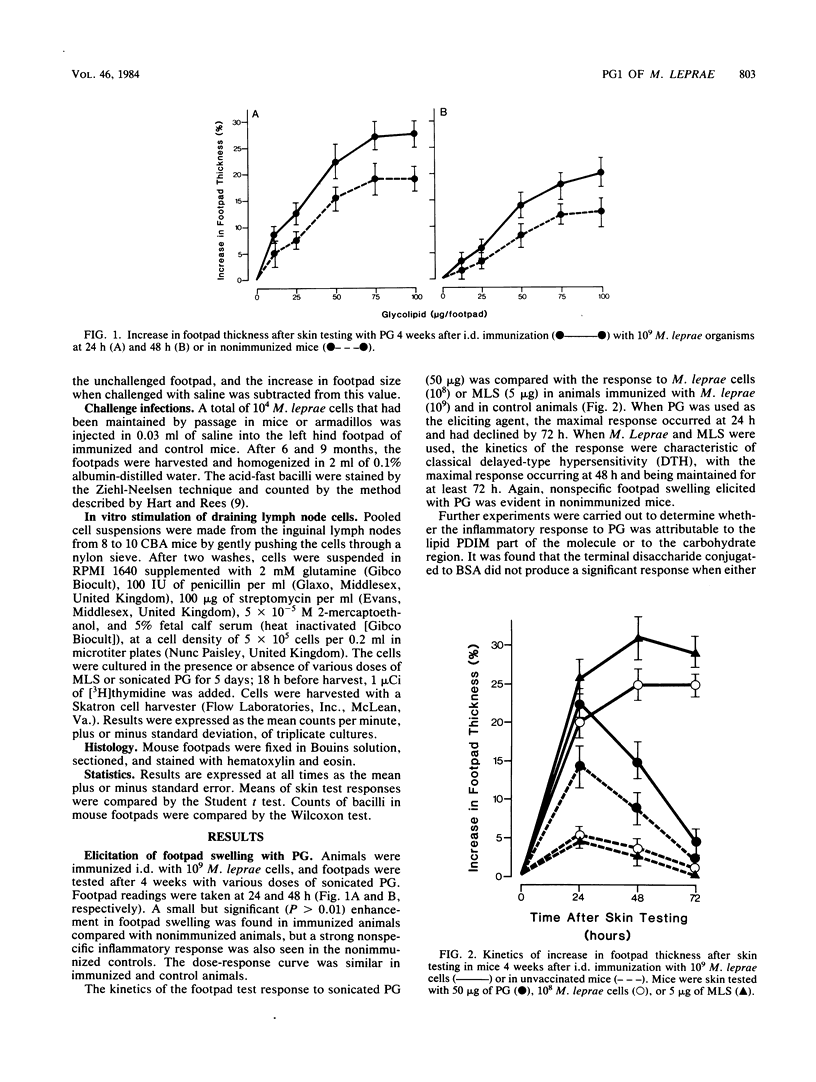
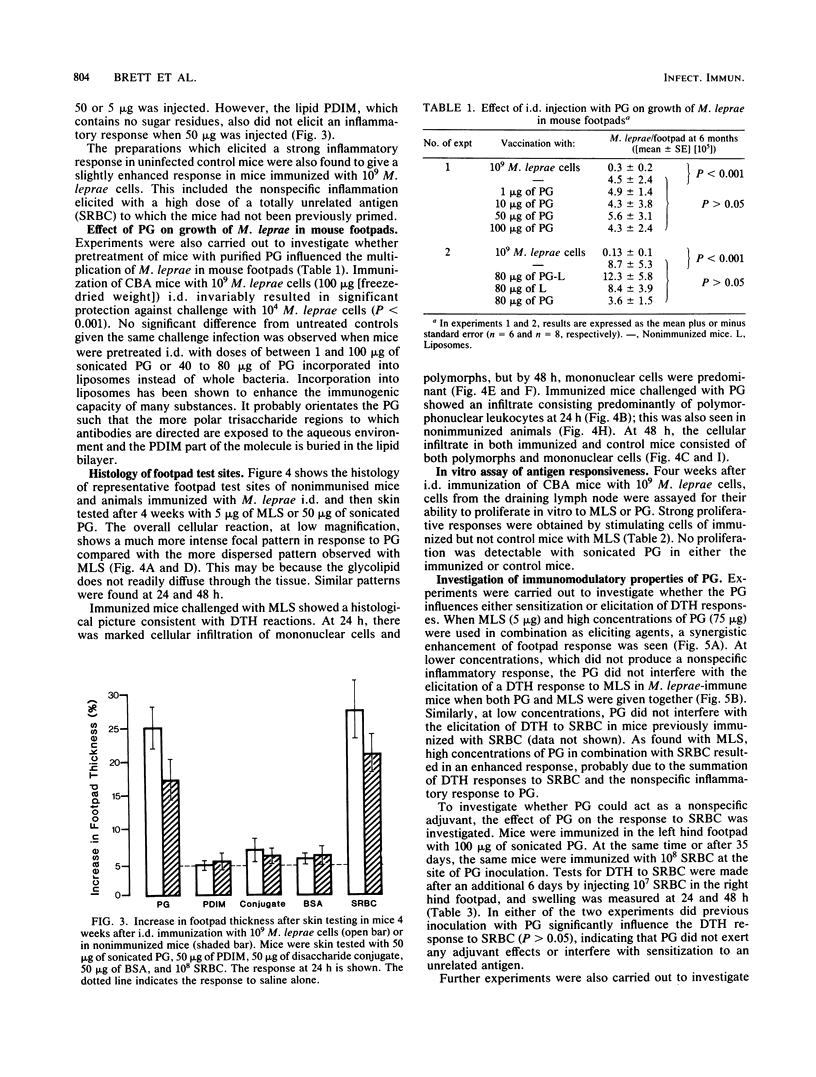
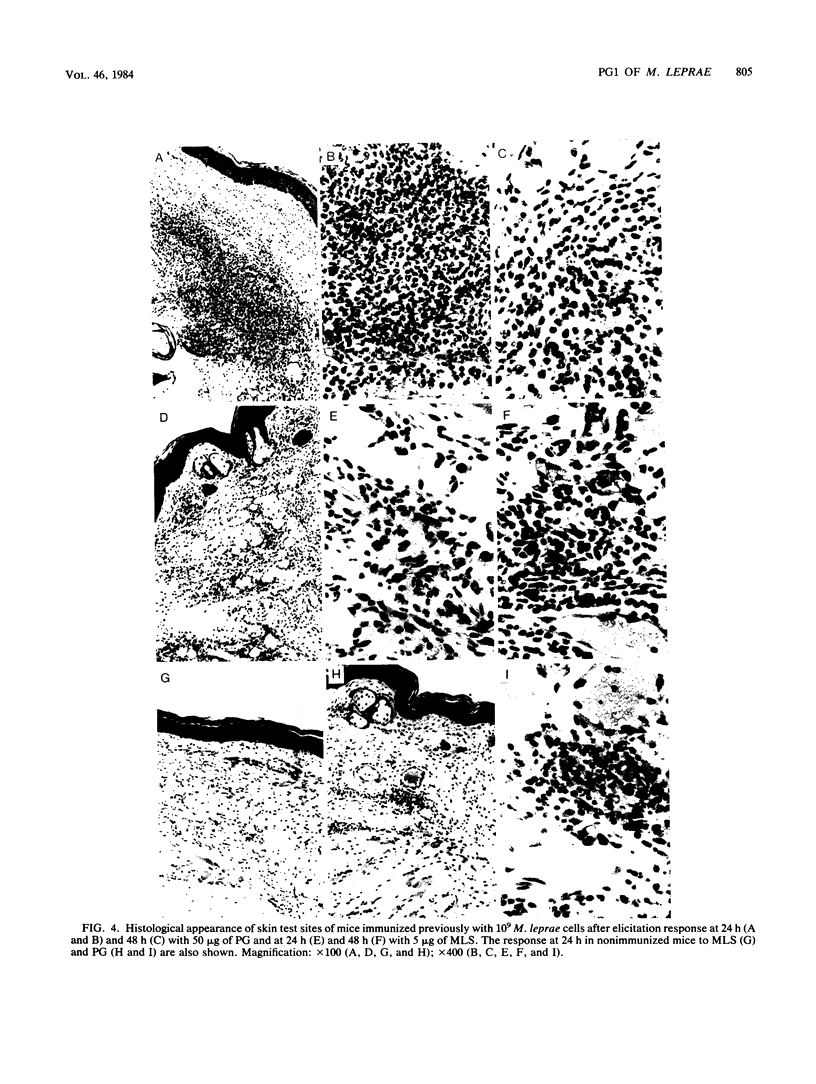
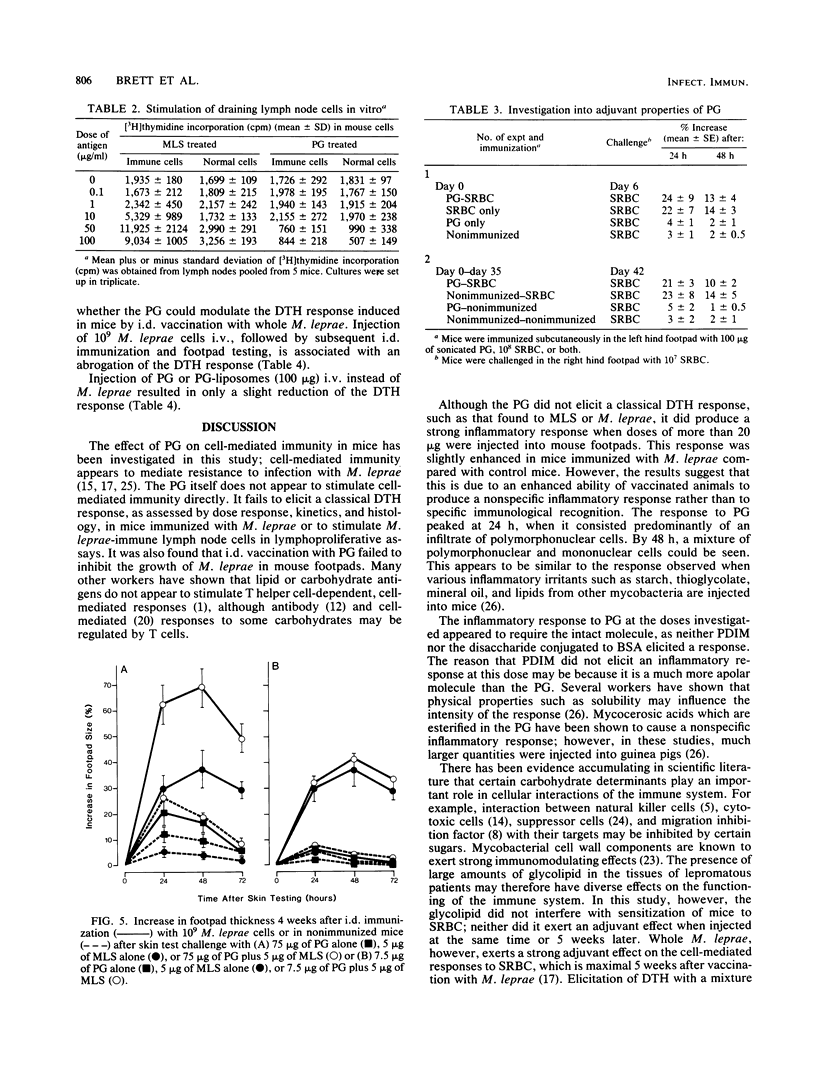
The involvement of the phenolic glycolipid from Mycobacterium leprae in cell-mediated immunity has been investigated in this study. The phenolic glycolipid itself does not appear to stimulate cell-mediated immunity directly, as shown by its failure to elicit a classical delayed-type hypersensitivity response in mice immunized with M. leprae or to stimulate M. leprae-immune lymph node cells in a lymphoproliferative assay. Intradermal vaccination with the phenolic glycolipid failed to influence the growth of M. leprae in mouse footpads. A nonspecific inflammatory response to the sonicated glycolipid was observed in mice vaccinated with whole M. leprae and in control animals. No evidence was obtained for any adjuvant or suppressive effect on cell-mediated immunity by the phenolic glycolipid either to M. leprae or an unrelated antigen (sheep erythrocytes); neither sensitization nor elicitation to either antigen was affected.
Full text
PDF
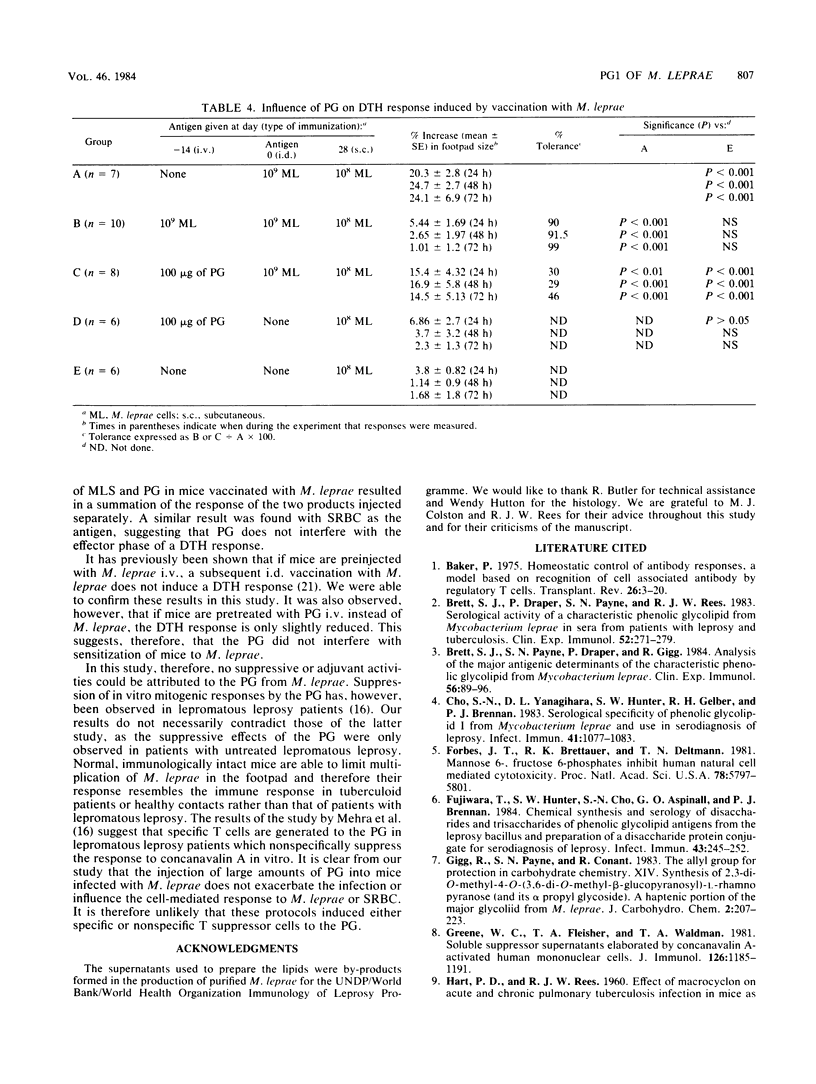

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J. Homeostatic control of antibody responses: a model based on the recognition of cell-associated antibody by regulatory T cells. Transplant Rev. 1975;26:3–20. doi: 10.1111/j.1600-065x.1975.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Draper P., Payne S. N., Rees R. J. Serological activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin Exp Immunol. 1983 May;52(2):271–279. [PMC free article] [PubMed] [Google Scholar]
- Brett S. J., Payne S. N., Draper P., Gigg R. Analysis of the major antigenic determinants of the characteristic phenolic glycolipid from Mycobacterium leprae. Clin Exp Immunol. 1984 Apr;56(1):89–96. [PMC free article] [PubMed] [Google Scholar]
- Cho S. N., Yanagihara D. L., Hunter S. W., Gelber R. H., Brennan P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect Immun. 1983 Sep;41(3):1077–1083. doi: 10.1128/iai.41.3.1077-1083.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes J. T., Bretthauer R. K., Oeltmann T. N. Mannose 6-, fructose 1-, and fructose 6-phosphates inhibit human natural cell-mediated cytotoxicity. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5797–5801. doi: 10.1073/pnas.78.9.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Hunter S. W., Cho S. N., Aspinall G. O., Brennan P. J. Chemical synthesis and serology of disaccharides and trisaccharides of phenolic glycolipid antigens from the leprosy bacillus and preparation of a disaccharide protein conjugate for serodiagnosis of leprosy. Infect Immun. 1984 Jan;43(1):245–252. doi: 10.1128/iai.43.1.245-252.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene W. C., Fleisher T. A., Waldmann T. A. Soluble suppressor supernatants elaborated by concanavalin A-activated human mononuclear cells. I. Characterization of a soluble suppressor T cell proliferation. J Immunol. 1981 Mar;126(3):1185–1191. [PubMed] [Google Scholar]
- Hunter S. W., Brennan P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J Bacteriol. 1981 Sep;147(3):728–735. doi: 10.1128/jb.147.3.728-735.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Fujiwara T., Brennan P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J Biol Chem. 1982 Dec 25;257(24):15072–15078. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Letvin N. L., Benacerraf B., Germain R. N. B-lymphocyte responses to trinitrophenyl-conjugated Ficoll: requirement for T lymphocytes and Ia-bearing adherent cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5113–5117. doi: 10.1073/pnas.78.8.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Cerottini J. C. Inhibition of T cell-mediated cytolysis by 2-deoxy-D-glucose (2-DG): differential effect of 2-DG on effector cells isolated early or late after alloantigenic stimulation in vitro. J Immunol. 1979 Mar;122(3):1067–1072. [PubMed] [Google Scholar]
- Mehra V., Bloom B. R. Induction of cell-mediated immunity to Mycobacterium leprae in guinea pigs. Infect Immun. 1979 Mar;23(3):787–794. doi: 10.1128/iai.23.3.787-794.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Brennan P. J., Rada E., Convit J., Bloom B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature. 1984 Mar 8;308(5955):194–196. doi: 10.1038/308194a0. [DOI] [PubMed] [Google Scholar]
- Patel P. J., Lefford M. J. Induction of cell-mediated immunity to Mycobacterium leprae in mice. Infect Immun. 1978 Jan;19(1):87–93. doi: 10.1128/iai.19.1.87-93.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. N., Draper P., Rees R. J. Serological activity of purified glycolipid from Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 1982 Jun;50(2):220–221. [PubMed] [Google Scholar]
- Schwartz B. A., Gray G. R. Proteins containing reductively aminated disaccharides. Synthesis and chemical characterization. Arch Biochem Biophys. 1977 Jun;181(2):542–549. doi: 10.1016/0003-9861(77)90261-2. [DOI] [PubMed] [Google Scholar]
- Shapiro M. E., Onderdonk A. B., Kasper D. L., Finberg R. W. Cellular immunity to Bacteroides fragilis capsular polysaccharide. J Exp Med. 1982 Apr 1;155(4):1188–1197. doi: 10.1084/jem.155.4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard C. C., Walker L. L., Van Landingham R. M., Ye S. Z. Sensitization or tolerance to Mycobacterium leprae antigen by route of injection. Infect Immun. 1982 Nov;38(2):673–680. doi: 10.1128/iai.38.2.673-680.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelt A. H., Rees R. J., Liew F. Y. Induction of delayed-type hypersensitivity to Mycobacterium leprae in healthy individuals. Clin Exp Immunol. 1981 Jun;44(3):501–506. [PMC free article] [PubMed] [Google Scholar]
- Tosato G., Pike S. E., Blaese R. M. Reversal of infectious mononucleosis-associated suppressor T cell activity by D-mannose. J Exp Med. 1983 Oct 1;158(4):1048–1060. doi: 10.1084/jem.158.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk J. L., Bryceson A. D. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266. doi: 10.1016/s0065-2776(08)60185-6. [DOI] [PubMed] [Google Scholar]
- Young D. B., Buchanan T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science. 1983 Sep 9;221(4615):1057–1059. doi: 10.1126/science.6348948. [DOI] [PubMed] [Google Scholar]
- Young D. B. Detection of mycobacterial lipids in skin biopsies from leprosy patients. Int J Lepr Other Mycobact Dis. 1981 Jun;49(2):198–204. [PubMed] [Google Scholar]
- Young D. B., Khanolkar S. R., Barg L. L., Buchanan T. M. Generation and characterization of monoclonal antibodies to the phenolic glycolipid of Mycobacterium leprae. Infect Immun. 1984 Jan;43(1):183–188. doi: 10.1128/iai.43.1.183-188.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



