Abstract
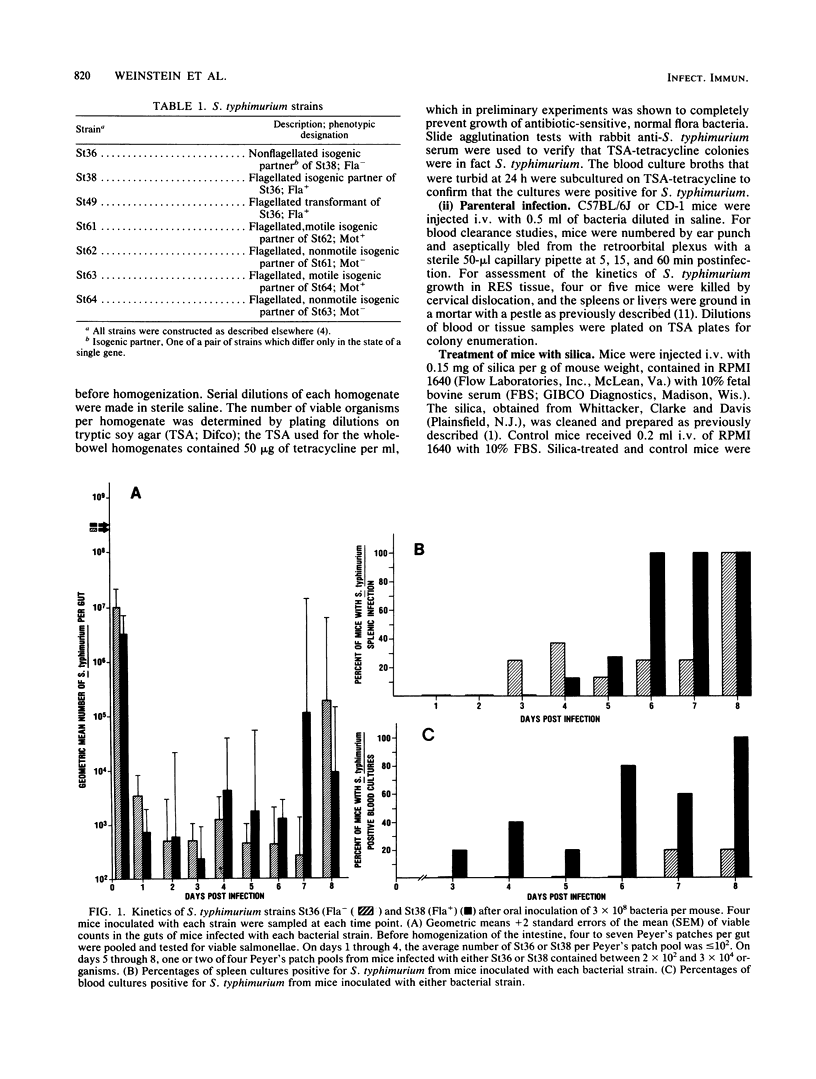
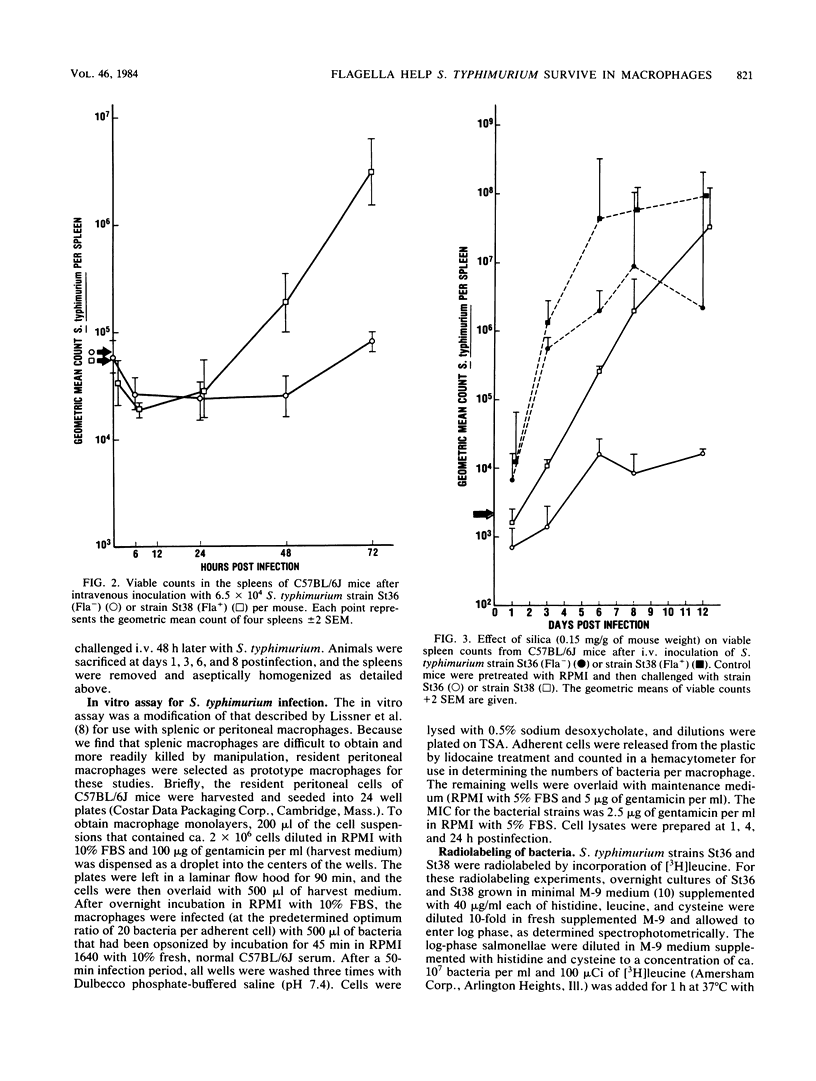
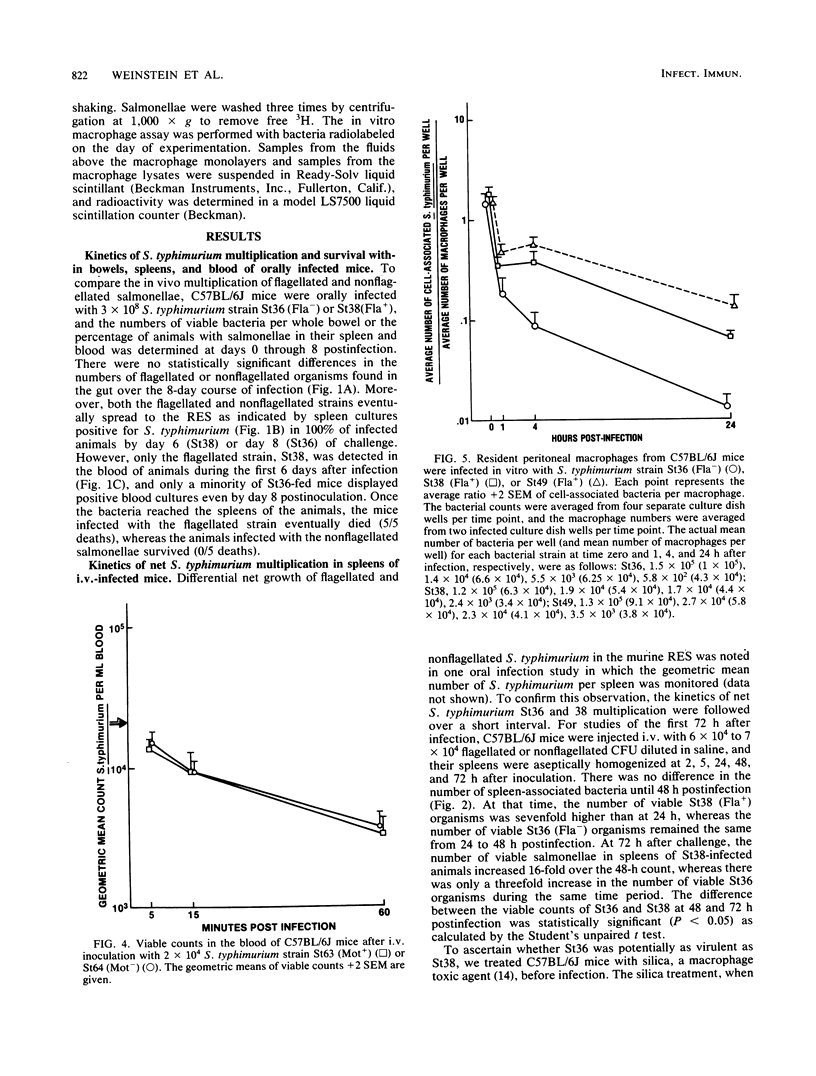
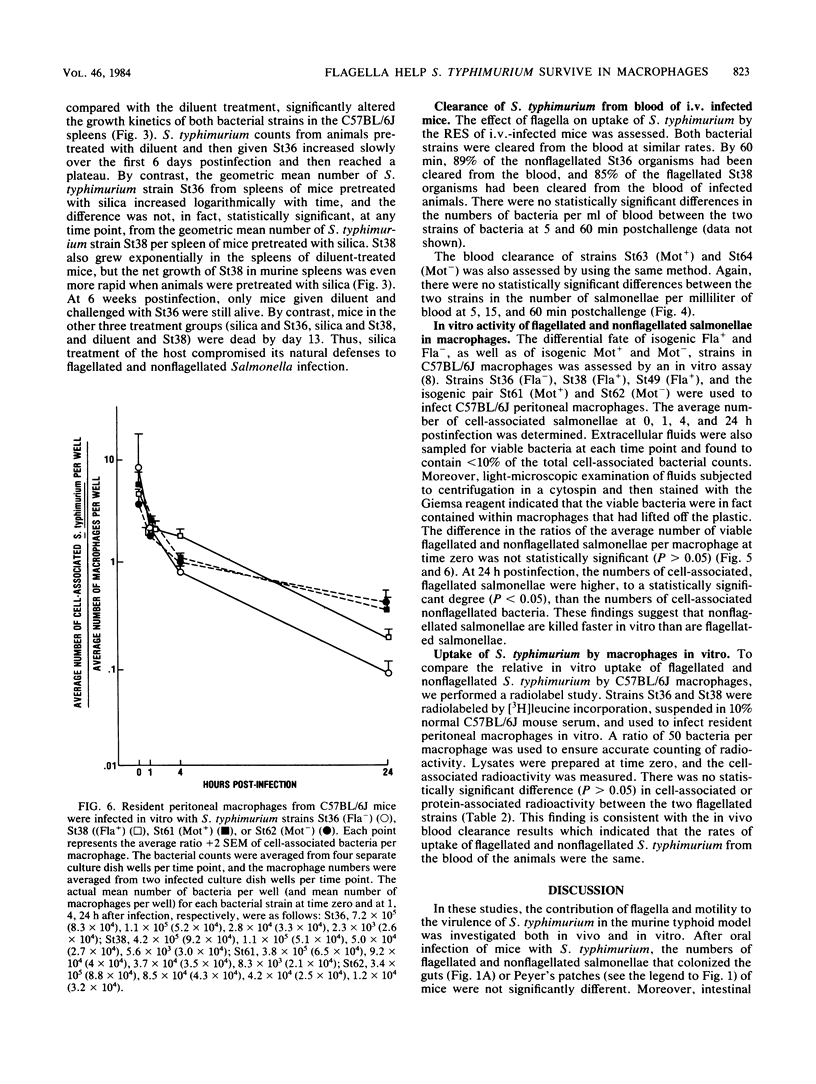
In this study, we evaluated how flagella enhance the pathogenicity of Salmonella typhimurium in strain C57BL/6J mice. When mice were infected orally with flagellated or nonflagellated S. typhimurium, equivalent numbers of bacteria colonized the gastrointestinal tracts of the animals, but the number of flagellated organisms increased faster once colonization began in the spleens and livers. To evaluate this differential rate of Salmonella growth, the rate of blood clearance, and the kinetics of net multiplication of salmonellae in splenic tissue after intravenous challenge, the two groups of mice were compared. We found that clearance of bacteria from the blood was the same for flagellated or nonflagellated strains. However, the number of flagellated bacteria in the spleen increased logarithmically until the death of the animals, whereas the number of nonflagellated salmonellae increased only slightly. In contrast, both flagellated and nonflagellated strains grew exponentially in the spleens of mice pretreated with silica, a macrophage toxic agent. In an in vitro macrophage assay, flagellated salmonellae survived longer than nonflagellated organisms. These results indicate that flagella either protect S. typhimurium from the intracellular killing mechanisms of murine macrophages or that flagella enhance the ability of S. typhimurium to multiply within murine macrophages.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. J., Taylor B. A., Blackwell J., Evans E. P., Freeman J. Regulation of Leishmania populations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin Exp Immunol. 1979 Jul;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- Brown I. N., Glynn A. A., Plant J. Inbred mouse strain resistance to Mycobacterium lepraemurium follows the Ity/Lsh pattern. Immunology. 1982 Sep;47(1):149–156. [PMC free article] [PubMed] [Google Scholar]
- Carsiotis M., Weinstein D. L., Karch H., Holder I. A., O'Brien A. D. Flagella of Salmonella typhimurium are a virulence factor in infected C57BL/6J mice. Infect Immun. 1984 Dec;46(3):814–818. doi: 10.1128/iai.46.3.814-818.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Deakins L. W., Killar L., Saluk P. H., Sultzer B. M. Dissociation of innate susceptibility to Salmonella infection and endotoxin responsiveness in C3HeB/FeJ mice and other strains in the C3H lineage. Infect Immun. 1982 May;36(2):696–703. doi: 10.1128/iai.36.2.696-703.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann A. W., Schmidt G., Rowley D. Intestinal colonization and virulence of Salmonella in mice. Infect Immun. 1978 Dec;22(3):763–770. doi: 10.1128/iai.22.3.763-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick R. B., Greisman S. E., Woodward T. E., DuPont H. L., Dawkins A. T., Snyder M. J. Typhoid fever: pathogenesis and immunologic control. N Engl J Med. 1970 Sep 24;283(13):686–691. doi: 10.1056/NEJM197009242831306. [DOI] [PubMed] [Google Scholar]
- Lissner C. R., Swanson R. N., O'Brien A. D. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983 Dec;131(6):3006–3013. [PubMed] [Google Scholar]
- Maier T., Oels H. C. Role of the macrophage in natural resistance to salmonellosis in mice. Infect Immun. 1972 Oct;6(4):438–443. doi: 10.1128/iai.6.4.438-443.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Metcalf E. S. Control of early Salmonella typhimurium growth in innately Salmonella-resistant mice does not require functional T lymphocytes. J Immunol. 1982 Oct;129(4):1349–1351. [PubMed] [Google Scholar]
- O'Brien A. D., Rosenstreich D. L. Genetic control of the susceptibility of C3HeB/FeJ mice to Salmonella typhimurium is regulated by a locus distinct from known salmonella response genes. J Immunol. 1983 Dec;131(6):2613–2615. [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Campbell G. H., MacDermott R. P., Formal S. B. Susceptibility of CBA/N mice to infection with Salmonella typhimurium: influence of the X-linked gene controlling B lymphocyte function. J Immunol. 1979 Aug;123(2):720–724. [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Formal S. B. Effect of silica on the innate resistance of inbred mice to Salmonella typhimurium infection. Infect Immun. 1979 Aug;25(2):513–520. doi: 10.1128/iai.25.2.513-520.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson H. G., Vas S. I. Resistance of inbred mice to Salmonella typhimurium. J Infect Dis. 1972 Oct;126(4):378–386. doi: 10.1093/infdis/126.4.378. [DOI] [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P. A., St Charles C., Taylor B. A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982 Jun 10;297(5866):506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- Tannock G. W., Blumershine R. V., Savage D. C. Association of Salmonella typhimurium with, and its invasion of, the ileal mucosa in mice. Infect Immun. 1975 Feb;11(2):365–370. doi: 10.1128/iai.11.2.365-370.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T., Blumenstock E., Kanegasaki S. Phagocytic and chemiluminescent responses of mouse peritoneal macrophages to living and killed Salmonella typhimurium and other bacteria. Infect Immun. 1981 Jun;32(3):1242–1248. doi: 10.1128/iai.32.3.1242-1248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T., Kanegasaki S. Enhanced phagocytic response of macrophages to bacteria by physical impact caused by bacterial motility or centrifugation. Infect Immun. 1982 Dec;38(3):865–870. doi: 10.1128/iai.38.3.865-870.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Jeney N., Günther E., Jann K. Mitogenic stimulation of murine spleen cells: relation to susceptibility to Salmonella infection. Infect Immun. 1977 Jan;15(1):26–33. doi: 10.1128/iai.15.1.26-33.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


