Abstract
Secondary amyloidosis is a common disease of water fowl and is characterized by the deposition of extracellular fibrils of amyloid A (AA) protein in the liver and certain other organs. Neither the normal role of serum amyloid A (SAA), a major acute phase response protein, nor the causes of secondary amyloidosis are well understood. To investigate a possible genetic contribution to disease susceptibility, we cloned and sequenced SAA cDNA derived from livers of domestic ducks. This revealed that the three C-terminal amino acids of SAA are removed during conversion to insoluble AA fibrils. Analysis of SAA cDNA sequences from several animals identified a distinct genetic dimorphism that may be relevant to susceptibility to secondary amyloid disease. The duck genome contained a single copy of the SAA gene that was expressed in liver and lung tissue of ducklings, even in the absence of induction of acute phase response. Genetic analysis of heterozygotes indicated that only one SAA allele is expressed in livers of adult birds. Immunofluorescence staining of livers from adult ducks displaying early symptoms of amyloidosis revealed what appear to be amyloid deposits within hepatocytes that are expressing unusually high amounts of SAA protein. This observation suggests that intracellular deposition of AA may represent an early event during development of secondary amyloidosis in older birds.
Amyloidosis of the secondary type occurs in most mammals as an occasional consequence of chronic inflammatory disease, though among water fowl, including domestic ducks, amyloidosis is relatively common (1–4). The disease is characterized by the deposition, primarily in the liver and to a lesser extent in other organs, of extracellular fibrils composed of amyloid A protein. Amyloid A protein (AA) is a form of serum amyloid A protein (SAA) and is generally found to be modified by proteolytic removal of C-terminal amino acids (5–7). SAA is an acute phase response protein synthesized in the liver (8–12). The normal physiological function of SAA, which constitutes a major component of the high density lipoprotein 3 complex in humans (13), remains unclear. The causes of secondary amyloidosis in water fowl are also unknown, especially in view of suggestive evidence that duck amyloid A protein has the same sequence as SAA (14). It remains a possibility that the disease is inherited, is due to chronic stimulation of the immune system by infectious agents and/or diet, or results from some other form of stress. In domestic flocks of Pekin ducks, the incidence of amyloid disease of the liver can reach >50% by sexual maturity (7–8 months) (15). The disease is apparently not linked to persistent infection with duck hepatitis B virus, which is common in domestic duck populations (16).
In the current study, we have undertaken a preliminary characterization of SAA expression, as a first step in determining if there are differences between ducks that could correlate with the rate of formation of AA deposits. To do this, antibody to purified AA protein was generated, the mRNA for SAA was cDNA cloned and sequenced, and expression patterns of SAA in duck tissues was determined. The amino acid sequence reported for duck AA protein in fibrils and comparison with human and murine SAA sequences has raised the possibility that AA deposition does not require C-terminal proteolytic processing (14). However, sequencing of the cDNA for duck SAA indicated that the reported amino acid sequence for duck AA protein present in fibrils lacks three C-terminal residues that are present in the soluble precursor protein. Thus, duck SAA is similar at its C end to human and murine SAA. Preliminary characterization of the SAA sequences within chromosomal DNA suggested that there is only a single gene encoding this product. Evidence for genetic dimorphism in the SAA gene was obtained and may be relevant to susceptibility to amyloid disease. Examination of livers from ducks showing early symptoms of amyloid disease indicated that intracellular deposition of amyloid protein may precede the appearance of extracellular amyloid fibrils.
MATERIALS AND METHODS
Purification of AA Protein from Fibrils.
AA protein was partially purified from the liver of a duck with severe amyloid disease following published procedures (17, 18) (details upon request). Antibodies to purified AA protein were raised in rabbits by following standard procedures. The characteristic pattern of AA fibril deposits was observed using immunofluorescence microscopy in various tissues from ducks with amyloid disease of the liver.
Immunofluorescence Staining and Confocal Microscopy.
Tissues were fixed with 95% (vol/vol) ethanol or ethanol/glacial acetic acid (95:5) and embedded in paraffin. Deparaffinated sections were incubated first with AA-specific rabbit antisera (see above) followed by goat antirabbit IgG conjugated to fluorescein-5-isothiocyanate (Cappel). Samples were examined and photographed using a Nikon Diaphot UV microscope and a Bio-Rad MRC 600 confocal microscope.
Subcloning and Sequencing of Duck SAA cDNA.
An oligo(dT)-primed cDNA expression library in phage λ gt22A DNA was prepared from poly(A)-selected duck liver RNA using a SuperScript kit (BRL). Approximately 5 × 105 plaque-forming units were plated and recombinant phage proteins were screened with a polyclonal rabbit antiserum to duck amyloid fibril protein. Several positive clones were isolated and two, clones 3.1 (nt 207–495) and 1.2 (nt 255–495), were selected for further analysis. cDNAs were subcloned into NotI/SalI cut pSPORT (BRL) to create plasmids pAmy 3.1 and 1.2, respectively. A 32P-labeled RNA prepared from pAmy 3.1 was used to screen ≈105 plaque-forming units from the same duck liver cDNA library that yielded six positive clones. The approximate size of each SAA cDNA was determined by PCR using λ gt11 forward and reverse primers (Invitrogen), and the largest of these (clone 3), which included the complete SAA coding sequence, was then subcloned into pGEM-T (Promega) and the nucleotide sequence determined (see below).
Southern and Northern Blot Analyses.
Genomic DNA was prepared from Pekin duck liver by a standard procedure and 10–30 μg was digested to completion with restriction endonucleases (see Fig. 2). DNA was fractionated on a 1% (wt/vol) agarose gel, transferred to nylon membrane (Hybond N; Amersham) and hybridized with a radiolabeled 32P duck SAA RNA of antisense polarity, prepared by in vitro transcription with T7 RNA polymerase of plasmid pGEM-SAA5′ linearized with SalI restriction endonuclease. pGEM-SAA5′ was created by subcloning the DNA product generated by PCR using the λ gt11 forward primer (Invitrogen) and a SAA-specific oligonucleotide (nt 276–296, Fig. 1, antisense polarity) with clone 3 phage DNA template into plasmid vector pGEM-T (Promega). To distinguish SAA types A and B, genomic DNA prepared from duck liver was digested with restriction endonucleases EcoRI and MluI, or with EcoRI alone and SAA DNA detected by Southern hybridization using a radiolabeled RNA prepared from pGEM-SAA5′, as described above.
Figure 2.
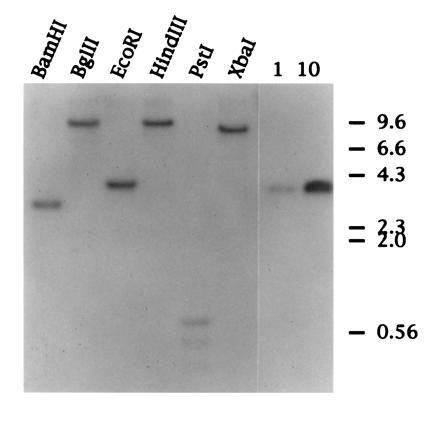
Southern blot hybridization of duck genomic DNA. Approximately 30 μg of duck genomic DNA was digested overnight with the restriction endonucleases indicated. DNA was resolved on a 1% (wt/vol) agarose gel and transferred to Hybond N membrane (Amersham). The lanes on the right represent 1 and 10 picograms of cloned duck SAA cDNA. The filter was hybridized with a radiolabeled RNA complementary to the 5′ of SAA cDNA (see Materials and Methods).
Figure 1.
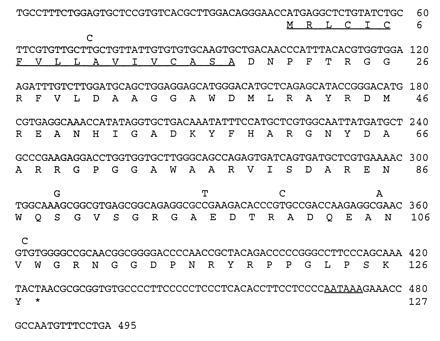
Nucleotide sequence of duck SAA cDNA aligned with the predicted amino acid sequence. The predicted N-terminal signal peptide and polyadenylylation signal sequence are underlined. The amino acid sequence derived from duck SAA genotype A is shown complete and the six conserved nucleotide changes in type B are shown above.
Poly(A)-selected RNA was prepared directly from ≈50-100 mg of frozen tissue by affinity chromatography of a proteinase K lysate on oligo(dT) cellulose (Invitrogen, Fast Track). For analysis of SAA expression in duck tissues, ≈1 μg of poly(A) selected RNA was resolved on a 1.5% formaldehyde agarose gel, transferred to nylon membrane (Hybond-N; Amersham) and hybridized with an antisense 32P-labeled SAA RNA prepared by in vitro transcription of pGEM-SAA5′ with T7 RNA polymerase, as described above.
Reverse Transcription (RT)–PCR and Subtyping of SAA mRNAs.
mRNA was prepared as described above, and ≈200 ng used for cDNA synthesis using oligo(dT) primer (0.4 μM), and (null)/1;50th of the cDNA was used directly in a standard PCR reaction using oligonucleotide JP61 (nt 2–22; Fig. 1, sense polarity) and JP62 (nt 475–495, Fig. 1, antisense polarity), respectively. The 500-bp PCR product was resolved on a 1% (wt/vol) agarose minigel and extracted from the gel using Qiaex resin (Qiagen, Chatsworth, CA). DNA was dissolved in water and subjected to automated dideoxy nucleotide sequencing in an Applied Biosystems “373 Stretch” machine using oligonucleotides JP61 and JP62. The two subtypes of SAA mRNA were detected by electrophoresis on a 4% (wt/vol) NuSieve (3:1) agarose gel (FMC) of DNA products generated by digestion of the RT-PCR DNA described above with MluI restriction endonuclease, followed by staining of the gel with ethidium bromide.
Sequencing of the Duck SAA Genomic DNA.
The SAA gene was amplified (LA PCR Kit, Takara Shuzo, Kyoto) from genomic duck DNA using oligonucleotide primers JP83 (nt 31–66; Fig. 1, sense) and JP84 (nt 458–593; Fig. 1, antisense), respectively, according to the manufacturer’s recommended conditions. The 1700-bp PCR product was cloned in plasmid pGEMT (Promega) and the nucleotide sequence determined using an Applied Biosystems machine and additional primers (details available upon request).
RESULTS
Comparative Sequence Analysis of the Duck SAA cDNA.
Details of the strategy we used to isolate a cDNA clone for duck SAA are described fully in Materials and Methods. The nucleotide sequence of this cDNA revealed that the precursor SAA protein contains 127 amino acids (Fig. 1). Comparison with the published protein amino acid sequence shows that the precursor includes an 18-residue signal peptide that is presumably removed during secretion from hepatocytes by signal peptidase. In addition, the cDNA sequence predicts the presence of three C-terminal residues (S-K-Y) in the precursor that are absent from protein that is deposited in amyloid fibrils.
A comparison of the predicted amino acid sequence of the duck SAA precursor protein with SAA from other animals illustrates that the SAA protein sequence has been extraordinarily well conserved during evolution (data not shown). This may suggest that SAA performs the same essential function in birds and mammals. Sequence conservation among SAAs between residues 59–79 (Fig. 1) is especially striking, which has led to speculation that this region may be important for fibril formation (13, 19).
The Duck SAA DNA Is Present in the Genome as a Single-Copy Gene.
To determine if there are multiple SAA genes in the duck genome as has been reported for several species, including humans, mice, rats, and dogs, we performed a Southern blot of Pekin duck genomic DNA digested with restriction endonucleases as detailed in Fig. 2. Hybridization was with a radiolabeled RNA complementary to the 5′ 296 nt of the 495-nt duck SAA cDNA (see Materials and Methods). Digestion of genomic DNA with restriction endonucleases that cleave outside of the SAA cDNA generated a single discrete DNA species that hybridized specifically with the radiolabeled RNA. Southern hybridization of DNA from four other duck liver DNA samples confirmed this result (data not shown), which indicates that only a single copy of the gene that encodes SAA is present within the duck genome. This is in marked contrast to the human and mouse genomes in which as many as five SAA genes have been reported. The data also suggest that there may have been a single SAA gene at the time when birds and mammals began to diverge, and that amplification and mutation of SAA genes within mammalian species occurred during evolution (20, 21). The presence of several SAA proteins that may exhibit subtle differences in structure and function may be linked to the more complex immune systems of mammals.
To further characterize the SAA gene we used primers representing the 5′ and 3′ ends of our cDNA to PCR amplify the SAA coding region from genomic duck DNA (see Materials and Methods). This generated a 1633-bp DNA that was then cloned and sequenced. Fig. 3 shows a schematic of the duck SAA gene structure and organization based upon both the nucleotide sequence and Southern hybridization data (Fig. 2) we have presented here. Comparison with the cDNA sequence identified three exons within this region of the SAA gene. The splice sites in the duck SAA mRNA coding region are located in precisely the same region relative to those in the mouse SAA genes, with highly conserved nucleotide sequence at the exon boundaries (data not shown), suggesting that the overall structure of the SAA gene has been conserved during evolution (20). The murine SAA gene has in addition a short untranslated 5′exon. We did not map the precise 5′ end of the duck SAA mRNA in this study and hence the possibility exists that the duck SAA gene also includes a similar small 5′exon.
Figure 3.

Schematic of structure and organization of duck SAA gene. Comparison of the duck SAA cDNA and genomic coding sequences. Exons I, II, and III are 146, 140, and 209 nt, respectively; introns are 720 (5′) and 446 (3′) nt, respectively. B, BamHI; E, EcoRI; P, PstI; and M, MluI.
The Duck SAA Gene Is Expressed in Liver and Lung Tissue.
The SAA acute phase response proteins are known to be synthesized principally by hepatocytes in response to interleukins 1 and 6 and tumor necrosis factor (22). Thus, while SAA deposits are found in heart, kidney and several other tissues during secondary amyloidosis, the primary source of SAA is thought to be the liver. We performed a Northern blot hybridization to determine the pattern of expression of SAA in various duck tissues (Fig. 4). While no SAA mRNA was detected in muscle, heart, intestine, kidney or pancreas, approximately equivalent amounts of a 500- to 600-nt SAA mRNA were present in liver and lung. These tissue mRNAs were all isolated from the same duckling without prior induction of an acute phase response, by injection of lipopolysaccharide for example. Therefore, the first feature of interest in Fig. 4 is that SAA appears to be expressed at quite high levels in normal duck liver. Studies in mice have shown that after induction of acute phase response there is, in addition to a huge increase in levels of SAA mRNA, a shortening of the poly(A) tail which effects not only the size but possibly also the stability of the SAA mRNA (23). Second, expression of SAA in lung was surprising, as this has not been reported in other animals in the absence of an acute phase response (13). This difference may reflect the fact that most studies are with specific pathogen-free-rodents, whereas our ducks are obtained from commercial flocks and may have a higher level of exposure to pathogen. Immunofluorescence staining of duck lung tissue with rabbit antisera generated to purified amyloid A protein suggested that smooth muscle cells surrounding the bronchioles express high levels of SAA (Fig. 5).
Figure 4.
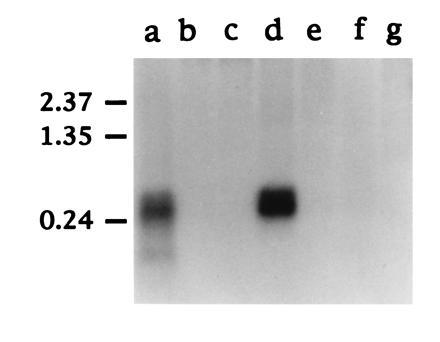
Northern blot hybridization of SAA mRNA. Approximately 1 μg of polyadenylylated RNA from each duck tissue indicated was fractionated on a 1.5% (wt/vol) formaldehyde agarose gel and transferred to nylon membrane (Hybond N; Amersham). The filter was hybridized with a 32P-labeled antisense RNA prepared by in vitro transcription of pGEM.SAA5′ with T7 RNA polymerase (see Materials and Methods). The tissues analyzed were liver (lane a), muscle (lane b), heart (lane c), lung (lane d), intestine (lane e), kidney (lane f), and pancreas (lane g). The position of RNA molecular weight size markers (in kb) are shown on the left.
Figure 5.
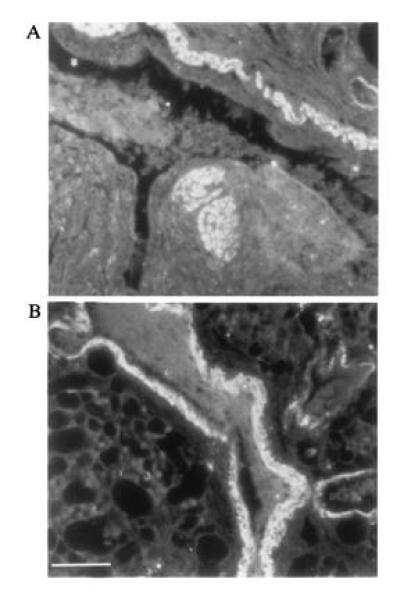
Expression of AA protein in duck lung tissue. Immunofluorescence staining of ethanol fixed Pekin duck lung tissue with rabbit antisera specific for duck liver AA protein (see Materials and Methods). A and B are two fields on the same section showing strong staining in smooth muscle around the bronchiole. (Bar = ≈200 μm.)
Genetic Dimorphism of Duck SAA Primary Protein Sequence and Susceptibility to Secondary Amyloidosis.
One possible explanation for why some animals develop secondary amyloidosis while others do not may be because they express a form of SAA protein that is more prone to processing into the insoluble fibrilar form associated with disease. Hence, we were interested to investigate if there were differences in primary SAA protein sequence between adult ducks with secondary amyloidosis and ducks with no liver disease. We performed a pilot study with a small number of adult domestic ducks housed at a large poultry farm. Several of the adult ducks showed pronounced symptoms of amyloid disease with distended abdomens caused by the accumulation of ascites in the peritoneum. Autopsy confirmed that some of these birds did indeed have advanced liver disease with substantial amyloid deposits in the liver and associated pericarditis. Liver material was taken from diseased animals and healthy adult ducks for immunohistochemistry and isolation of RNA. We examined the SAA protein sequence in these animals by an indirect method using RT-PCR with SAA-specific oligonucleotide primers (see Materials and Methods). Rapid automated nucleotide sequencing was used to analyze the PCR products directly without a cloning step. This revealed that among the total of 15 animals that were examined, two distinct types of SAA protein (A and B) could be detected with six conserved nucleotide differences in mRNA sequence (Fig. 1). Two of these nucleotide changes resulted in alterations in the primary sequence of the predicted SAA protein (Ser-89 → Gly and Val-107 → Ala, respectively) (Fig. 1). Types A and B were represented at approximately equal frequency (8 type A, 7 type B) and of the four birds with advanced disease, 1 was type A and 3 were type B.
In none of the animals examined did we detect both SAA forms A and B expressed in the liver, though heterozygotes were most likely represented at high incidence in the population. To confirm this, we made use of a restriction enzyme polymorphism for MluI that cleaves type B SAA DNA only, at nt 359 (Fig. 1). This analysis revealed that of 15 adult duck liver samples examined eight were of AB genotype and seven were BB. Interestingly, all of the heterozygotes identified in this limited survey appeared to express only the A allele (examples shown in Fig. 6). To test whether failure to detect expression of type B was due to preferential amplification of type A cDNA, type A and B cDNAs were mixed in different ratios before PCR amplification and MluI digestion. This showed that type B cDNA could readily be detected, even when present at a 100-fold reduced level compared with type A cDNA (data not shown). The absence of AA homozygotes in our population sample may be due to skewed distribution of SAA alleles arising from a preponderance of adult females in commercial flocks. While we cannot conclude from this pilot study whether type A or B SAA is more commonly associated with development of secondary amyloidosis, analysis of large numbers of animals using the above methodology may reveal such an association.
Figure 6.
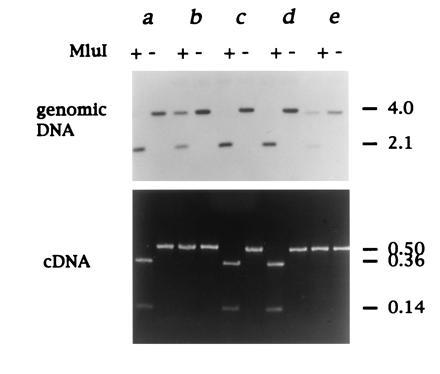
Expression of a single SAA locus in heterozygotes. The SAA genotype was determined by digestion of genomic duck DNA with EcoRI followed by MluI as shown, and SAA DNA detected by Southern hybridization). Type B SAA gene is cleaved by MluI, whereas type A is not (Figs. 1 and 3). SAA gene expression was analyzed by RT-PCR and subsequent MluI restriction endonuclease digestion). Analysis of 5 matched duck liver DNA and RNA samples (a–e) is shown from 15 adult ducks examined in total. Ducks a, c, and d are homozygous for the B allele. Ducks b and e are AB heterozygotes (Upper) but only express the A allele (Lower). The approximate sizes of DNA fragments are shown on the right in kb.
Amyloid Deposits in Hepatocytes of Diseased Adult Duck Livers.
Among the adult duck livers we examined, four showed early symptoms of secondary amyloidosis. Immunofluorescence staining of one of these livers with a polyclonal antiserum to AA protein indicated that while most hepatocytes appeared to stain at low levels, ≈5% of hepatocytes contained relatively increased levels of AA protein (Fig. 7A). Interestingly, UV microscopy also identified autofluorescing structures that appeared coincident with this subset of strongly staining hepatocytes (Fig. 7 A and B), indicating that these structures might be amyloid deposits. Occasional hepatocytes contained elevated levels of AA protein but no amyloid deposits, though the converse did not appear to be true. We used confocal microscopy to directly compare specific AA staining and autofluorescence of amyloid deposits in the same field (Fig. 7C). This confirmed that amyloid deposits colocalized within hepatocytes and were not extracellular. Similar deposits were identified by this method in liver samples from each of the four adult ducks that had shown early signs of secondary amyloidosis, but not in livers from ducklings or apparently healthy adult ducks (data not shown).
Figure 7.

Amyloid deposits in isolated hepatocytes in duck livers at early stage of amyloid disease. (A and B) Photos of the same field on a section cut from ethanol-fixed liver from a duck that exhibited early symptoms of amyloidosis. (A) Rabbit anti-AA fluorescence staining pattern (fluorescein filter). (B) Autofluorescence signal alone (rhodamine filter). (Bar = ≈150 μm.) (C) Three photos of the same specimen obtained using a confocal microscope in which specific AA antibody staining (green) appears colocalized with autofluorescing amyloid deposits (red). (Bar = ≈50 μm.)
DISCUSSION
We have presented here the first characterization of an avian SAA gene. There is striking homology between the amino acid sequence of SAA protein from duck and other animals. Comparison between the reported amino acid sequence of the duck amyloid fibril protein (14) and the amino acid sequence predicted from the cDNA that we describe here, shows that an 18-residue hydrophobic N-terminal signal sequence is cleaved from the protein during secretion. In addition, it appears that the “SKY” peptide has been cleaved from the extreme C terminus during formation of the fibril AA protein. However, it is not clear if this processing contributes to protein deposition. Southern hybridization analysis revealed that, in contrast to most other animals, there is a single copy of the SAA gene in the genome of the domestic duck. This is transcribed to produce a polyadenylylated mRNA of about 550 nt. Interestingly, in addition to the liver that is the normal site of SAA synthesis, we detected similarly large amounts of SAA mRNA in lung tissue. Consistent with this observation, immunofluorescence staining showed high levels of AA protein in smooth muscle cells surrounding bronchioles. We did not investigate if levels of lung mRNA increase in response to injection of lipopolysaccharide that induces an acute phase response in ducks and other animals.
The relative abundance of the SAA mRNA in normal duck liver and the small size of the mRNA make this gene an especially suitable candidate for analysis by RT-PCR and rapid automated nucleotide sequencing. Our limited analysis identified two distinct forms of SAA protein (A and B) in domestic ducks (Fig. 1). Type B SAA corresponds to the previously reported duck AA protein sequence (14). RT-PCR sequence analysis of four adult ducks with advanced symptoms of amyloidosis showed three to be type B and one to be type A. Of the 15 ducks examined in total, eight were of type A and seven of type B. Surprisingly, we did not detect evidence of expression of both SAA forms in individual duck livers using this method. We demonstrated that AB heterozygotes represented greater than 50% of the sample of ducks we examined, though RT-PCR analysis suggested that the A allele was expressed exclusively in the livers of these animals. We have not investigated the mechanism by which expression of the B allele is suppressed in heterozygotes, but one possible explanation of our data may be that the SAA gene is a target for imprinting (24). While the small number of animals tested in this study does not allow us to draw conclusions regarding one form of SAA being more associated with the development of secondary amyloidosis than the other, our results indicate that there may be significant differences in the distribution of types A and B among normal and diseased animals. This could be tested in a follow-up study using many more samples.
Our data suggest that an early event during development of secondary amyloidosis in the duck may be intracellular deposition of AA protein. Deposition was associated with hepatocytes in which there were relatively high levels of AA protein, though whether elevated levels of AA protein were due to overexpression of the SAA gene, failure to rapidly secrete AA protein, or some other factor was not determined. Extracellular AA would likely remain following cell death and it is possible that the extensive AA deposits characteristic of advanced stage amyloid disease may eventually form by this route. Why intracellular accumulation of AA protein would lead to formation of deposits is not clear. Early peptide analyses of protein from amyloid fibrils revealed that the insoluble protein lacked C-terminal amino acid residues that were present on the soluble protein. This led to the hypothesis that proteolytic processing of a precursor form may be responsible for formation of insoluble fibers. However, an improved understanding of the mechanism of action of mutant prion proteins in neurodegenerative diseases such as scrapie, has influenced current thinking on amyloidosis (25). SAA is produced in very large amounts during acute phase response and rapid secretion and clearance from the blood are likely to be critical. This process is proposed to involve conformational changes during conversion from the native form to a denatured form via a partially unfolded intermediate. If some event, such as binding to a chaperone protein, were to perturb this delicate balance such that the intermediate is shunted into an alternative pathway that favors production of an insoluble polymeric form of SAA, amyloidosis may result. Since amyloidosis can arise from deposition of many different proteins, a model such as this, which invokes a common pathway, is clearly appealing. It is reasonable to speculate that certain SAA molecules may be more likely to form amyloid deposits than others, which may account for differences in susceptibility to amyloidosis among domestic duck populations. Alternatively, molecules that associate with SAA in high density lipoprotein complexes in blood may play a critical role. Recent reports have shown that in addition to preventing aggregation of newly synthesized protein molecules, heat shock proteins can also function to disassemble protein aggregates in the cell (26). Consequently, it seems likely that molecules that control the normal folding pathway of SAA protein may have a role in the disease process. It has been reported that overcrowding of ducks will increase the incidence of amyloid disease, which may indicate a link with heat shock proteins or with transmission of pathogens (27).
Acknowledgments
We are grateful to Heidi Simmons and Jeff Saputelli for excellent technical assistance; Anita Cywinski for nucleotide sequencing; Dr. Andres Klein-Szanto and colleagues in the Fox Chase Cancer Center histopathology facility; and the laboratory animal, special services, oligonucleotide synthesis and confocal microscope facilities at Fox Chase. We thank Dr. Zheko V. Kounev at Maple Leaf Farms, Milford, IN, for providing us with amyloid diseased duck tissues for analysis. We are also grateful to Maureen Climaldi for secretarial assistance. This work was supported by U.S. Public Health Service Grants CA-40737, CA-06927, and AI-18641 from the National Institutes of Health and by an appropriation from the Commonwealth of Pennsylvania.
Footnotes
References
- 1.Rigdon R H. Am J Pathol. 1961;39:369–378. [PMC free article] [PubMed] [Google Scholar]
- 2.Cowan D F. Pathol Vet. 1968;5:51–58. [PubMed] [Google Scholar]
- 3.Cowan D F. Pathol Vet. 1968;5:59–66. [PubMed] [Google Scholar]
- 4.Dougherty E, Rickard C G, Scott M L. Avian Dis. 1963;7:217. [Google Scholar]
- 5.Gorevic P D, Greenwald M, Frangione B, Pras M, Franklin E C. J Immunol. 1977;118:1113–1118. [PubMed] [Google Scholar]
- 6.Rosenthal C J, Franklin E C, Frangione B, Greenspan J. J Immunol. 1976;116:1415–1418. [PubMed] [Google Scholar]
- 7.Sipe J D, Ignaczak T F, Pollock P S, Glenner G G. J Immunol. 1976;116:1151–1156. [PubMed] [Google Scholar]
- 8.Benson M D, Kleiner E. J Immunol. 1980;124:495–499. [PubMed] [Google Scholar]
- 9.Hoffman J S, Benditt E P. J Biol Chem. 1982;257:10518–10522. [PubMed] [Google Scholar]
- 10.Kisilevsky R, Benson M D, Axelrad M A, Boudreau L. Lab Invest. 1979;41:206–210. [PubMed] [Google Scholar]
- 11.Kisilevsky R, Benson M D. Lab Invest. 1981;44:84–86. [PubMed] [Google Scholar]
- 12.Morrow J F, Stearman R S, Peltzman C B, Potter D A. Proc Natl Acad Sci USA. 1981;78:4718–4722. doi: 10.1073/pnas.78.8.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meek R L, Benditt E P. Proc Natl Acad Sci USA. 1989;86:1890–1894. doi: 10.1073/pnas.86.6.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ericsson L H, Eriksen N, Walsh K A, Benditt E P. FEBS Lett. 1987;218:11–16. doi: 10.1016/0014-5793(87)81008-6. [DOI] [PubMed] [Google Scholar]
- 15.Rigdon R H. Poult Sci. 1967;46:698–705. doi: 10.3382/ps.0460168. [DOI] [PubMed] [Google Scholar]
- 16.Mason W S, Lien J-M, Petcu D J, Coates L, London W T, O’Connell A, Aldrich C, Custer R P. In: Hepadna Viruses. Robinson W, Koike K, Will H, editors. New York: Liss; 1987. pp. 3–16. [Google Scholar]
- 17.Benditt E P, Eriksen N, Hanson R H. Proc Natl Acad Sci USA. 1979;76:4092–4096. doi: 10.1073/pnas.76.8.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pras M, Schubert M, Zucker-Franklin D, Rimon A, Franklin E C. J Clin Invest. 1968;47:924–705. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sellar G C, DeBeer M C, Lelias J M, Snyder P W, Glickman L T, Felsburg P J, Whitehead A S. J Biol Chem. 1991;266:3505–3510. [PubMed] [Google Scholar]
- 20.Yamamoto K, Goto N, Kosaka J, Shiroo M, Yeul Y, Migita S. J Immunol. 1987;139:1683–1688. [PubMed] [Google Scholar]
- 21.Uhlar C M, Burgess C J, Sharp P M, Whitehead A S. Genomics. 1994;19:228–235. doi: 10.1006/geno.1994.1052. [DOI] [PubMed] [Google Scholar]
- 22.Rienhoff H J, Huang J H, Li X X, Liao W S. Mol Biol Med. 1990;7:287–298. [PubMed] [Google Scholar]
- 23.de Beer M C, Kindy M S, Lane W S, de Beer F C. J Biol Chem. 1994;269:4661–4667. [PubMed] [Google Scholar]
- 24.Barlow D. Trends Genet. 1994;10:194–199. doi: 10.1016/0168-9525(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 25.Kelly J W. Curr Opin Struct Biol. 1996;6:11–17. doi: 10.1016/s0959-440x(96)80089-3. [DOI] [PubMed] [Google Scholar]
- 26.Parsell D A, Kowal A S, Singer M A, Lindquist S. Nature (London) 1994;372:475–478. doi: 10.1038/372475a0. [DOI] [PubMed] [Google Scholar]
- 27.Cowan D F, Johnson W C. Lab Invest. 1970;23:551–555. [PubMed] [Google Scholar]


