Abstract
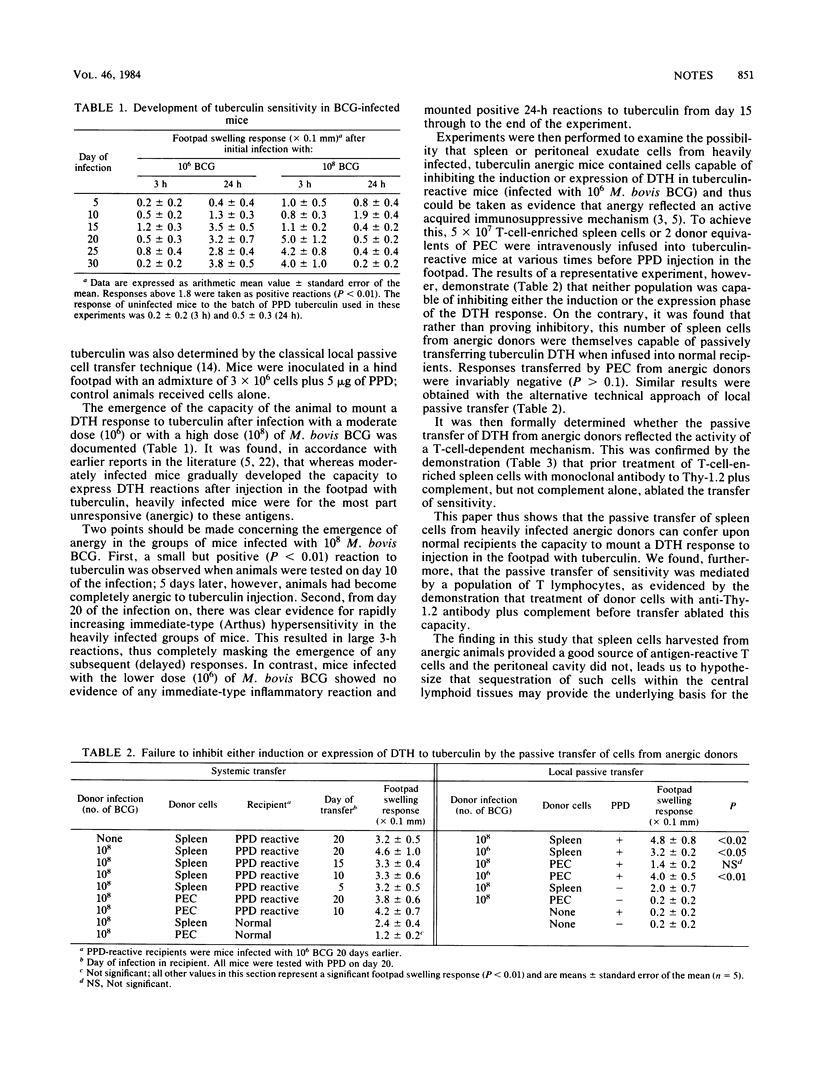
Mice heavily infected with Mycobacterium bovis BCG rapidly became anergic to cutaneous injection with tuberculin. Evidence is presented suggesting that this anergy reflects an adaptive physiological change within the host in which antigen-reactive Thy-1.2+ cells become sequestered in central lymphoid tissues, with a concomitant reduction in the circulating pool. No evidence could be provided to support the suggestion that anergy was a consequence of an acquired immunosuppressive mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba T., Yoshida T., Cohen S. Desensitization. III: The role of lymphokines in the maintenance of the anergic state during desensitization. J Exp Pathol. 1983;1(1):39–48. [PubMed] [Google Scholar]
- Bullock W. E. Leprosy: a model of immunological perturbation in chronic infection. J Infect Dis. 1978 Mar;137(3):341–354. doi: 10.1093/infdis/137.3.341. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. I. Tuberculin sensitivity and resistance to reinfection in BCG-vaccinated mice. Cell Immunol. 1970 Sep;1(3):253–265. doi: 10.1016/0008-8749(70)90047-x. [DOI] [PubMed] [Google Scholar]
- Collins F. M. The immunology of tuberculosis. Am Rev Respir Dis. 1982 Mar;125(3 Pt 2):42–49. doi: 10.1164/arrd.1982.125.3P2.42. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Watson S. R. Suppressor T-cells in BCG-infected mice. Infect Immun. 1979 Aug;25(2):491–496. doi: 10.1128/iai.25.2.491-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer J. M., Parker D., Turk J. L. Suppression of delayed hypersensitivity to tuberculin by antigenic competition. A positive immunoregulatory mechanism sensitive to cyclophosphamide. Immunology. 1981 Apr;42(4):549–559. [PMC free article] [PubMed] [Google Scholar]
- Ford W. L., Atkins R. C. Specific unresponsiveness of recirculating lymphocytes ater exposure to histocompatibility antigen in F 1 hybrid rats. Nat New Biol. 1971 Dec 8;234(49):178–180. doi: 10.1038/newbio234178a0. [DOI] [PubMed] [Google Scholar]
- Holden M., Dubin M. R., Diamond P. H. Frequency of negative intermediate-strength tuberculin sensitivity in patients with active tuberculosis. N Engl J Med. 1971 Dec 30;285(27):1506–1509. doi: 10.1056/NEJM197112302852704. [DOI] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzini L., Rottoli P., Rottoli L. The spectrum of human tuberculosis. Clin Exp Immunol. 1977 Feb;27(2):230–237. [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METAXAS M. N., METAXAS-BUEHLER M. Studies on the cellular transfer of tuberculin sensitivity in the guinea pig. J Immunol. 1955 Nov;75(5):333–347. [PubMed] [Google Scholar]
- McConnell I., Lachmann P. J., Hobart M. J. Restoration of specific immunological virginity. Nature. 1974 Jul 12;250(462):113–116. doi: 10.1038/250113a0. [DOI] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- North R. J., Mackaness G. B. Immunological control of macrophage proliferation in vivo. Infect Immun. 1973 Jul;8(1):68–73. doi: 10.1128/iai.8.1.68-73.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983 Jul 1;158(1):74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Carswell J. W., Stanford J. L. Preliminary evidence for the trapping of antigen-specific lymphocytes in the lymphoid tissue of 'anergic' tuberculosis patients. Clin Exp Immunol. 1976 Oct;26(1):129–132. [PMC free article] [PubMed] [Google Scholar]
- Rowley D. A., Gowans J. L., Atkins R. C., Ford W. L., Smith M. E. The specific selection of recirculating lymphocytes by antigen in normal and preimmunized rats. J Exp Med. 1972 Sep 1;136(3):499–513. doi: 10.1084/jem.136.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossman S. F., Levin H. A., Rocklin R. E., David J. R. The compartmentalization of antigen-reactive lymphocytes in desensitized guinea pigs. J Exp Med. 1971 Sep 1;134(3 Pt 1):741–750. doi: 10.1084/jem.134.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Miller J. F., Mitchell G. F. Antigen-induced selective recruitment of circulating lymphocytes. Cell Immunol. 1971 Apr;2(2):171–181. doi: 10.1016/0008-8749(71)90036-0. [DOI] [PubMed] [Google Scholar]
- Turcotte R., Forget A. Cutaneous unresponsiveness to Mycobacterium bovis BCG in intravenously infected mice. Infect Immun. 1983 Aug;41(2):453–461. doi: 10.1128/iai.41.2.453-461.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman J., Lockshin M. In vitro and in vivo cellular immunity in anergic miliary tuberculosis. Am Rev Respir Dis. 1973 Apr;107(4):661–664. doi: 10.1164/arrd.1973.107.4.661. [DOI] [PubMed] [Google Scholar]
- Zeitz S. J., Ostrow J. H., Van Arsdel P. P., Jr Humoral and cellular immunity in the anergic tuberculosis patient. A prospective study. J Allergy Clin Immunol. 1974 Jan;53(1):20–26. doi: 10.1016/0091-6749(74)90095-5. [DOI] [PubMed] [Google Scholar]


