Abstract
Neutrophil influx is an early inflammatory response that is essential for the clearance of bacteria and cellular debris during cutaneous wounding. A non-invasive real-time fluorescence imaging technique was developed to examine the kinetics of enhanced green fluorescence protein-polymorphonuclear leukocyte (EGFP-PMN) influx within a wound. We hypothesized that infection or systemic availability would directly regulate the dynamics of EGFP-PMN recruitment and the efficiency of wound closure. Neutrophil recruitment increased dramatically over the first 24 hours from 106 at 4 hours up to a maximum of 5×106 EGFP-PMNs at 18 hours. A high rate of EGFP-PMN turnover was evidenced by ∼80% decrease in EGFP signal within 6 hours. In response to wound colonization by Staphylococcus aureus or injection of GM-CSF, systemic PMNs increased twofold above saline control. This correlated with an increase in EGFP-PMN recruitment up to ∼107 within the wound. Despite this effect by these distinct inflammatory drivers, wound closure occurred at a rate similar to the saline-treated control group. In summary, a non-invasive fluorescence-based imaging approach combined with genetic labeling of neutrophils provides a dynamic inner view of inflammation and the kinetics of neutrophil infiltration into the wounded skin over extended durations.
INTRODUCTION
Skin wounding triggers a cascade of inflammatory events that leads to rapid recruitment of phagocytes from the circulation to the site of injury (Singer and Clark, 1999; Li et al., 2005). A tenuous balance is thought to exist between the protective mechanisms exerted by phagocyte infiltration and the potential for aberrant wound healing under condition that promotes excessive or impaired infiltration. For instance, prolonged release of proteolytic enzymes, oxygen-free radicals, and proinflammatory cytokines owing to excessive leukocyte infiltration can lead to chronic wound recurrence as observed in peripheral vascular disease and diabetes (Feiken et al., 1995; Pierce, 2001; Dovi et al., 2003). In addition, impairment of leukocyte recruitment is also associated with delayed wound healing (Devalaraja et al., 2000; Mori et al., 2004; Miller et al., 2006). The development of effective inflammatory models that track the dynamic balance between changes in systemic neutrophil availability and their recruitment to the wound is important in identifying the mechanism that leads to normal or aberrant wound healing. In this study, we present a new experimental model that allows us to continuously track neutrophil influx and colonization of bioluminescent bacteria within dermal wounds.
Quantitative measurement of neutrophil infiltration to the site of inflammation is typically performed on histological sections of tissue samples by either immunostaining with specific antibody or biochemical detection of myeloperoxidase activity (Feiken et al., 1995; Engelhardt et al., 1998; Agaiby and Dyson, 1999; Baskaran et al., 2000). These approaches require the sacrifice of animals at each time point. However, neither they provide high temporal resolution of dynamic changes in neutrophil infiltration (Hardy et al., 2001) nor do they lend themselves to spatial analysis of inflammatory events in wound resolution. Recently, fluorescence-based non-invasive tissue imaging approaches have been reported for assessment of keratinocyte activation in skin wound (Pan and Sanes, 2004) and macrophage infiltration in peritonitis (Lisy et al., 2006); however, real-time analysis of neutrophil recruitment during wound healing is lacking.
The objective of this study was to assess how dynamics of neutrophil influx to the wound correlate with circulating neutrophil numbers and efficiency of wound healing using a non-invasive whole-animal fluorescence approach. Our strategy was to quantify dynamic changes of wound fluorescence and healing over the time course of 10 days and correlate this with the circulating neutrophil count. Fluorescence imaging of neutrophil influx was achieved using a transgenic mouse model, in which enhanced green fluorescent protein (EGFP) was knocked into the lysozyme gene resulting in preferential labeling of mature neutrophils (Faust et al., 2000). We evaluated the effects of modulations of circulating neutrophils on dynamic patterns of neutrophil influx using experimental models of systemic injection of GM-CSF or local colonization of Staphylococcus aureus, a Gram-positive aerobic bacterial pathogen. These data show that an increase in systemic neutrophil count in GM-CSF treated- and S. aureus-infected mice closely correlated with an increase in the number of neutrophils infiltrating the wound, but not a significant change in the time course of wound closure.
RESULTS
GFP fluorescence intensity directly correlates with the number of EGFP-PMNs within a wound
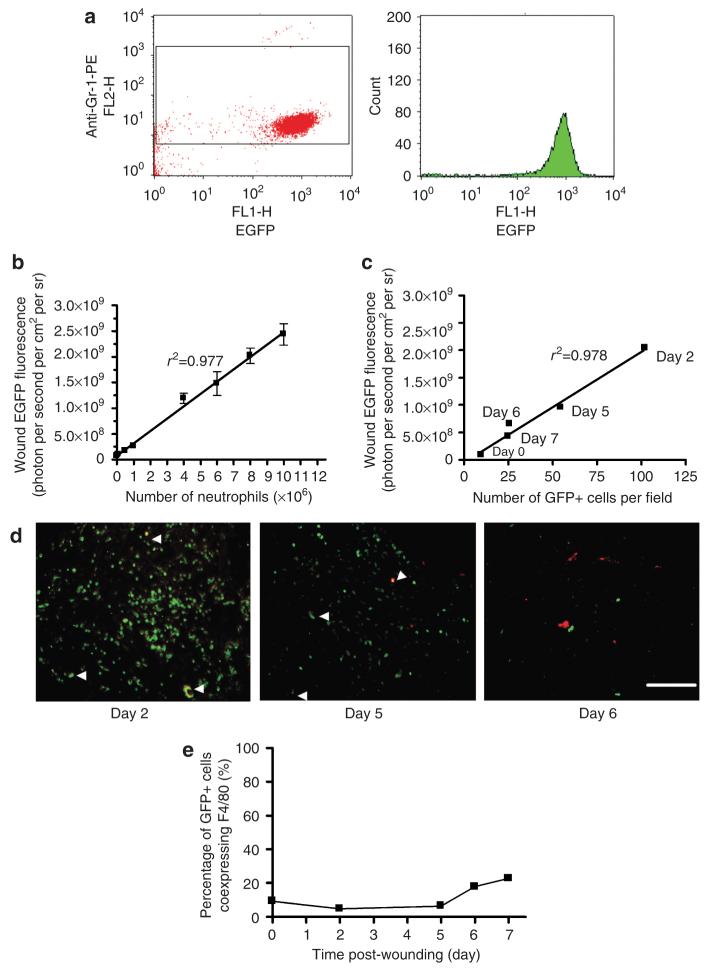
To validate the sensitivity of the imaging system (Xenogen IVIS 100) in the detection of neutrophil-expressed GFP, we correlated the average intensity of wound fluorescence as a function of the number of neutrophils placed into the wound. Flow cytometric analysis of bone marrow-isolated neutrophils confirmed that approximately 97% of mature neutrophils were GFP positive (Figure 1a). To correlate wound fluorescence with the absolute count, EGFP polymorphonuclear leukocytes (EGFP-PMNs) were placed on the dorsal skin wound of wild-type C57BL/6J mice over a range from 5.0×104 to 1×107 cells (Figure 1b). The minimum number of EGFP-PMNs that could be resolved by Xenogen imaging was 3.5×104mm-2, which corresponded to ∼1×106 EGFP-PMNs over a wound area of 28 mm2. Fluorescence intensity increased linearly with the number of EGFP-PMNs placed in the wound up to 1×107 cells (r2=0.997). Histological skin sections of the wound site were collected to confirm that EGFP fluorescence was an accurate determination of the number of PMNs recruited to the wound area (Figure 1c and d). EGFP-positive cells were counted on en face sections, including cells in deep granulation tissue (∼500 μm deep), over the time course of wounding, and this was plotted against wound EGFP fluorescence measured just before skin histology. A linear correlation confirmed that real-time EGFP fluorescence intensity closely correlates with PMN recruitment into the wound. As the lysozyme EGFP gene can potentially be expressed in monocyte/macrophages as well as in neutrophils, we next examined the relative percentage of GFP-positive macrophages by counting the numbers of GFP-positive cells coexpressing a red fluorescence-conjugated macrophage marker (F4/80 antibody) in histological skin sections collected after wounding (Figure 1e). F4/80-positive macrophages constituted as many as 10% of total GFP-positive cells up to day 5, and this increased up to 25% during wound closure. These data indicate that the majority of phagocytes (that is >75%) within the wound area are infiltrated neutrophils and the absolute number in the wound can be enumerated using the correlation between EGFP-PMN number and fluorescence intensity.
Figure 1. Tissue fluorescence intensity correlates with EGFP-PMN recruitment into the wound area.
(a) Flow cytometric detection of bone marrow-isolated neutrophils (Gr-1-positive cells) expressing EGFP fluorescence. Left panel is an EGFP/anti-Gr-1-PE plot and right panel is a cell count histogram showing EGFP+ cells, in which Gr-1+ cells were gated to determine the percentage of EGFP+ cells from total Gr-1+ cells. Representatives of two separate experiments. (b) In vivo titration of bone marrow-isolated neutrophils. GFP fluorescent intensity correlates linearly with number of EGFP neutrophils placed in back skin wound site of wild-type mice (n=2, fluorescence intensity=241*PMN+7.57×107). Data are expressed as mean±SEM. (c) GFP fluorescence intensity correlates linearly with number of GFP+ cells in histological skin sections of wounded site viewed by fluorescence microscopy, in which counted GFP+ cells in histological sections of skin at days 0, 2, 5, 6, and 7 after wounding were correlated with GFP fluorescence intensity measured using Xenogen imaging system just before the preparation of histological skin sections. (d) Immunofluorescent detection of macrophages (F4/80+ cells, red) expressing EGFP fluorescence in histological sections of skin at days 0, 2, 5, 6, and 7 after wounding. White arrow indicates EGFP+ cells coexpressing F4/80 (shown as yellow). Bar=100 μm. (e) Percentage of GFP+ monocyte/macrophage coexpressing F4/80 total GFP+ cells. Either GFP+ or F4/80+ cells was counted from 5 to 6 different regions of each samples and average was taken for mean value.
In vivo imaging of neutrophil infiltration during wound healing
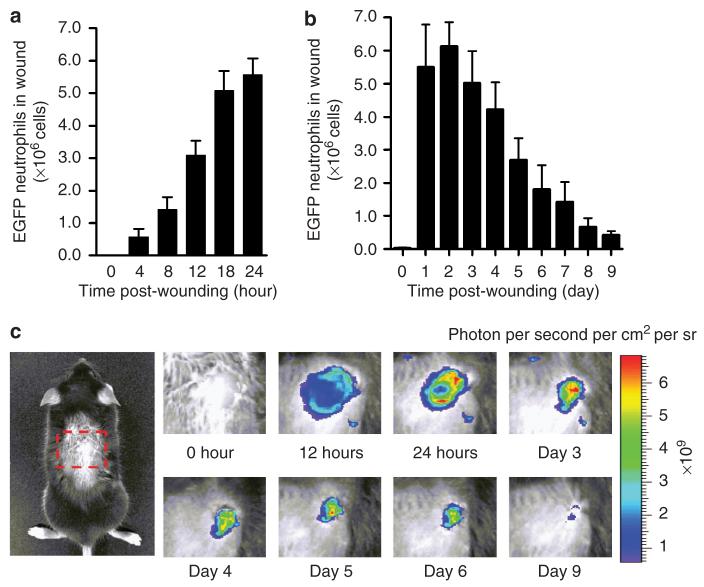
Neutrophil influx after wounding increased most rapidly over the initial 12 hours and reached a maximum value between day 1 and 2, the level plateaued up to day 3, and decreased precipitously at day 5 (Figure 2). Accumulation in the wound reached ∼6×106 by day 2, a value ∼6-fold greater than that contained within the entire systemic circulation assuming a blood volume of 2 ml and measurement of ∼5×105 PMN ml-1. EGFP-PMN fluorescence intensity decreased progressively to baseline levels from day 5 through wound closure at day 9. A particular strength of this technique is the capacity to dynamically map EGFP neutrophil fluorescence within the ∼3 mm radius of the wound area during the course of wounding (Figure 3a). At day 1, EGFP neutrophil fluorescence intensity at the wound edge (r=3 mm) was slightly greater than toward the center regions (Figure 3b). However, by day 2, the relative distribution shifted such that EGFP neutrophils were concentrated toward the center of the wound, forming a relatively uniform distribution within the wound. We next quantified the persistence of the fluorescence signal from EGFP neutrophils placed in the wounds of wild-type mice from EGFP-PMN donors. The time-dependent decay of EGFP intensity provided a measure of the lifetime of PMNs within the wound. Fluorescence decayed exponentially down to 20% of the initial intensity within 6 hours (Figure 3c). This decrease in EGFP fluorescence over time of observation was not a function of bleaching and loss of signal as confirmed by snapshot imaging of wounds over the 6 hours, which revealed an identical rate of fluorescence decay as wounds measured continuously. These data suggest that EGFP neutrophils undergo apoptosis or phagocytic clearance on a timescale commensurate with their lifetime in the circulation (Coxon et al., 1999). Moreover, the redistribution of signal from the wound periphery toward the center indicates that influx is a dynamic process fed by additional EGFP neutrophil from the circulation or tissue surrounding the wound.
Figure 2. Dynamics of neutrophil infiltration over time course of wound healing.
(a) Time course of wound EGFP fluorescence during initial 24 hours after wounding (n=4). (b) Time course of wound EGFP fluorescence during initial 10 days after wounding (n=5). (c) Representative fluorescent images of EGFP neutrophil infiltration during entire wound healing process. Data were expressed as means±SEM.
Figure 3. Spatial mapping and lifetime of EGFP-PMN in the wound.
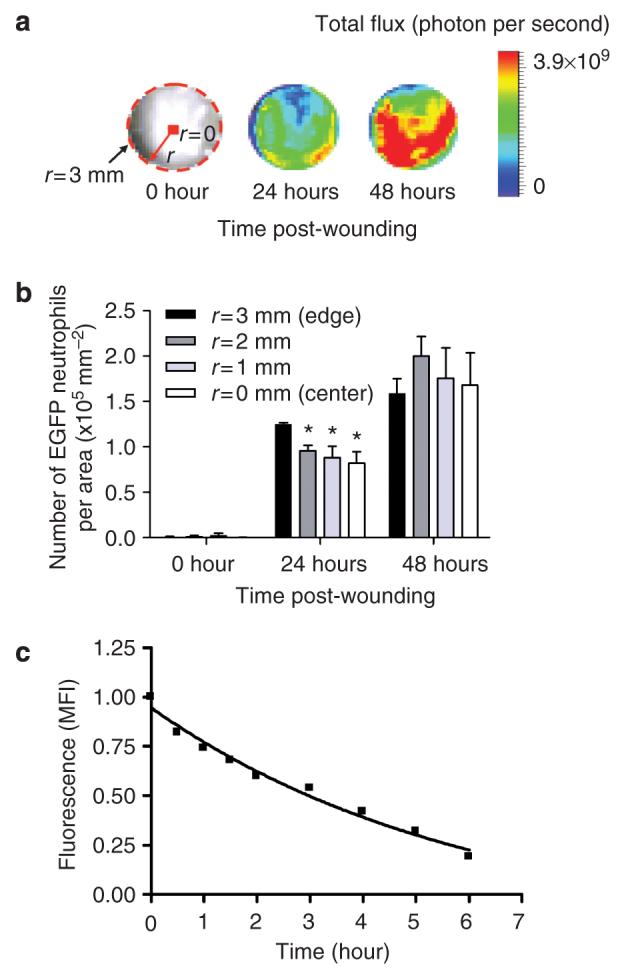
(a) Representative fluorescent image of GFP intensity (photon per second per cm2 per sr) emitted from infiltrated EGFP-PMN in circular (3 mm in radius) full thickness wound at 0, 24, and 48 hours after wounding. Where r and dotted line indicate radius and boundary of wound edge, respectively. (b) Dynamic changes in number of EGFP-PMN per area at regions from edge (r=3 mm) to center (r=0) within wound area at 0, 24, and 48 hours after wounding (n=3). *Significant difference between r=3 vs r=2, r=1, and r=0mm (P<0.05). (c) Ex vivo time-dependent decay of GFP fluorescence emitted from bone marrow-isolated EGFP-PMN (1×106 cells) on back skin wound of WT mice. Fluorescence intensity in the presence of EGFP-PMN was normalized to the value before application (normalized fluorescence intensity=1.136 exp(-0.17t)-0.1892). Data are expressed as mean±SEM.
Effects of systemic injection of GM-CSF and local colonization of S. aureus on systemic neutrophil count, neutrophil infiltration, and wound healing
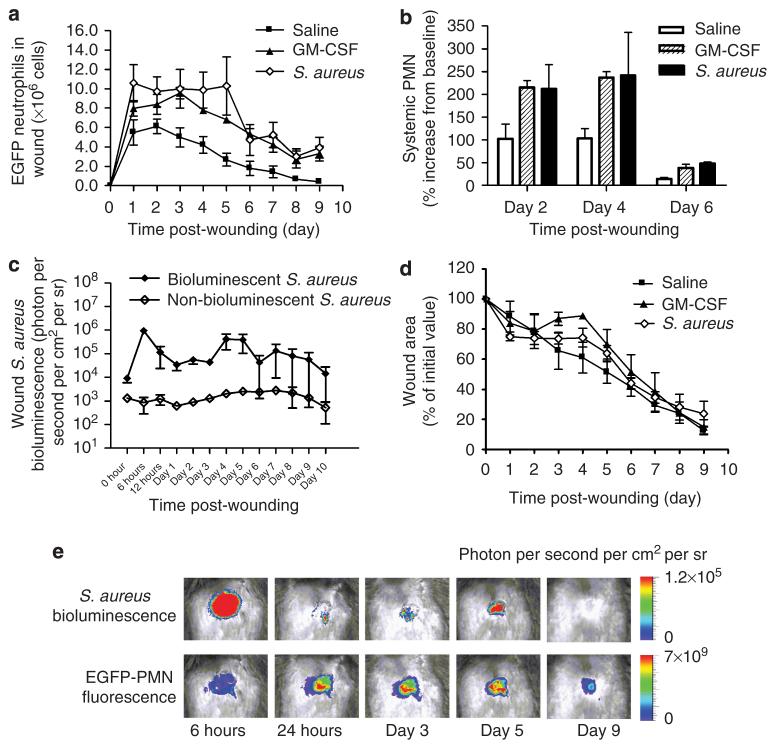
We next examined how wound infection or an increase in the availability of circulating PMNs released from bone marrow stores would regulate the dynamics of EGFP-PMN recruitment and the efficiency of wound closure. GM-CSF was injected (intraperitoneally) each day following wounding, and in separate experiments, a bioluminescent strain of S. aureus was directly inoculated into the wound and the kinetics of EGFP-PMN influx was measured (Figure 4). Local skin wounding resulted in a significant increase in both circulating and recruited neutrophils in the three experimental groups over the initial 2 days. Treatment with GM-CSF or S. aureus resulted in the sustained presence of higher numbers of EGFP-PMNs in the wound (Figure 4a). Compared with a 100% increase in the systemic neutrophil count and influx of ∼4×106 EGFP-PMNs to the wound between days 2 and 4 in the saline-injected control group, GM-CSF and S aureus treatment stimulated a ∼250% increase in circulating PMNs and recruitment of up to 107 EGFP-PMNs (Figure 4b). A higher level of EGFP-PMN was sustained through day 5 in S. aureus-infected wounds, despite a dramatic decrease in the circulating count at this time point. The kinetics of S. aureus colonization in wounded skin was monitored directly by quantifying the intensity of a bioluminescent strain of S. aureus (2.5×106 CFU (colony-forming units) per 100 μl) along with EGFP-PMN fluorescence signal (Figure 4c and e). This allowed us to non-invasively quantitate the in vivo kinetics of neutrophil influx and bacterial colonization during the wound healing process. S. aureus burden significantly increased within 6 hours of inoculation and decreased precipitously through day 3 and completely disappeared at day 9, after transiently rebounding at days 4 and 5. The EGFP fluorescence remained significantly high up to day 5 and its decrease correlated in time with the disappearance of bacterium. Unlike the control group in which EGFP-PMN recruitment substantially decreased by 50% at day 5, recruitment in GM-CSF and S. aureus groups remained significantly higher than saline-injected control even up to wound closure at day 9. However, despite a significant boost in rate and extent of PMN influx into the wound, there was no statistically significant difference in wound area closure over the time course of healing among saline, GM-CSF, and S. aureus groups of acutely inflamed skin wounds (Figure 4d).
Figure 4. GM-CSF and S. aureus inoculation increase systemic neutrophil count and wound recruitment, but not wound healing time.
(a) Dynamics of neutrophil infiltration over time course of wound healing for saline-injected control (n=5), GM-CSF (n=5), and S. aureus(n=4) groups. (b) Percentage increase of circulating neutrophil numbers from basal value at day 0 (baseline value: 0.28±0.08×106PMN ml-1 for saline control, 0.21±0.01×106PMN ml-1 for GM-CSF, and 0.15±0.15×=106PMN ml-1 for S. aureus group). (c) Dynamics of in vivo bioluminescence of actively metabolizing bacteria in wounded skin of EGFP mice inoculated with bioluminescent and non-bioluminescent strain of S. aureus.(d) Wound area over time course of wound healing for saline-injected control (n=5), GM-CSF (n=4), and S. aureus (n= 4) groups. (e) Representative images of in vivo S. aureus bioluminescence and EGFP neutrophil fluorescence. Data are expressed as mean±SEM.
DISCUSSION
A non-invasive real-time imaging method was combined with genetic tagging of neutrophils to quantify their recruitment into a skin wound over the time course of infection and wound healing. Using this method, we demonstrated that (1) local skin injury stimulated systemic increase of circulating neutrophils and subsequent infiltration into a skin wound, (2) both responses were further enhanced in equal amounts by systemic injection of GM-CSF or local colonization of S. aureus bacterium, but (3) wound healing in these groups was not significantly delayed compared to saline-injected controls.
Compared with other cell trafficking studies that use secondary fluorescent labeling of cells or addition of contrast agents (Hardy et al., 2001; Lisy et al., 2006), one advantage of real-time detection of EGFP-PMN is the ability to characterize the temporal and spatial localization of neutrophils over the duration of injury and repair without administration of exogenously labeled cells. Flow cytometric analysis of bone marrow-isolated EGFP-PMNs confirmed that ∼95% of mature neutrophils were green fluorescence positive, a level consistent with a previous study (Faust et al., 2000). Histological analysis of tissue sections through the wound revealed that macrophages also express lysozyme EGFP and constitute ∼10% of fluorescent phagocyte infiltration up to day 5, increasing up to 25% during wound closure.
Several recent studies have also quantified the dynamics of inflammatory cell infiltration to skin wounds using histological analysis of fixed tissue sections (Feiken et al., 1995; Engelhardt et al., 1998; Agaiby and Dyson, 1999). The consensus is that neutrophil influx reaches a maximum within the initial 1–2 days after skin wounding and their numbers return to baseline in the absence of gross infection. Neutrophil clearance is accomplished by tissue macrophage phagocytosis over the entire duration of healing, and our data show a correlation between increased clearance and proportion of phagocytes within the wound (Sylvia, 2003). At days 3–4 after wounding in the absence of sepsis, the wound enters a late inflammatory phase, which precedes granulation tissue formation from days 4–5 (Singer and Clark, 1999). Our imaging system proved to be highly sensitive in detection of as few as 5.0×105 EGFP-PMNs over the 6-mm-diameter circular wound area. The fluorescence signal increased linearly up to 107 EGFP-PMNs, and tissue histology confirmed a direct correlation between resident and detected PMNs. Kinetic analysis showed that the rate of EGFP-PMN influx increased most rapidly between 8 and 18 hours. A maximum number was detected between days 1–2, which correlated with a tissue density of ∼2×105PMN mm-2 in the wound. On the basis of this correlation, the neutrophil accumulation in the wound at day 2 was estimated to be 6.1×106 EGFP-PMNs, a value ∼6-fold greater than the total number in the circulation. On the basis of the fluorescence decay of EGFP-PMNs placed in the wound, we predict that neutrophil turnover in the wound to be on the order of ∼106 cells per 3 hours. Spatial mapping revealed that neutrophils initially enter the wound primarily at the site of the severed dermal microvessels over the first day. Neutrophil density increased from the wound edge toward the center within 48 hours, presumably due to inward migration and continuous influx of new neutrophils from tissue underlying the wound.
An important finding of this study was that dynamic changes in the systemic neutrophil count closely correlated with subsequent neutrophil influx into the wound area. A major source of neutrophils entering the circulation is the bone marrow pool, as recently reported by Metcalf et al. (1996), who showed that neutrophil accumulation in the peritoneal cavity correlated closely with the total number of mature bone marrow neutrophils available for release. This pool can be significantly increased by either cytokine treatment such as GM-CSF/G-CSF or bacterial infection. We found that GM-CSF or local colonization with S. aureus effectively stimulated a 250% increase in circulating neutrophils, and this correlated with a ∼100% increase in neutrophil recruitment. S. aureus tended to increase neutrophil recruitment more rapidly within the first 24 hours, but GM-CSF elicited an equivalent influx by day 3. A decrease in circulating neutrophils was also accompanied by a concomitant decrease in neutrophil influx between day 4 and day 6. Again, neutrophil numbers diminished more slowly for S. aureus as compared to GM-CSF till day 5, but were equivalent by day 6. One explanation for the enhanced neutrophil influx and maintenance in the wound is that GM-CSF or S. aureus can prime neutrophils for enhanced functional response, thereby facilitating subsequent adhesion to inflamed endothelium and increasing sensitivity to chemotactic stimulus at the site of injury. Studies have shown that GM-CSF can stimulate neutrophil respiratory burst, induce the production of platelet activating factor and other cytokines (Weisbart et al., 1987; Wirthmueller et al., 1989), increase surface expression of CD11b on circulating neutrophils (Socinski et al., 1988; Devereux et al., 1989), and enhance neutrophil adhesion to vascular endothelial cells (Yong et al., 1992). Similarly, local S. aureus infection can elicit an increase in circulating cytokines, a rapid mobilization of bone marrow pool of neutrophils, and an increase in surface expression of cell adhesion molecules, which lead to subsequent neutrophil infiltration to the site of infection (Yao et al., 1995; Lowy, 1998; Bengtson et al., 2006; Escotte et al., 2006).
A remarkable finding was that systemic injection of GM-CSF and S. aureus inoculation induced comparable kinetics of neutrophil influx and phagocyte clearance. In addition, wound closure occurred normally at a rate similar to the saline-treated control group. Several recent studies have reported that the local application of GM-CSF enhanced wound healing (Cianfarani et al., 2006; Mann et al., 2006; Siddiqui et al., 2007); however, there was no significant difference in the rate of decrease in neutrophil influx and wound closure between the experimental groups in our study. In the case of S. aureus-inoculated mice, it appears that sustained and continuous increase of neutrophil influx during the initial few days contributed to the efficient clearance of bacteria to a level that enabled normal wound healing. This is consistent with our recent report of normal healing in wild-type mice, but impaired bacterial clearance and healing during infection with S. aureus in MyD88 and IL-1 receptor-deficient mice (Miller et al., 2006). The latter study revealed a severe defect in neutrophil recruitment, suggesting that bacterial clearance mediated by the neutrophilic response is a key event required for eventual wound healing. Taken together, this implies that wound healing involves an efficient process of bone marrow release, tissue recruitment, bacteriocidal function, and appropriate apoptotic and phagocytic clearance of neutrophils. We hypothesize that there is dynamic feedback between the wound and systemic compartments that maintains a dynamic balance in neutrophil recruitment via stimulation of bone marrow production and release of mature phagocytes. Our data demonstrate that the equilibrium set point of phagocyte infiltration is regulated by the extent of acute infection within the wound or by increased systemic release of neutrophils as driven by GM-CSF. Future studies will use real-time fluorescent imaging of the wound to delineate how the virulence and amount of S. aureus colonizing the wound can influence this equilibrium and the tendency toward wound chronicity.
In conclusion, we have demonstrated that a fluorescence-based imaging approach combined with genetic labeling of neutrophils is a sensitive and convenient tool for non-invasive quantification of inflammation and for the kinetics of neutrophil infiltration into the wounded skin over extended durations without need for animal sacrifice. Using this whole-animal tissue fluorescence imaging, we demonstrated that dynamic changes of circulating neutrophil numbers in response to local skin injury closely correlated with subsequent neutrophil influx into a wound area, possibly due to the release of mediators that can increase circulating neutrophils from bone marrow pool and enhance functional response for subsequent adhesion and transmigration.
MATERIALS AND METHODS
Animal preparation
EGFP mice were generated by cross-breeding 129Sv lys-EGFP mice (generously provided by Dr Thomas Graf, Albert Einstein College of Medicine, New York) with C57BL/6J (Jackson Lab, Bar Harbor, ME) in animal facility at University of California at Davis and were housed in the same facility. Female mice between 8 and 12 weeks of age were used in all the experiments. In this study, experimental groups were divided into three, saline, GM-CSF, and S. aureus groups. Saline-injected group (0.9% saline, 200 μl) was used as a control. In GM-CSF group, mice were injected (intraperitoneally) with 10 μgkg-1 (200 μl) GM-CSF (Invitrogen, Carlsbad, CA) each day following back skin wound. In S. aureus group, mice were inoculated locally with S. aureus bacterium (2.5×106CFU per 100μl) at site of back skin wound. In this study, mice were euthanized using two methods. For isolation of neutrophils from bone marrow, mice were euthanized by cervical dislocation after being anesthetized with 100 mg per kg ketamine/xylazine (intraperitoneally, 10:1; Sigma, St Louis, MO). For all other experiments, mice were euthanized by exposure of CO2 gas at the end of experiment. All animal experiments were approved by Institutional Animal Care and Use Committee (IACUC) of the University of California at Davis and performed by following the guidelines of Animal Welfare Act and the Health Research Extension Act.
Back skin wound
Mice were anesthetized with 100 mg per kg ketamine/xylazine (intraperitoneally, 10:1; Sigma) and then the back skin hair was shaved with a mechanical shaver. After sterilizing with 10% w/v povidone—iodine and 70% alcohol, 6-mm-diameter circular full-thickness wound was made using a skin biopsy punch (Robbins Instruments Inc., Chatham, NJ).
In vivo fluorescence imaging of EGFP neutrophils
For in vivo imaging of EGFP neutrophils appearing on the site of back skin wound, the whole-body small animal fluorescence imaging system (Xenogen IVIS 100 system, Xenogen Inc., Hopkinton, MA) equipped with a charge-coupled device camera was used. Mice were put into the imaging chamber of the system after being anesthetized by either ketamine/xylazine (at day 0) or isoflurane (from day 1 to day 9 after wounding). The EGFP-expressing neutrophils within wound area were visualized using GFP filter for excitation (445–490 nm) and emission (515–575 nm) at an exposure time of 0.5 seconds. Analysis of the images were performed using Live image Pro. 2.0 software (Caliper Life Science, Hopkinton, MA), and fluorescence intensities expressed as average radiance (photons per cm2 per sr) were measured by drawing circular region of interest over the entire wound area.
Bone marrow neutrophil isolation
Mice were euthanized and the femurs and tibiae from both hind limbs were removed. After cutting off the end caps of the bones, a 10 ml syringe with 25-gauge needle filled with HEPES balanced saline solution (without calcium and magnesium) was used to flush bone marrow from each bone. The collected bone marrow was then pelleted by centrifugation (∼500 g for 5 minutes), filtered with cell strainer, and resuspended in 6 ml of HBSS. The suspension was gently laid over 6 ml of separation media (62% Percoll, 0.35 mg ml-1 sodium bicarbonate, 0.5% fetal bovine serum, 2% HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) 1 M stock solution (30 mM HEPES, pH 7.4, 110 mM NaCl, 10 mM KCl, 10 mM glucose, 1mM MgCl2)) in a 15 ml conical tube. The bone marrow cells were centrifuged for 30 minutes at 1,300 g, and mature neutrophils were isolated by collecting bottom-layered cells. The neutrophil concentration was determined using Coulter counter with a cell size ranging between 5.8 and 7.6 μm.
Flow cytometric detection of GFP neutrophils
Bone marrow-isolated neutrophils (1×106PMN ml-1) were incubated with phycoerythrin (PE)-anti-mouse Gr-1 (BD Pharmingen, San Diego, CA) for identification of neutrophils. The cell suspension was analyzed with a FACScan flow cytometry (Beckton Dickson, San Jose, CA) using excitation at 488 nm and emission either at 525 nm (GFP) or 575 nm (PE). Gr-1-positive cells were gated to examine the relative percentage of neutrophils expressing green fluorescence.
Ex vivo titration of EGFP neutrophils
Full thickness wound (6 mm in diameter) was made on top of back skin in C57BL6 mice using a skin biopsy punch. Neutrophils were isolated from bone marrow of EGFP mice and a known number of cells (5×104–1×107) were placed on the skin wound site in C57BL/6J mice. Then the mice were put in the imaging chamber of Xenogen IVIS 100 immediately and image was taken using GFP filter set. The average GFP fluorescence intensity values were measured using Live image Pro. 2.0 software (Caliper Life Science). A linear relationship was used to calculate the total number of EGFP-PMNs in wound area from fluorescence intensity.
Histological detection of EGFP+ PMN and monocyte/macrophages within wounds
On days 0, 2, 5, 6, and 7 after wounding in EGFP mice, GFP intensity within a wound was measured using Xenogen imaging system just before euthanization with CO2 gas. The entire wounded skin was excised with 8 mm skin biopsy punch, and the excised skin was then fixed with 3.7% formalin, processed, and paraffin-embedded. Skin sections were cut into 5 μm in thickness and placed on slides. Slides were heated in boiling citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) for 30 min and cooled down to room temperature for antigen retrieval. Skin samples were blocked with blocking buffer (2% goat serum, 1% BSA, 0.1% cold fish skin gelatin, 0.1% Triton X-100, 0.05% Tween 20, 0.01% sodium azide, 0.01 M phosphate-buffered saline, and pH 7.2) and incubated with 10 μgml-1 rat anti-mouse F4/80 antibody (eBiosciences, San Diego, CA) for 1 hour at room temperature for monocyte/macrophages identification. Samples were washed three times with buffer (1×phosphate-buffered saline pH 7.4, 0.05% Tween 20), followed by incubation with secondary antibody, rat IgG Alexa 647 (5 μgml-1 Invitrogen) for 1 hour at room temperature. Then slides were mounted with Fluormount G (Southern Biotech Associates, Birmingham, AL) and fluorescent images were taken under the Olympus BX61 motorized microscope at×20 magnification with the excitation filter bandpass for GFP (460–490 nm) and Cy 5 (650 nm).
EGFP-PMN fluorescence signal decay
To measure the duration, an aliquot of EGFP-PMN can emit a green fluorescence signal on skin wound, 1×106 of neutrophils isolated from bone marrow of EGFP mice were applied on top of back skin wound site in C57BL/6J mice, and images were collected taken using GFP filter at selected time points through 6 hours.
Spatial mapping of EGFP neutrophil fluorescence
To determine temporal and spatial re-localization of infiltrated neutrophils within wound area, the circular wound area was divided into 96 regions of interest of rectangular segments and the total flux (photon/s) was measured from all segments. Additionally, the 96 rectangular segments (area of each segment=0.375 mm2) were divided into four groups as a function of the distance from the center of circular wound (that is, r = 0–3 mm groups) and average was taken to obtain mean value of total flux from each group.
Systematic PMN count
Whole blood samples (20 μl) were taken via tail vein from saline-injected, GM-CSF-injected, and S. aureus-inoculated mice at day 0, 2, 4, and 6 after wounding and put into EDTA- or heparin-coated capillary tubes (Microvette CB300LH; Sarstedt Inc., Newton, NC). The total number of leukocytes was counted using automatic cell counter (Coulter counter, Beckman Coulter Inc., Fullerton, CA) and PMN concentration was determined by blood smear differential analysis.
Preparation and in vivo bioluminescent imaging of S. aureus
Bioluminescent (SH1000 strain) and non-bioluminescent strain (control vector of SH1000 strain) of S. aureus was prepared as described previously (Miller et al., 2006). In brief, S. aureus was streaked onto Tryptic soy agar (Tryptic soy broth + 1.5% Bacto Agar). Colonies of S. aureus were grown overnight at 37°C in a shaking incubator (240 r.p.m.) in Tryptic soy broth. Mid-logarithmic phase bacteria were obtained after a 3-hour subculture of 1:100 dilution of the overnight culture and cultures were performed in the presence of chloramphenicol (10 μgml-1). Bacterial cells were pelleted, resuspended, and washed three times in phosphate-buffered saline. Bacterial concentrations were estimated with a spectrophotometer by determining the absorbance at 600 nm (A600). CFUs were verified by plating dilutions of the inoculum onto Tryptic soy broth agar±chloramphenicol overnight. The 100 μl of mid-logarithmic growth phase S. aureus strain (2.5×106CFU per 100 μl) was inoculated into the wounded site of EGFP mice. Dynamic changes in actively metabolizing S. aureus within a wound area were visualized using Xenogen imaging system (Xenogen IVIS 100 system; Xenogen Inc.) at an exposure time of 2 minutes. For simultaneous imaging of EGFP neutrophils corresponding to dynamic changes in S. aureus bioluminescence, EGFP-expressing neutrophils within wound area were visualized following bioluminescent imaging. Analysis of the images were performed using Live image Pro. 2.0 software (Caliper Life Science), and bioluminescence intensities expressed as average radiance (photons per cm2 per sr) were measured by drawing circular region of interest over the entire wound area.
Statistical analysis
Data analysis was performed using GraphPad Prism version 4.0 software (GraphPad Software, San Diego, CA). Variation of data between different time points were analyzed using repeated measure one-way analysis of variance followed by Tukey post-test for secondary analysis for significance. Statistical significance between two groups was determined by two-tailed unpaired t-tests. P-values of <0.05 were considered statistically significant.
ACKNOWLEDGMENTS
We thank Dr Thomas Graf for generously providing the EGFP-lysozyme transgenic mice. Dr Simon is supported by NIH AI42794, Dr Curry is supported by NIH HL28607, Dr Liu is supported by NIH RO1 AI20958, and Dr Isseroff is supported by NIH AR44518 and Shriners Hospital Grant no. 8550.
Abbreviations
- EGFP-PMN
enhanced green fluorescence protein-polymorphonuclear leukocyte
- GFP
green fluorescent protein
Footnotes
CONFLICT OF INTEREST The authors state no conflict of interest.
REFERENCES
- Agaiby AD, Dyson M. Immuno-inflammatory cell dynamics during cutaneous wound healing. J Anat. 1999;195(Part 4):531–42. doi: 10.1046/j.1469-7580.1999.19540531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskaran H, Yarmush ML, Berthiaume F. Dynamics of tissue neutrophil sequestration after cutaneous burns in rats. J Surg Res. 2000;93:88–96. doi: 10.1006/jsre.2000.5955. [DOI] [PubMed] [Google Scholar]
- Bengtson SH, Phagoo SB, Norrby-Teglund A, Pahlman L, Morgelin M, Zuraw BL, et al. Kinin receptor expression during Staphylococcus aureus infection. Blood. 2006;108:2055–63. doi: 10.1182/blood-2006-04-016444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianfarani F, Tommasi R, Failla CM, Viviano MT, Annessi G, Papi M, et al. Granulocyte/macrophage colony-stimulating factor treatment of human chronic ulcers promotes angiogenesis associated with de novo vascular endothelial growth factor transcription in the ulcer bed. Br J Dermatol. 2006;154:34–41. doi: 10.1111/j.1365-2133.2005.06925.x. [DOI] [PubMed] [Google Scholar]
- Coxon A, Tang T, Mayadas TN. Cytokine-activated endothelial cells delay neutrophil apoptosis in vitro and in vivo. A role for granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:923–34. doi: 10.1084/jem.190.7.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devalaraja RM, Nanney LB, Du J, Qian Q, Yu Y, Devalaraja MN, et al. Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol. 2000;115:234–44. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux S, Bull HA, Campos-Costa D, Saib R, Linch DC. Granulocyte macrophage colony stimulating factor induced changes in cellular adhesion molecule expression and adhesion to endothelium: in-vitro and in-vivo studies in man. Br J Haematol. 1989;71:323–30. doi: 10.1111/j.1365-2141.1989.tb04287.x. [DOI] [PubMed] [Google Scholar]
- Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 2003;73:448–55. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- Engelhardt E, Toksoy A, Goebeler M, Debus S, Brocker EB, Gillitzer R. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol. 1998;153:1849–60. doi: 10.1016/s0002-9440(10)65699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escotte S, Al Alam D, Le Naour R, Puchelle E, Guenounou M, Gangloff SC. T cell chemotaxis and chemokine release after Staphylococcus aureus interaction with polarized airway epithelium. Am J Respir Cell Mol Biol. 2006;34:348–54. doi: 10.1165/rcmb.2005-0191OC. [DOI] [PubMed] [Google Scholar]
- Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000;96:719–26. [PubMed] [Google Scholar]
- Feiken E, Romer J, Eriksen J, Lund LR. Neutrophils express tumor necrosis factor-alpha during mouse skin wound healing. J Invest Dermatol. 1995;105:120–3. doi: 10.1111/1523-1747.ep12313429. [DOI] [PubMed] [Google Scholar]
- Hardy J, Edinger M, Bachmann MH, Negrin RS, Fathman CG, Contag CH. Bioluminescence imaging of lymphocyte trafficking in vivo. Exp Hematol. 2001;29:1353–60. doi: 10.1016/s0301-472x(01)00756-1. [DOI] [PubMed] [Google Scholar]
- Li W, Dasgeb B, Phillips T, Li Y, Chen M, Garner W, et al. Wound-healing perspectives. Dermatol Clin. 2005;23:181–92. doi: 10.1016/j.det.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Lisy MR, Schuler E, Lehmann F, Czerney P, Kaiser WA, Hilger I. Diagnosis of peritonitis using near-infrared optical imaging of in vivo labeled monocytes—macrophages. J Biomed Opt. 2006;11:064014. doi: 10.1117/1.2409310. [DOI] [PubMed] [Google Scholar]
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–32. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- Mann A, Niekisch K, Schirmacher P, Blessing M. Granulocyte—macrophage colony-stimulating factor is essential for normal wound healing. J Invest Dermatol. 2006;126(Suppl):87–92. doi: 10.1038/sj.jidsymp.5650013. [DOI] [PubMed] [Google Scholar]
- Metcalf D, Robb L, Dunn AR, Mifsud S, Di Rago L. Role of granulocyte—macrophage colony-stimulating factor and granulocyte colony-stimulating factor in the development of an acute neutrophil inflammatory response in mice. Blood. 1996;88:3755–64. [PubMed] [Google Scholar]
- Miller LS, O’Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, et al. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Mori R, Kondo T, Nishie T, Ohshima T, Asano M. Impairment of skin wound healing in beta-1,4-galactosyltransferase-deficient mice with reduced leukocyte recruitment. Am J Pathol. 2004;164:1303–14. doi: 10.1016/s0002-9440(10)63217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YA, Sanes JR. Non-invasive visualization of epidermal responses to injury using a fluorescent transgenic reporter. J Invest Dermatol. 2004;123:888–91. doi: 10.1111/j.0022-202X.2004.23486.x. [DOI] [PubMed] [Google Scholar]
- Pierce GF. Inflammation in nonhealing diabetic wounds: the space—time continuum does matter. Am J Pathol. 2001;159:399–403. doi: 10.1016/S0002-9440(10)61709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui FH, Mokhashi MH, Boathman A. Recombinant granulocyte—macrophage colony-stimulating factor in the treatment of indolent ulcers with Klippel—Trenaunay—Weber syndrome: a case report. J Pediatr Surg. 2007;42:558–60. doi: 10.1016/j.jpedsurg.2006.10.093. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Socinski MA, Cannistra SA, Sullivan R, Elias A, Antman K, Schnipper L, et al. Granulocyte—macrophage colony-stimulating factor induces the expression of the CD11b surface adhesion molecule on human granulocytes in vivo. Blood. 1988;72:691–7. [PubMed] [Google Scholar]
- Sylvia CJ. The role of neutrophil apoptosis in influencing tissue repair. J Wound Care. 2003;12:13–6. doi: 10.12968/jowc.2003.12.1.26458. [DOI] [PubMed] [Google Scholar]
- Weisbart RH, Kwan L, Golde DW, Gasson JC. Human GM-CSF primes neutrophils for enhanced oxidative metabolism in response to the major physiological chemoattractants. Blood. 1987;69:18–21. [PubMed] [Google Scholar]
- Wirthmueller U, De Weck AL, Dahinden CA. Platelet-activating factor production in human neutrophils by sequential stimulation with granulocyte—macrophage colony-stimulating factor and the chemotactic factors C5A or formyl-methionyl-leucyl-phenylalanine. J Immunol. 1989;142:3213–8. [PubMed] [Google Scholar]
- Yao L, Bengualid V, Lowy FD, Gibbons JJ, Hatcher VB, Berman JW. Internalization of Staphylococcus aureus by endothelial cells induces cytokine gene expression. Infect Immun. 1995;63:1835–9. doi: 10.1128/iai.63.5.1835-1839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong KL, Rowles PM, Patterson KG, Linch DC. Granulocyte—macrophage colony-stimulating factor induces neutrophil adhesion to pulmonary vascular endothelium in vivo: role of beta 2 integrins. Blood. 1992;80:1565–75. [PubMed] [Google Scholar]