Abstract
The E2 protein of high risk human papillomavirus type 16 (HPV16) contains an amino-terminal (N) domain, a hinge (H) region and a carboxyl-terminal (C) DNA binding domain. Using enhanced green fluorescent protein (EGFP) fusions with full length E2 and E2 domains in transfection assays in HeLa cells we found that the C domain is responsible for the nuclear localization of E2 in vivo, whereas the N and H domains do not contain additional nuclear localization signals (NLSs). Deletion analysis of EGFP-E2 and EGFP-cE2 determined that the C domain contains an alpha helix cNLS that overlaps with the DNA binding region. Mutational analysis revealed that the arginine and lysine residues in this cNLS are essential for nuclear localization of HPV16 E2. Interestingly, these basic amino acid residues are well conserved among the E2 proteins of BPV-1 and some high risk HPV types but not in the low risk HPV types, suggesting that there are differences between the NLSs and corresponding nuclear import pathways between these E2 proteins.
The papillomavirus E2 proteins have major functions during the viral life cycle in the regulation of viral DNA replication together with E1 and in transcriptional activation or repression. E2 can function either as an activator of viral transcription at low concentrations or as a repressor at high concentrations when it interferes with the binding of transcription factors such as TFIID and Sp1 (reviewed in Longworth and Laimins, 2004). In addition, E2 plays an essential role in viral genome segregation by tethering the viral DNA to the mitotic chromosomes and in the case of bovine papillomavirus (BPV) type 1 E2 protein this process is mediated by the bromodomain protein Brd4 (Baxter et al., 2005; McBride, McPhillips, and Oliveira, 2004; McPhillips, Ozato, and McBride, 2005; Oliveira, Colf, and McBride, 2006; You et al., 2004). The high risk types HPV16 and 18 E2 proteins have also proapoptotic activities (Demeret, Garcia-Carranca, and Thierry, 2003; Webster et al., 2000). For HPV18 E2 this proapoptotic activity depends on the induction of the extrinsic pathway via caspase 8 activation in the cytoplasm (Blachon et al., 2005; Demeret, Garcia-Carranca, and Thierry, 2003). Recently, the high risk HPV16/18 E2 proteins, but not the low risk types, have been shown to have the ability to induce genomic instability via a strong interaction with two activators of the Anaphase Promoting Complex (APC), Cdc20 and Cdh1 (Bellanger et al., 2005).
The full length E2 protein contains two functional domains connected by a flexible hinge (H) region and forms dimers via the C terminal domain. The structures of both the N-terminal and C-terminal domains have been determined: the N domain consists of a bundle of α-helixes packed against a β-sheet framework (Harris and Botchan, 1999) whereas the C -DNA binding domain consists of a dimeric β-barrel structure with a pair of symmetrically disposed α-helices that bind and bend the DNA (Hegde and Androphy, 1998; Hegde et al., 1992; Kim et al., 2000). The N domain functions as a transactivation domain and can also recruit and bind the E1 helicase. The structure and functions of the N and C domains are relatively conserved among papillomaviruses, whereas the H region is variable both in sequence and length. Alternative promoter usage and mRNA splicing generate additional forms of the E2 polypeptides that contain intact H and C domains and either a truncated or substituted N domain (reviewed in Longworth and Laimins, 2004).
Nucleocytoplasmic transport occurs through the nuclear pore complex, which is a large structure with an eight fold symmetry. Nuclear import of macromolecules is signal-mediated, energy-dependent and highly selective. The first identified nuclear localization signals (NLSs), referred to as classical NLSs, fall into two categories: monopartite NLSs consisting of a simple sequence of 3-5 basic amino acid residues, and bipartite NLSs consisting of a basic dipeptide upstream (10-12 amino acids) from a simple basic sequence. Other types of NLSs have been identified in hnRNP proteins, ribosomal proteins, U snRNPs, viral proteins while many others remain to be characterized. Most NLSs do not fit a well defined consensus, and for many, no consensus has been proposed (reviewed in Fried and Kutay, 2003; Moroianu, 1999). Two NLSs have been identified in the BPV-1 E2 protein: residues 107 to 115 (RKCFKKGAR) in the N domain and residues 339-352 (KCYRFRVKKNHRHR) in the C domain (Skiadopoulos and McBride, 1996). Partially homologous sequences are present in the E2 proteins of HPV types, although it is not clear if they actually function as NLSs. The E2 protein of low risk type HPV11 has been shown to contain a classic monopartite NLS in the hinge region, required for nuclear localization of HPV11 E2 and for its interaction with the nuclear matrix (Zou et al., 2000). This HPV11 E2’ NLS is not conserved in the high risk HPV16 and HPV18 E2 proteins. In this study we have identified and characterized the NLS of high risk HPV16 E2 protein using both in vivo and in vitro analysis of the intracellular localization of E2, E2 domains and different deletion and substitution variants.
RESULTS
The C domain of HPV16 E2 is required for E2 nuclear localization in vivo
To determine the E2 domain(s) that mediates the nuclear localization of HPV16 E2 we generated EGFP fusion proteins containing either the full length E2, or the N domain together with the hinge (called nhE2) or the C domain (called cE2). HeLa cells were transiently transfected with either EGFP-E2, EGFP-nhE2, EGFP-cE2 or EGFP itself and after 24 h the intracellular localization of the corresponding EGFP fusion proteins was examined by fluorescence microscopy. EGFP-E2 had strong nuclear localization whereas EGFP itself was diffusely localized throughout the cell (Fig. 2, panels A and G). The EGFP-cE2 had a similar nuclear localization as the full length E2, whereas the EGFP-nhE2 was mostly cytoplasmic (Fig. 2, panels E and C). Note that although the EGFP is small enough to passively diffuse through the nuclear pore complex, the EGFP-E2 and EGFP-cE2 (both as dimers) are above the limit of passive diffusion and their nuclear localization requires NLS(s). Immunoblot analysis with an anti-GFP antibody of the translated EGFP-nhE2 and EGFP-cE2 fusion proteins indicated that they were intact (data not shown), excluding the possibility that the cytoplasmic localization of EGFP-nhE2 is due to protein degradation. These data suggest that the C domain is responsible for the nuclear localization of HPV16 E2 and that the N domain and hinge region do not contain additional NLSs. Note that in some cells the nuclear localization of EGFP-E2 and EGFP-cE2 showed some accumulation in sub-nuclear structures that are most likely nucleoli (Fig. 2, panels A and E). Nucleolar GFP fluorescence has been previously observed with GFP-E2 proteins from both the high risk types (16 and 18) and low risk types (6 and 11) (Blachon et al., 2005).
Fig. 2. Nuclear localization of HPV16 E2 and its C domain in vivo.
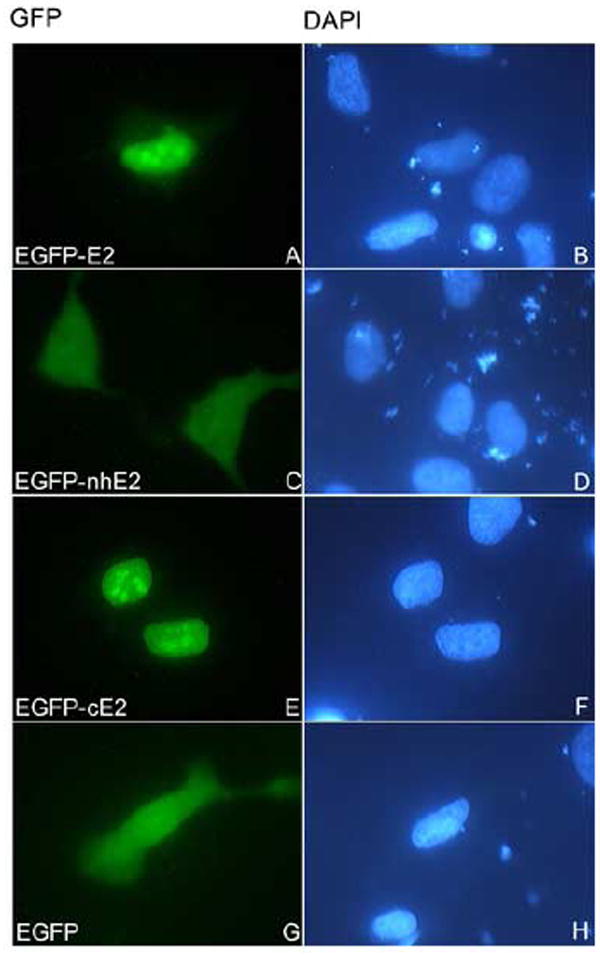
HeLa cells were transfected with either EGFP-E2 (panels A and B), or EGFP-nhE2 (panels C and D), or EGFP-cE2 (panels E and F), or EGFP (panels G and H) plasmids and examined by fluorescence microscopy at 24 hours post transfection. Panels A, C, E and G represent the fluorescence of the EGFP and panels B, D, F and H the DAPI staining of the nuclei. Note the nuclear localization of EGFP-E2 and EGFP-cE2 (panels A and E).
To investigate if the C domain can mediate active nuclear import we made a GST-cE2 fusion protein and tested it in in vitro nuclear import assays. Digitonin-permeabilized HeLa cells were incubated with either GST-cE2, or GST-NLS16L1 (as a positive control) or GST (as a negative control) in the presence of either only transport buffer or exogenous HeLa cytosol. Both the GST-cE2 and GST-NLS16L1 were efficiently imported into the nucleus in the presence of cytosol (Fig. 3, panels B and D), whereas the GST itself was not (Fig. 3, panel F). These data show that the C domain of HPV16 E2 can mediate active nuclear import of a GST reporter indicating the presence of a strong NLS. GST-cE2 was not imported in the absence of cytosol (Fig. 3, panel A) suggesting that E2 nuclear import is mediated by nuclear import receptor(s).
Fig. 3. Nuclear import of the C domain of E2 in in vitro assays in digitonin-permeabilized cells.

Digitonin-permeabilized HeLa cells were incubated with either GST-cE2 (panels A and B), or GST-NLS16L1 (panels C and D), or GST, as a negative control (panels E and F) in the presence of either only transport buffer (panels A, C and E) or HeLa cytosol (panels B, D and F). Nuclear localization of the GST fusion proteins was detected by immunofluorescence microscopy with an anti-GST antibody. Note the nuclear import of GST-cE2 and GST-NLS16L1 in panels B and D.
An alpha helix NLS located in the C domain mediates HPV16 E2 nuclear import
It has been previously shown that in addition to an NLS located in the N domain, BPV1 E2 contains a cNLS (KCYRFRVKKNHRHR) in the C domain (Skiadopoulos and McBride, 1996). This cNLS is only partially conserved in HPV16 E2 protein (298LKCLRYRFKKH308). To test if this potential cNLS is required for nuclear localization of HPV16 E2 we generated a deletion mutant, EGFP-E2ΔcNLS lacking this sequence. Analysis of the intracellular localization of EGFP-E2ΔcNLS after transfection of HeLa cells revealed that the deletion mutant is mislocalized in the cytoplasm in contrast to the wild type EGFP-E2 which is in the nucleus (Fig. 4A, compare panels C and A). Moreover, the same cytoplasmic localization of EGFP-E2ΔcNLS versus nuclear localization of EGFP-E2 was obtained after transfection of COS-1 cells (Fig. 4B, compare panels C and A). Immunoblot analysis with an anti-GFP antibody of the expressed EGFP-E2 and EGFP-E2ΔcNLS revealed that the proteins were intact and not degraded (data not shown) indicating that the cytoplasmic localization of EGFP-E2ΔcNLS is not due to degradation. Deletion of the cNLS also led to the cytoplasmic mislocalization of the C domain upon transfection of HeLa cells with the EGFP-cE2ΔcNLS expression plasmid (Fig. 5A, compare panels C and A). Together these data suggest that the cNLS (298LKCLRYRFKKH308) is required for nuclear localization of HPV16 E2 and that there are no additional NLSs present in this E2 protein.
Fig. 4. Deletion of a potential cNLS located in the C domain leads to cytoplasmic mislocalization of HPV16 E2 in both HeLa and Cos-1 cells.

HeLa cells (A) or Cos-1 cells (B) were transfected with either EGFP-E2 (panels A and B) or EGFP-E2ΔcNLS (panels C and D) plasmids and examined by fluorescence microscopy at 24 hours post transfection. Panels A and C, represent the fluorescence of the EGFP and panels B and D the DAPI staining of the nuclei.
Fig. 5. Deletion of the cNLS leads to cytoplasmic mislocalization of the C domain of HPV16 E2.
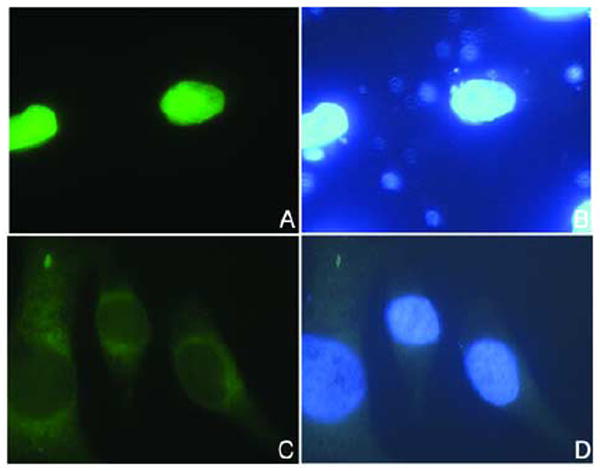
HeLa cells were transfected with either EGFP-cE2 (panels A and B), or EGFP-cE2ΔcNLS (panels C and D) plasmids and examined by fluorescence microscopy at 24 hours post transfection. Panels A and C represent the fluorescence of the EGFP and panels B and D the DAPI staining of the nuclei. Note the cytoplasmic localization of the EGFP-cE2ΔcNLS (panel C).
To determine if the cNLS is sufficient for active nuclear import we made a GST-cNLS fusion protein and tested it in nuclear import assays. Digitonin-permeabilized HeLa cells were incubated with either GST-cNLS, or GST-NLS16L1 or GST in the presence of either transport buffer or HeLa cytosol. Although the GST-cNLS was imported into the nucleus in the presence of cytosol, the efficiency of import was reduced in comparison with the GST-NLS16L1 (Fig. 6 compare panels B and D) or GST-cE2 (Fig. 3, panel B).
Fig. 6. Analysis of the nuclear import of GST-cNLS in digitonin-permeabilized cells.
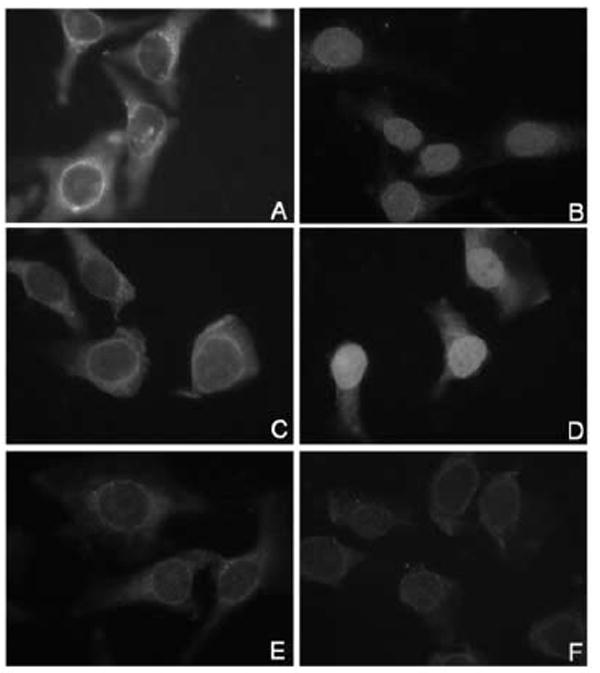
Digitonin-permeabilized HeLa cells were incubated with either GST-cNLSE2 (panels A and B), or GST-NLS16L1 (panels C and D), or GST (panels E and F) in the presence of either only transport buffer (panels A, C and E) or HeLa cytosol (panels B, D and F). Nuclear localization of the GST fusion proteins was detected by immunofluorescence microscopy with an anti-GST antibody.
To determine the amino acids in the cNLS required for nuclear localization we generated the following alanine and glutamic acid substitutions of the basic residues in the cNLS (in bold) in the context of both EGFP-E2 and EGFP-cE2: LKCLAAAFKKH (called sb1), LKCLRYRFAAH (called sb2), LKCLEEEFKKH (called sb3) and LKCLRYRFEEH (called sb4). We then analyzed the effect of these mutations on the intracellular localization of EGFP-E2 and EGFP-cE2 after transient transfection of HeLa cells.
The sb1 mutation in the cNLS (RYR to AAA) inhibited the nuclear localization of both E2 and the cE2 domain leading to a diffuse localization throughout the cell of the EGFP-E2(sb2) and EGFP-cE2(sb2) mutants (Figs. 7A and 7B, panels C), in contrast with the nuclear localization of EGFP-E2 and EGFP-cE2 (Figs. 7A and 7B, panels A). The sb2 mutation in the cNLS (KK to AA) disrupted the nuclear localization of E2 leading to mostly cytoplasmic mislocalization of the EGFP-E2(sb2) (Fig. 8, panel C). Surprisingly, when we analyzed the intracellular localization of EGFP-E2(sb2) in comparison with the wild type EGFP-cE2 in HeLa cells, there was only a modest effect of the sb2 mutation on the nuclear localization of EGFP-cE2(sb2) ((Fig. 8, compare panels G and E).
Fig. 7. Mutational-function analysis of the cNLS of HPV16 E2 in vivo.

HeLa cells were transfected with either EGFP-E2 (A, panels A and B), or EGFP-E2(sb1) (A, panels C and D), or EGFP-cE2 (B, panels A and B), or EGFP-cE2(sb1) (B, panels C and D), or EGFP (B, panels E and F) plasmids and examined by fluorescence microscopy at 24 hours post transfection. Panels A, C, and E represent the fluorescence of the EGFP and panels B, D and F the DAPI staining of the nuclei.
Fig. 8. Mutational-function analysis of the cNLS of HPV16 E2 in vivo.
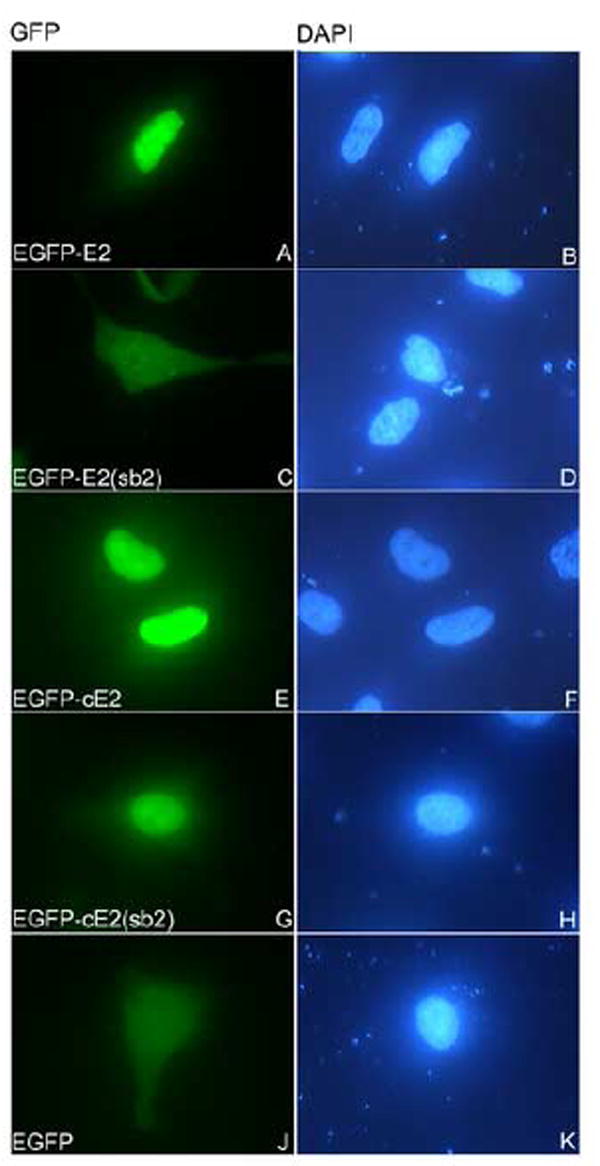
HeLa cells were transfected with either EGFP-E2 (panels A and B), or EGFP-E2(sb2) (panels C and D), or EGFP-cE2 (panels E and F), or EGFP-cE2(sb2) (panels G and H), or EGFP (panels J and K) plasmids and examined by fluorescence microscopy at 24 hours post transfection. Panels A, C, E, G and J represent the fluorescence of the EGFP and panels B, D, F, H and K the DAPI staining of the nuclei.
Substitutions of either the RYR or KK residues to glutamic acid residues completely disrupted the nuclear localization of E2 leading to cytoplasmic mislocalization of the EGFP-E2(sb3) and EGFP-E2(sb4) in HeLa cells(Fig. 9, panels B and D).
Fig. 9. Effect of substitutions of basic amino acids to glutamic acid in the cNLS on HPV16 E2 nuclear localization.

HeLa cells were transfected with either EGFP-E2 (panels A and D), or EGFP-E2(sb3) (panels B and E), or EGFP-E2(sb4) (panels C and F) plasmids and examined by fluorescence microscopy at 24 hours post transfection. Panels A-C represent the fluorescence of the EGFP and panels D-F the DAPI staining of the nuclei. Note the nuclear localization of EGFP-E2 in panel A, and the cytoplasmic localization of EGFP-E2(sb3) and EGFP-E2(sb4) variants in panels B and C.
Discussion
In this study we have identified a cNLS (298LKCLRYRFKKH308) located in the C domain that is essential for the nuclear localization of HPV16 E2 protein in vivo. HPV16 E2’ cNLS overlaps with the alpha recognition helix which contains the amino acids involved in the interaction with the DNA (Hegde and Androphy, 1998). Using mutational-function analysis in vivo we determined that both the RYR motif and the KK residues in the cNLS (298LKCLRYRFKKH308) are critical for the nuclear localization of HPV16 E2. The difference in the effect of the mutations of KK to AA in the cNLS on the intracellular localization of the EGFP-cE2(sb2) (mostly nuclear) versus EGFP-E2(sb2) (mostly cytoplasmic) could be due to the fact that these substitutions may impair cE2 dimerization. In such a case the EGFP-cE2(sb2) (as a monomer) would be below the passive diffusion limit of the nuclear pore complex, and may enter the nucleus even with a defective NLS, whereas the dimeric EGFP-E2 is above this limit and requires a functional NLS. Interestingly, the K residues are conserved in the E2 proteins of BPV-1 and some high risk HPV types (16, 18, 45 and 70) (Table 1) but not in other high risk HPV types (such as 31, 33, 52 and 56). These basic K residues are also not conserved in the low risk HPV E2 proteins; in the HPV11 E2 and HPV6A/6B E2 the KK residues are substituted with ND residues (Table 1). This correlates with the finding that in HPV11 E2 the sequence (LKCFRYRLND) does not function in nuclear localization of E2; instead HPV11 E2 contains a classical monopartite NLS (hNLS = RKRAR) located in the H region that mediates nuclear localization of this E2 protein (Zou et al., 2000). This hNLS is conserved in other low risk types, but not in the high risk types HPV16 and 18 E2 proteins (Zou et al., 2000). No additional NLSs were found in the N domain and hinge region of HPV16 E2. The E2 protein of BPV-1 contains a similar alpha helix cNLS located in the C domain (Table 1) which is conserved among fibropapillomaviruses (Skiadopoulos and McBride, 1996). BPV-1 E2 contains also an NLS located in the N domain (nNLS = RKCFKKGAR) (Skiadopoulos and McBride, 1996) which is not conserved in either the high risk HPV16 E2 or the low risk HPV11 E2. The difference in the NLSs of different papillomavirus E2 proteins suggests that they use different karyopherins/importins and nuclear import pathways.
Table 1.
| HPV type | cNLS homologies |
|---|---|
| HPV16 E2 | LKCLRYRFKKH |
| HPV18 E2 | LKCLRYRLRKH |
| HPV45 E2 | LKCLRYRLRK |
| HPV70 E2 | LKCLRYRLRK |
| HPV11 E2 | LKCFRYRLNDK |
| HPV6A/6B E2 | LKCFRYRLNDK |
| BPV1 E2 | VKCYRFRVKKNHRHR |
Although the alpha helix cNLS is essential for nuclear localization of HPV16 E2, we cannot exclude the possibility that additional residues outside this sequence may contribute to the complete cNLS function. In this respect, the nuclear import of the GST-cNLS in digitonin-permeabilized cells was less efficient than that of the GST-cE2. At least two reasons, which are not exclusive, could account for this: 1) additional residues are involved in the cNLS function and 2) most likely the α-helix structure of the cNLS is not maintained when isolated out of the E2 protein context, and may be essential for the interaction with the corresponding nuclear import receptor.
The E2 proteins exist in solution and bind to DNA as dimers. However, it is not clear if in the cell they dimerize in the cytoplasm and enter the nucleus as dimers or they enter as monomers and dimerize in the nucleus. In the first case dimerization will lead to the presence of two NLSs which may function cooperatively in the nuclear import of E2.
Although the cNLS of HPV16 E2 contains basic residues and has sequence resemblance with classical monopartite NLSs it is different in its structure as it is an alpha helix, whereas the classical NLSs bind to the karyopherins α in an extended conformation, as shown by structural studies (Chook and Blobel, 2001). The transcription factor PacC from the fungus Aspergillus nidulans also contains an alpha helix NLS that overlaps with the DNA-binding residues within the zinc finger domain of PacC (Fernandez-Martinez et al., 2003). Although the PacC’s NLS contains basic residues and resembles in sequence a monopartite NLS it does not interact with the A. nidulans importin α (Fernandez-Martinez et al., 2003). Future studies will lead to the identification of the nuclear import receptor(s) for the alpha helix cNLS of HPV16 E2 protein and the biochemical characterization of this nuclear import pathway.
Materials and Methods
Generation of EGFP-E2, EGFP-nhE2 and EGFP-cE2 plasmids and mutagenesis
The enhanced green fluorescent protein (EGFP) expression plasmid pEGFP-C1 (Clontech, Inc.) was used to construct the EGFP fusion protein expression plasmids (EGFP-E2, EGFP-cE2, GFP-E2ΔcNLS, EGFP-cE2ΔcNLS (Fig. 1)) as follows. The pEGFP-C1 plasmid was double digested with EcoRI and SalI, run on a 0.7% agarose gel and the digested vector was extracted using the protocol from the QIAquick Gel Extraction Kit (Qiagen). DNA fragments spanning the full length HPV16 E2 or the C domain (cE2), or E2ΔcNLS, cE2ΔcNLS (lacking the potential cNLS, aa 298-308) were amplified using PCR oligonucleotides that added EcoRI and SalI restriction endonuclease sites and the PCR products were digested with EcoR1 and Sal1 restriction enzymes and then ligated using T4 DNA ligase into the EcoR1 and Sal1 cloning sites of pEGFP-C1. The resulting plasmids were used to transform XL1-Blue cells and the purified plasmids were confirmed by sequencing (MGH DNA Sequencing Department).
Fig. 1.

Schematic representation of deletion and substitution mutants of HPV16 E2 and its C domain used to make EGFP fusion proteins.
The EGFP-nhE2 (containing the N domain and H region of E2, aa 1-284) was generated using the QuikChange™ Site-Directed Mutagenesis Kit from Stratagene and mutated primers containing a stop codon at aa 285. The EGFP fusions containing mutations of the cNLS: EGFP-E2(sb1, substitution of RYR with AAA), EGFP-E2(sb2, substitution of KK with AA), EGFP-cE2(sb1), EGFP-cE2(sb2) (see Fig. 1), E2(sb3, substitution of RYR with EEE) and EGFP-E2(sb4, substitution of KK with EE), were generated using the the QuikChange™ Site-Directed Mutagenesis Kit and the corresponding mutated primers. The constructs were used to transform XL1 blue bacteria and the purified plasmids were confirmed by sequence analysis (MGH DNA Sequencing Department). The different E2 proteins used to make EGFP fusions are schematically represented in Fig. 1.
Preparation of GST-cE2 and GST-cNLS
The GST-cNLSE2 fusion protein construct was prepared by fusing the cNLS sequence (298LKCLRYRFKKH308) to a GST reporter. To make the GST-cNLS construct, the corresponding forward and reverse oligonucleotides containing an EcoR I and Xho I restriction-cutting sites were annealed and inserted directly into the pGEX4T-1 vector which had been double cut with the same enzymes. The GST-cE2 construct was generated by inserting the PCR amplified cE2 fragment containing EcoR1 and Xho 1 sites into the pGEX4T-1 vector which had been double cut with the same enzymes. Both the GST-cNLS and GST-cE2 constructs were transformed in XL1 blue bacteria and confirmed by automated sequence analysis (MGH DNA sequencing department). For protein expression, the GST-cNLS and GST-cE2 constructs were used to transform E.coli BL21 CodonPlus. After induction of the bacteria with 1 mM IPTG for 2h at 30°, the GST-cNLS and GST-cE2 fusion proteins were purified in native state on glutathione-Sepharose beads using a standard procedure. The GST-NLSHPV16L1 containing the monopartite NLS (AKRKKRKL) of HPV16 L1 major capsid protein was prepared as previously described (Nelson, Rose, and Moroianu, 2003). The proteins were checked for purity and lack of proteolytic degradation by SDS-PAGE and Coomassie Blue staining. The purified proteins were dialyzed in transport buffer (20 mM HEPES-KOH, pH 7.3, 110 mM potassium acetate, 2 mM magnesium acetate, 1 mM EGTA, 2 mM DTT, plus protease inhibitors), and stored in aliquots at −80°C until use.
Transient expressions and fluorescence microscopy
HeLa cells (ATCC) and COS-1 cells (ATCC) were plated on 12 mm poly-L-lysine-coated glass coverslips to 50 – 70% confluency 24 hours prior to transfection. Cells in each well were transfected with the corresponding EGFP-E2 plasmids (as indicated in Fig. legends) and FUGENE 6 (Roche Applied Science, IN) in 500 μl DMEM prepared following product protocol. Media was changed to DMEM with 10% FBS and pen-strep after 6 hours and the cells were fixed 24 hours after the initial transfection with 10% formaldehyde in PBS (10 min). Coverslips were mounted using Vectashield-Dapi mounting medium (Vector Labs, CA) to identify the nuclei by DAPI staining and examined by fluorescence microscopy using a Nikon Eclipse TE 300 Microscope. All images were taken at the same exposure with a Sony DKC-5000 camera. The lower cytoplasmic level of the expressed EGFP-E2ΔcNLS and EGFP-cE2ΔcNLS (Figs. 4 and 5) could be due to either lower expression or less stability of these proteins when they are located in the cytoplasm. We did not notice that the EGFP-E2 fusion proteins induce apoptosis of the transfected HeLa cells in our conditions. This is in agreement with previous results were apoptosis was detectable only in cells infected at higher m.o.i. of HPV18 and HPV16 GFP E2-expressing recombinant adenoviruses (Blachon et al., 2005).
Nuclear Import assays
The nuclear import assays were carried out as previously described (Nelson, Rose, and Moroianu, 2002). Briefly, subconfluent HeLa cells, grown on poly-L-lysine coated glass coverslips for 24 h, were permeabilized with 70 μg/ml digitonin for 5 min on ice, and washed with cold transport buffer. All import reactions contained an energy regenerating system (1 mM GTP, 1mM ATP, 5 mM phosphocreatine, and 0.4 U creatine phosphokinase), HeLa cytosol and the different GST-fusion proteins (as indicated in the Fig. legends). Final import reaction volume was adjusted to 20 μl with transport buffer. For visualization of nuclear import the GST fusion proteins were detected by immunofluorescence with an anti-GST antibody, as previously described (Nelson, Rose, and Moroianu, 2002). Coverslips were mounted using Vectashield-Dapi mounting medium (Vector Labs, CA) to identify the nuclei by DAPI staining. Nuclear import was analyzed with a Nikon Eclipse TE 300 Microscope that has a fluorescence attachment and a Sony DKC-5000 CCD camera.
Acknowledgments
We thank Dr. Peter Howley for his kind gift of GST-16E2 expression plasmid. This work was supported by a grant from the National Institutes of Health (RO1 CA94898) to JM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baxter MK, McPhillips MG, Ozato K, McBride AA. The mitotic chromosome binding activity of the papillomavirus E2 protein correlates with interaction with the cellular chromosomal protein, Brd4. J Virol. 2005;79(8):4806–18. doi: 10.1128/JVI.79.8.4806-4818.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellanger S, Blachon S, Mechali F, Bonne-Andrea C, Thierry F. High-risk but not low-risk HPV E2 proteins bind to the APC activators Cdh1 and Cdc20 and cause genomic instability. Cell Cycle. 2005;4(11):1608–15. doi: 10.4161/cc.4.11.2123. [DOI] [PubMed] [Google Scholar]
- Blachon S, Bellanger S, Demeret C, Thierry F. Nucleocytoplasmic shuttling of high risk human Papillomavirus E2 proteins induces apoptosis. J Biol Chem. 2005;280(43):36088–98. doi: 10.1074/jbc.M505138200. [DOI] [PubMed] [Google Scholar]
- Chook YM, Blobel G. Karyopherins and nuclear import. Curr Opin Struct Biol. 2001;11(6):703–15. doi: 10.1016/s0959-440x(01)00264-0. [DOI] [PubMed] [Google Scholar]
- Demeret C, Garcia-Carranca A, Thierry F. Transcription-independent triggering of the extrinsic pathway of apoptosis by human papillomavirus 18 E2 protein. Oncogene. 2003;22(2):168–75. doi: 10.1038/sj.onc.1206108. [DOI] [PubMed] [Google Scholar]
- Fernandez-Martinez J, Brown CV, Diez E, Tilburn J, Arst HN, Jr, Penalva MA, Espeso EA. Overlap of nuclear localisation signal and specific DNA-binding residues within the zinc finger domain of PacC. J Mol Biol. 2003;334(4):667–84. doi: 10.1016/j.jmb.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Fried H, Kutay U. Nucleocytoplasmic transport: taking an inventory. Cell Mol Life Sci. 2003;60(8):1659–88. doi: 10.1007/s00018-003-3070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SF, Botchan MR. Crystal structure of the human papillomavirus type 18 E2 activation domain. Science. 1999;284(5420):1673–7. doi: 10.1126/science.284.5420.1673. [DOI] [PubMed] [Google Scholar]
- Hegde RS, Androphy EJ. Crystal structure of the E2 DNA-binding domain from human papillomavirus type 16: implications for its DNA binding-site selection mechanism. J Mol Biol. 1998;284(5):1479–89. doi: 10.1006/jmbi.1998.2260. [DOI] [PubMed] [Google Scholar]
- Hegde RS, Grossman SR, Laimins LA, Sigler PB. Crystal structure at 1.7 A of the bovine papillomavirus-1 E2 DNA-binding domain bound to its DNA target. Nature. 1992;359(6395):505–12. doi: 10.1038/359505a0. [DOI] [PubMed] [Google Scholar]
- Kim SS, Tam JK, Wang AF, Hegde RS. The structural basis of DNA target discrimination by papillomavirus E2 proteins. J Biol Chem. 2000;275(40):31245–54. doi: 10.1074/jbc.M004541200. [DOI] [PubMed] [Google Scholar]
- Longworth MS, Laimins LA. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev. 2004;68(2):362–72. doi: 10.1128/MMBR.68.2.362-372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AA, McPhillips MG, Oliveira JG. Brd4: tethering, segregation and beyond. Trends Microbiol. 2004;12(12):527–9. doi: 10.1016/j.tim.2004.10.002. [DOI] [PubMed] [Google Scholar]
- McPhillips MG, Ozato K, McBride AA. Interaction of bovine papillomavirus E2 protein with Brd4 stabilizes its association with chromatin. J Virol. 2005;79(14):8920–32. doi: 10.1128/JVI.79.14.8920-8932.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroianu J. Nuclear import and export pathways. J Cell Biochem. 1999;Suppl(32-33):76–83. doi: 10.1002/(sici)1097-4644(1999)75:32+<76::aid-jcb10>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- Nelson LM, Rose RC, Moroianu J. Nuclear import strategies of high risk HPV16 L1 major capsid protein. J Biol Chem. 2002;23:23. doi: 10.1074/jbc.M200724200. [DOI] [PubMed] [Google Scholar]
- Nelson LM, Rose RC, Moroianu J. The L1 major capsid protein of human papillomavirus type 11 interacts with Kap beta2 and Kap beta3 nuclear import receptors. Virology. 2003;306(1):162–9. doi: 10.1016/s0042-6822(02)00025-9. [DOI] [PubMed] [Google Scholar]
- Oliveira JG, Colf LA, McBride AA. Variations in the association of papillomavirus E2 proteins with mitotic chromosomes. Proc Natl Acad Sci U S A. 2006;103(4):1047–52. doi: 10.1073/pnas.0507624103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiadopoulos MH, McBride AA. The bovine papillomavirus type 1 E2 transactivator and repressor proteins use different nuclear localization signals. J Virol. 1996;70(2):1117–24. doi: 10.1128/jvi.70.2.1117-1124.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster K, Parish J, Pandya M, Stern PL, Clarke AR, Gaston K. The human papillomavirus (HPV) 16 E2 protein induces apoptosis in the absence of other HPV proteins and via a p53-dependent pathway. J Biol Chem. 2000;275(1):87–94. doi: 10.1074/jbc.275.1.87. [DOI] [PubMed] [Google Scholar]
- You J, Croyle JL, Nishimura A, Ozato K, Howley PM. Interaction of the bovine papillomavirus E2 protein with Brd4 tethers the viral DNA to host mitotic chromosomes. Cell. 2004;117(3):349–60. doi: 10.1016/s0092-8674(04)00402-7. [DOI] [PubMed] [Google Scholar]
- Zou N, Lin BY, Duan F, Lee KY, Jin G, Guan R, Yao G, Lefkowitz EJ, Broker TR, Chow LT. The hinge of the human papillomavirus type 11 E2 protein contains major determinants for nuclear localization and nuclear matrix association. J Virol. 2000;74(8):3761–70. doi: 10.1128/jvi.74.8.3761-3770.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


