Abstract
Ischemia-induced extracellular glutamate accumulation and the subsequent excitotoxicity contribute significantly to ischemic brain injury. Volatile anesthetics have been shown to reduce ischemic brain injury. Here, we showed that oxygen-glucose deprivation (OGD, to simulate ischemia in vitro) increased extracellular glutamate accumulation in the corticostriatal slices of adult rats. This increased accumulation was reduced by dihydrokinate, a glutamate transporter type 2 inhibitor, and 4,4’-dinitrostilbene-2,2’-disulfonic acid, a blocker for volume-activated anion channels. The volatile anesthetics isoflurane, sevoflurane and desflurane at clinically relevant concentrations did not affect the OGD-induced extracellular glutamate accumulation from brain slices of adult rats. Isoflurane also did not change the OGD-induced extracellular glutamate accumulation from brain slices of newborn/young rats. These results suggest that the OGD-induced glutamate accumulation involves reversed transport of glutamate via glutamate transporters and volume-activated anion channels. Volatile anesthetics may not inhibit this extracellular glutamate accumulation.
Keywords: anion channel, glutamate, glutamate transporter, oxygen-glucose deprivation, volatile anesthetics
1. INTRODUCTION
Glutamate is the major excitatory neurotransmitter in the central nervous system. It also can cause excitotoxicity when the extracellular concentrations of glutamate are high (Choi, et al., 1987). Thus, extracellular glutamate concentrations are tightly regulated under physiological conditions. No extracellular enzymes have been found for glutamate. Glutamate transporters, also named excitatory amino acid transporters (EAATs) that transport glutamate from extracellular to intracellular spaces, play a critical role in maintaining extracellular homeostasis of glutamate (Danbolt, 2001). It has been generally accepted that increased extracellular glutamate concentrations and the subsequent glutamate-induced excitotoxicity contribute significantly to the pathophysiology of many human diseases including stroke and neurodegenerative diseases (Danbolt, 2001; Lipton, 1999). Malfunction of EAATs has been considered to be a major mechanism to increase extracellular glutamate concentrations under these pathological conditions (Danbolt, 2001). For example, brain ischemia and energy deprivation can cause reversed transport of glutamate via EAATs: glutamate is transported from intracellular to extracellular space according to glutamate concentration gradient due to the collapse of the electrical and ionic gradients required for the normal functions of EAATs (Jabaudon, et al., 2000; Rossi, et al., 2000).
Ischemia can cause cellular swelling that activates several volume regulatory mechanisms, including volume-activated anion channels. These channels are glutamate-permeable and have been proposed as a source of ischemia-evoked glutamate efflux (Estevez, et al., 1999; Kimelberg, et al., 1990).
Volatile anesthetics, such as isoflurane, can be neuroprotective when applied during ischemia (Sakai, et al., 2007). Multiple mechanisms including reduction of metabolic rate and blocking glutamate receptor activation may contribute to this effect (Wilson and Gelb, 2002). Reduction of the increase of extracellular glutamate accumulation during ischemia is a possible mechanism for volatile anesthetic-induced neuroprotection. Conflicting results on this mechanism are produced in in vivo studies (Illievich, et al., 1994; Patel, et al., 1995). An in vitro study using brain slices from neonatal rats showed that anoxia-induced glutamate release was reduced by volatile anesthetics (Bickler, et al., 1995). Thus, we designed this study to determine whether volatile anesthetics reduce ischemia-induced extracellular glutamate accumulation and if so, whether it is through inhibition of reversed transport of glutamate via EAATs or inhibition of volume-activated glutamate efflux. We used corticostriatal slices from adult rats and newborn/young rats and simulated ischemia in vitro by oxygen-glucose deprivation (OGD).
2. RESULTS
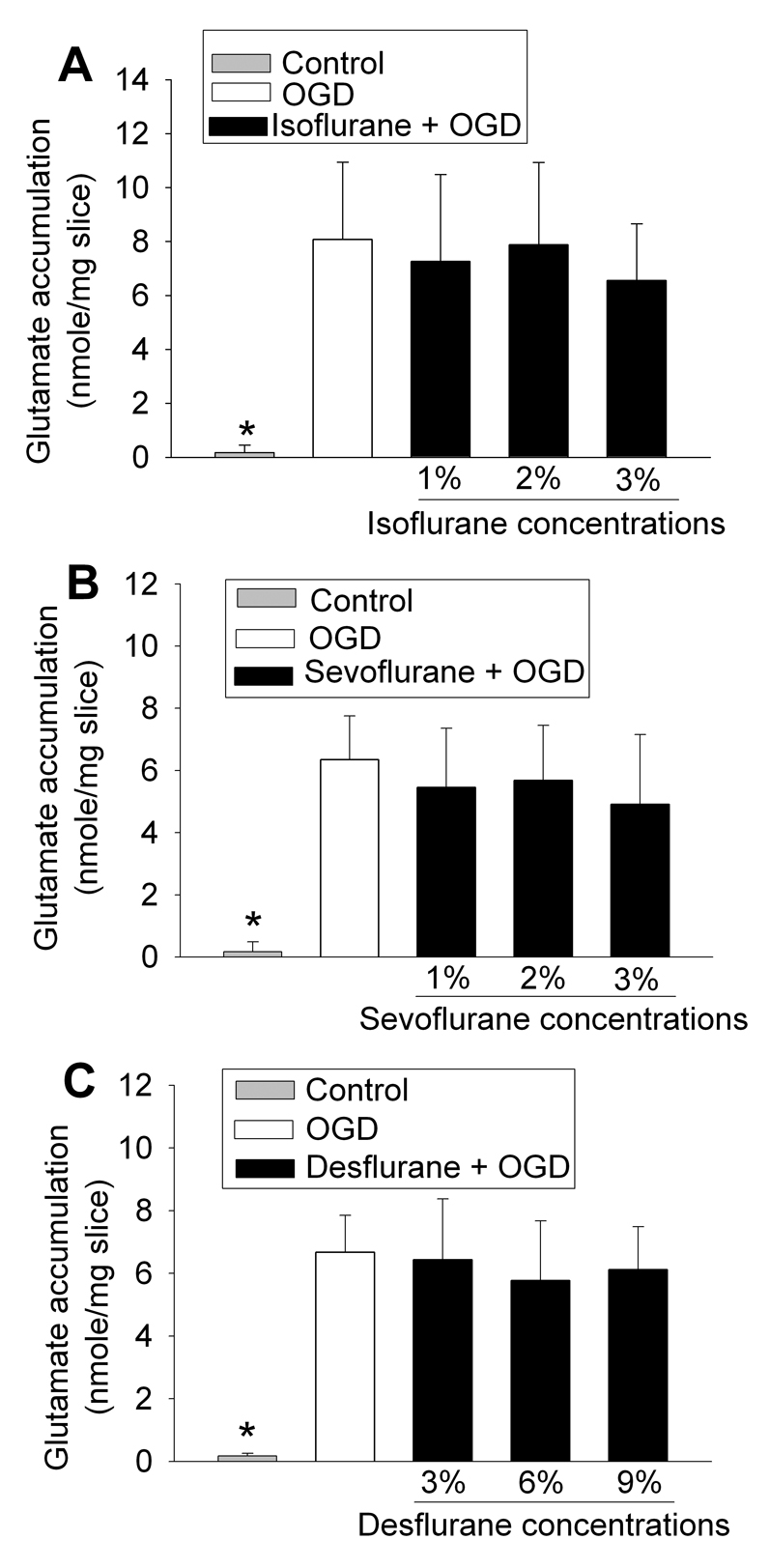
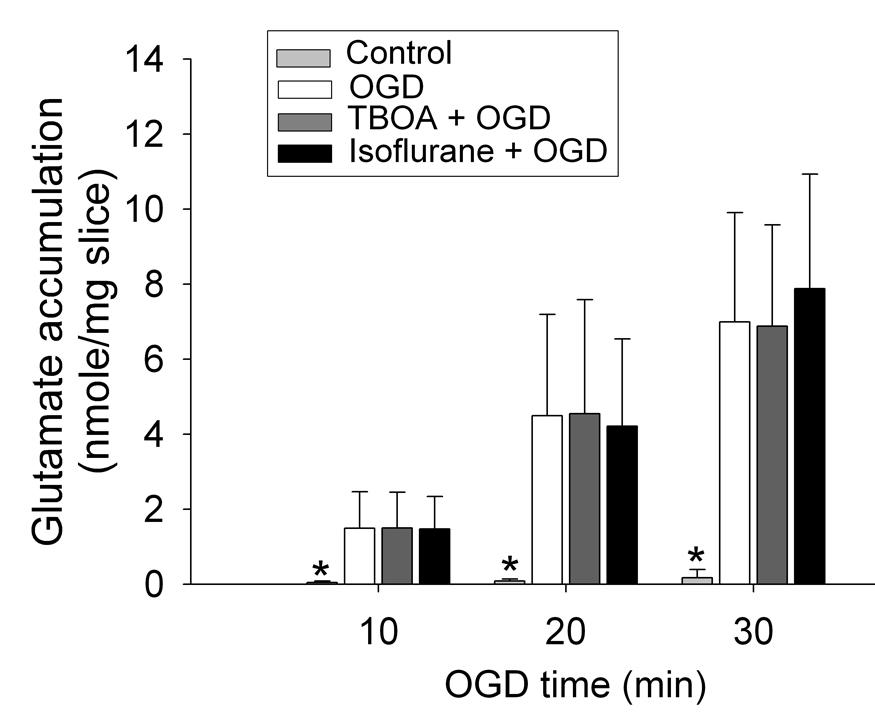
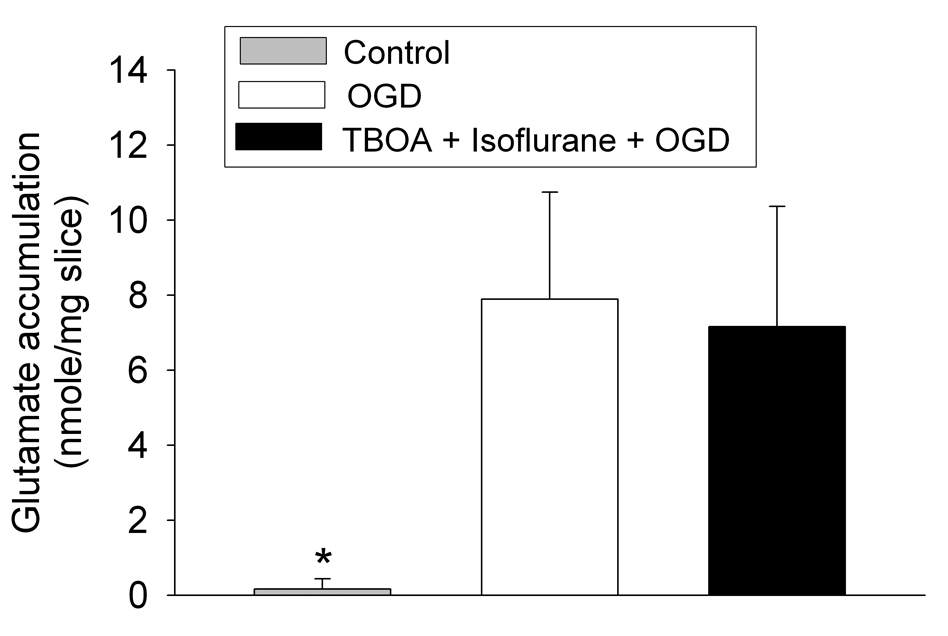
Application of a 30-min OGD to adult rat brain slices significantly increased the glutamate concentrations in the artificial cerebrospinal fluid (aCSF) (Fig. 1), suggesting that OGD increased the extracellular glutamate accumulation. This increase was attenuated by 300 µM dihydrokinate, a selective inhibitor for EAAT type 2 (EAAT2) (Anderson, et al., 2001; Seki, et al., 1999), or 1000 µM 4,4’-dinitrostilbene-2,2’-disulfonic acid (DNDS), an anion channel blocker (Seki, et al., 1999). These results indicate the involvement of EAAT2 and anion channels in the OGD-induced extracellular glutamate accumulation. Surprisingly, the 30-min OGD-induced extracellular glutamate accumulation was not attenuated by DL-threo-β-benzyloxyaspartate (TBOA), a broad spectrum EAAT antagonist, at any concentrations tested (Fig. 1). This accumulation was not affected by isoflurane, sevoflurane or desflurane at the concentrations tested (Fig. 2). Similarly, OGD for 10 min or 20 min also caused significant extracellular glutamate accumulation. The accumulation induced by these short episodes of OGD also was not inhibited by 100 µM TBOA or 2.0% isoflurane (Fig. 3). The combination of 100 µM TBOA plus 2.0% isoflurane also did not affect the 30-min OGD-induced extracellular glutamate accumulation (Fig. 4). Similar to these results from brain slices of adult rats, application of a 15-min OGD to brain slices of newborn/young rats also caused extracellular glutamate accumulation. This accumulation was not affected by various concentrations of isoflurane (Fig. 5).
Fig. 1.
Effects of glutamate transporter inhibitors and anion channel blocker on oxygen–glucose deprivation (OGD)-induced extracellular glutamate accumulation. Corticostriatal slices from adult male rats were exposed to OGD for 30 min in the presence or absence of 10 or 100 µM DL-threo-β-benzyloxyaspartate (TBOA) (Panel A), 50 or 300 µM dihydrokinate (DHK) (Panel B), or 100 or 1000 µM 4,4’-dinitrostilbene-2,2’-disulfonic acid (DNDS) (Panel C). Results are means ± S.D. (n = 26 for panel A, = 15 for panel B, and = 17 for panel C). * P < 0.05 compared with OGD.
Fig. 2.
Effects of volatile anesthetics on oxygen–glucose deprivation (OGD)-induced extracellular glutamate accumulation. Corticostriatal slices from adult male rats were exposed to OGD for 30 min in the presence or absence of 1, 2 or 3% isoflurane (Panel A), 1, 2 or 3% sevoflurane (Panel B), or 3, 6 or 9% desflurane (Panel C). Results are means ± S.D. (n = 14 for panel A, = 11 for panel B, and = 11 for panel C). * P < 0.01 compared with OGD.
Fig. 3.
Inability of DL-threo-β-benzyloxyaspartate (TBOA) or isoflurane to inhibit the oxygen–glucose deprivation (OGD)-induced extracellular glutamate accumulation. Corticostriatal slices from adult male rats were exposed to OGD for 10, 20 or 30 min in the presence or absence of 100 µM TBOA or 2.0% isoflurane. Results are means ± S.D. (n = 16 for TBOA and =15 for isoflurane treatment). * P < 0.01 compared with OGD.
Fig. 4.
Inability of the combination of DL-threo-β-benzyloxyaspartate (TBOA) plus isoflurane to inhibit the oxygen–glucose deprivation (OGD)-induced extracellular glutamate accumulation. Corticostriatal slices from adult male rats were exposed to OGD for 30 min in the presence or absence of the combination of 2.0% isoflurane plus 100 µM TBOA. Results are means ± S.D. (n = 15). * P < 0.01 compared with OGD.
Fig. 5.
Effects of isoflurane on oxygen–glucose deprivation (OGD)-induced extracellular glutamate accumulation. Corticostriatal slices from 16- to 30-days old rats were exposed to OGD for 15 min in the presence or absence of 1, 2, 3 or 4% isoflurane. Results are means ± S.D. (n = 15). * P < 0.01 compared with OGD.
3. DISCUSSION
It has been well established that ischemia causes extracellular glutamate accumulation (Patel, et al., 1995; Seki, et al., 1999). Consistent with this idea, OGD increased the glutamate concentrations in the aCSF in our study. Multiple mechanisms have been proposed to contribute to the ischemia-induced glutamate release. Nelson et al. showed that the early phase of glutamate release induced by OGD (< 25 min after the onset of OGD) involved Ca++-dependent synaptic release. The later phase of release (> 25 min of OGD) involved the volume-activated anion channels and reversed transport of glutamate via EAATs contributed to the glutamate release in both phases (Nelson, et al., 2003). However, Bickler et al. found that Ca++-dependent synaptic release of glutamate did not contribute to the anoxia-induced glutamate release during the first 10 min of the anoxia (Bickler, et al., 1995). Instead, in vitro and in vivo studies have shown that the reversed transport of glutamate and swelling-induced release of glutamate are the major mechanisms for ischemia-induced glutamate efflux (Jabaudon, et al., 2000; Rossi, et al., 2000; Seki, et al., 1999). Consistent with this conclusion, our results showed that dihydrokinate and DNDS attenuated the OGD-induced glutamate accumulation in the aCSF. Since dihydrokinate is a selective EAAT2 inhibitor, our results suggest that OGD can induce reversed transport of glutamate via EAAT2. In support of our findings, a previous study showed that dihydrokinate reduced ischemia-induced glutamate accumulation in rat brains (Seki, et al., 1999).
TBOA did not inhibit the OGD-induced glutamate accumulation. TBOA has been marketed as a non-transportable general inhibitor and should not cause heteroexchange, a phenomenon defined when the addition of one substrate stimulates the efflux of a second substrate that has accumulated in the cells (Koch, et al., 1999). A previous study showed that TBOA decreased glutamate release as detected by monitoring N-methyl-D-aspartate receptor activity in neurons of neonatal hippocampal slices after energy deprivation (Jabaudon, et al., 2000). However, TBOA has been shown to be a substrate of EAATs in astrocytes and can reduce energy deprivation-induced excitatory amino acid release at a low concentration (10 µM) but not at higher concentrations (Anderson, et al., 2001), presumably due to the compensation of the reduced release of excitatory amino acids in the presence of TBOA by the release of excitatory amino acids induced by higher concentrations of this agent. These results, along with our results, suggest that TBOA may not be a good agent for studying reversed transport of glutamate via EAATs.
Volatile anesthetics have been shown to be neuroprotective (Sakai, et al., 2007). Since volatile anesthetics can reduce metabolic rates (Wilson and Gelb, 2002), it is possible that volatile anesthetics can delay or reduce ischemia-induced glutamate release. Isoflurane reduced glutamate accumulation in rat brain after forebrain ischemia in one study (Patel, et al., 1995). However, isoflurane did not decrease glutamate accumulation during global cerebral ischemia in another study (Illievich, et al., 1994). The reasons for these conflicting in vivo findings are not known. The location of the microdialysis probe, such as in the ischemic core vs. in the penumbra, may contribute to the different findings. Our study showed that isoflurane, sevoflurane and desflurane, three volatile anesthetics in current clinical practice, at clinically relevant concentrations did not affect the OGD-induced extracellular glutamate accumulation from brain slices of adult rats. The inability to inhibit OGD-induced glutamate accumulation by isoflurane was not dependent on the severity of the OGD. The combination of isoflurane and TBOA (100 µM) did not decrease the OGD-induced glutamate accumulation. These results strongly suggest that volatile anesthetics do not affect reversed transport of glutamate and swelling-induced efflux of glutamate under ischemic condition. We also showed that isoflurane did not affect the OGD-induced extracellular glutamate accumulation from cortical brain slices of newborn/young rats. However, a previous study showed that the volatile anesthetics enflurane and halothane reduced anoxia-induced glutamate release from cortical brain slices of neonatal rats (Bickler, et al., 1995). Enflurane and halothane are no longer used in clinical practice in the USA. It is not known whether the different findings between our study and this previous study are because different agents or different insults (OGD vs. anoxia) were used or different parameters (extracellular glutamate accumulation vs. glutamate release) were monitored.
The lack of attenuation of OGD-induced glutamate accumulation by volatile anesthetics in our study is not because the concentrations of volatile anesthetics in the aCSF are too low to induce biological effects. Our previous study using identical method to apply volatile anesthetics to aCSF showed that the aqueous concentrations were ~247, 194 and 472 µM, respectively, for 1.1% isoflurane, 1.9% sevoflurane and 6% desflurane delivered in the gases (Wang, et al., 2007). These aqueous concentrations are similar to the estimated concentrations based on the partition coefficients of the anesthetics in gas versus electrolyte solutions (Honemann, et al., 1998). In addition, the concentration ranges used for isoflurane, sevoflurane and desflurane in this study induced a neuroprotective effect in our previous study (Wang, et al., 2007). All rats were anesthetized with isoflurane before their brains were removed for preparation of brain slices in this study. This prior isoflurane exposure could affect the responses of the slices to subsequent exposure to volatile anesthetics and OGD. However, the isoflurane exposure was short (< 3 min). Our previous studies showed that isoflurane exposure shorter than 5 min would not induce significant protection against subsequent OGD in rat brain slices (Zheng and Zuo, 2003) and that pretreatment with isoflurane did not affect the OGD-induced extracellular glutamate accumulation (Wang, et al., 2007).
In summary, we showed that reversed transport of glutamate via EAATs and volume-activated anion channels contributed to OGD-induced extracellular glutamate accumulation. Volatile anesthetics used in current clinical practice did not affect this glutamate accumulation.
4. EXPERIMENTAL PROCEDURES
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Virginia. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23) revised in 1996. All efforts were made to minimize the number of animals used and their suffering. All reagents unless specified below were obtained from Sigma (St. Louis, MO, USA).
4.1. Preparation of brain slices
Similar to the methods reported before (Wang, et al., 2007; Yuan, et al., 2004), corticostriatal slices were prepared from 2- to 3-month-old, 200- to 250-g, male Sprague-Dawley rats (adult rats) or 16- to 30-day old Sprague-Dawley rats (newborn/young rats) (Hilltop, Scotdale, PA, USA). Rats were anesthetized with isoflurane and then decapitated. Brain was removed rapidly and placed in ice-cold aCSF bubbled with 5% CO2 and 95% O2. The aCSF contained 116 mM NaCl, 26.2 mM NaHCO3, 5.4 mM KCl, 1.8 mM CaCl2, 0.9 mM MgCl2, 0.9 mM NaH2PO4, and 5.6 mM glucose, pH 7.4. The cortical slices (400 µm thick) of forebrain were prepared using a vibrating tissue slicer in ice-cold cutting solution containing 260 mM sucrose, 26.2 mM NaHCO3, 3 mM KCl, 1.2 mM NaH2PO4, 5 mM MgCl2, and 9 mM glucose, pH 7.4 and bubbled with 5% CO2 and 95% O2. After sectioning, slices were placed into a tissue holder. These slices were immersed in circulating aCSF continuously bubbled with 5% CO2 and 95% O2 (oxygenated aCSF) at room temperature for at least 1 h for recovery of the synaptic function.
4.2. Oxygen-glucose deprivation
Corticostriatal slices were transferred into a chamber containing glucose-free aCSF (also containing 1 mM dithionite, an oxygen absorbent) gassed with 5% CO2 and 95% N2. Gassing for 20-min before the placement of brain slices was allowed to reduce the oxygen content in the solution. Under these conditions, the PO2 in the aCSF was lower than 0.1 mmHg as measured by a Clark oxygen electrode (Cameron Instrument Co., Port Aransas, TX, U.S.A.). The chamber containing the slices was immersed in a water bath to keep the temperature of glucose-free aCSF at 37°C as monitored by a thermometer. In the time-course experiments, the OGD was maintained for 10, 20 or 30 min. The aCSF and the corticostriatal slices were collected immediately after the OGD. The aCSF was kept at −70°C until it was used to measure glutamate concentrations. The brain slices from adult rats were dried and their dry weights were measured. The slices from newborn/young rats were used to measure protein content.
4.3. Application of volatile anesthetics
aCSF (2 ml) was continuously pregassed with various concentrations of volatile anesthetics in the carrier gases (5% CO2–95% O2 for control and 5% CO2–95% N2 for OGD, respectively) for 20 min at 37°C. A single direction of gas delivery was used to deliver volatile anesthetics. Corticostriatal slices were then transferred into and submerged in the pregassed aCSF and the incubation was for 15 or 30 min at 37°C except for time course experiments. During the incubation, the solution was continuously gassed with the same pregases containing volatile anesthetics. The concentrations of volatile anesthetics used in the study were 1, 2, 3 and 4% for isoflurane and sevoflurane and 3, 6 and 9% for desflurane. They were delivered by agent-selective vaporizers and their concentrations in the gases were continuously monitored by a Datex infrared analyzer (Capnomac, Helsinki, Finland).
4.4. Application of glutamate transporters and anion channel blockers
To determine whether EAATs and anion channels were involved in ischemia-induced extracellular glutamate accumulation, corticostriatal slices were exposed to OGD for 30 min in the presence or absence of 10 or 100 µM TBOA, 50 or 300 µM dihydrokinate, or 100 or 1000 µM DNDS.
4.5. Analysis of glutamate levels by high performance liquid chromatography (HPLC)
The glutamate concentrations in the aCSF samples were measured by HPLC (Shimadzu, Kyoto, Japan) using the electrochemical detector Coulochem III (ESA, Inc., Chelmsford, MA) after a precolumn derivatization with OPA/βME solution (1,2-phthalic dicarboxaldehyde, Acros, New Jersey/β-mercapto-ethanol, Sigma). The reversed-phase column Supelcosil LC-8-DB, 5 µm, 5cm×4.6cm (Supelco Inc., Bellefonte, PA) was eluted isocratically with 0.1 M Na2HPO4 mobile phase in 35% methanol (pH = 6.4 adjusted with phosphoric acid) at 1 ml/min. Standard stock solution of glutamate (1 mg/ml in 50% methanol, kept refrigerated) was diluted with 0.05 M HClO4 (1:1) before analysis. Stock OPA/βME solution was prepared by dissolving 27 mg OPA in 1 ml of HPLC grade methanol and then adding 5 µl βME and 9 ml of 0.1 M sodium tetraborate. The solution was kept in amber glass at room temperature for up to 5 days. On the day of analysis, 1 ml of OPA/βME stock solution was diluted with 3 ml of 0.1 M sodium tetraborate solution to prepare OPA/βME working solution. Using autoinjector precolumn derivatization program, 10 µl of samples and 5 µl of OPA/βME working solution were injected to column for analysis. Electrochemical detector with analytical cell model 5011 was set as follows: electrode 1: −0.40 V, electrode 2: +0.60 V, guard cell: +0.65 V, filter: 5 second and current range: 200 nA. The aCSF samples were analyzed after the standard curve of glutamate has been established.
4.6. Statistical analysis
The amount of glutamate in the aCSF was normalized by the slice dry weight (adult rats) or slice protein content (newborn/young rats). Results are means ± S.D. Brain slices for various experimental conditions in each set of experiments were from the same rat brains. Each experimental condition was repeated at least 11 times with brain slices from different rats. Statistical analysis for the comparisons among the control, OGD and treatment groups was performed by one way repeated measures analysis of variance followed by the Student-Newman-Keuls method. A P < 0.05 was accepted as significant. The analysis was performed with the SPSS10.0 software (SPSS Inc., Chicago, IL).
Acknowledgement
This study was supported by grants (R01 GM065211 and R01 NS045983 to Z Zuo) from the National Institute of Health, Bethesda, Maryland.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- DNDS
4,4’-dinitrostilbene-2,2’-disulfonic acid
- EAAT
excitatory amino acid transporters
- OGD
oxygen-glucose deprivation
- TBOA
DL-threo-β-benzyloxyaspartate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The research work was performed in and should be attributed to the Department of Anesthesiology, University of Virginia, Charlottesville, VA22908, USA
REFERENCES
- Anderson CM, Bridges RJ, Chamberlin AR, Shimamoto K, Yasuda-Kamatani Y, Swanson RA. Differing effects of substrate and non-substrate transport inhibitors on glutamate uptake reversal. J. Neurochem. 2001;79:1207–1216. doi: 10.1046/j.1471-4159.2001.00668.x. [DOI] [PubMed] [Google Scholar]
- Bickler PE, Buck LT, Feiner JR. Volatile and intravenous anesthetics decrease glutamate release from cortical brain slices during anoxia. Anesthesiology. 1995;83:1233–1240. doi: 10.1097/00000542-199512000-00014. [DOI] [PubMed] [Google Scholar]
- Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J. Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Estevez AY, O'Regan MH, Song D, Phillis JW. Effects of anion channel blockers on hyposmotically induced amino acid release from the in vivo rat cerebral cortex. Neurochem. Res. 1999;24:447–452. doi: 10.1023/a:1020902104056. [DOI] [PubMed] [Google Scholar]
- Honemann CW, Washington J, Honemann MC, Nietgen GW, Durieux ME. Partition coefficients of volatile anesthetics in aqueous electrolyte solutions at various temperatures. Anesthesiology. 1998;89:1032–1035. doi: 10.1097/00000542-199810000-00032. [DOI] [PubMed] [Google Scholar]
- Illievich UM, Zornow MH, Choi KT, Strnat MA, Scheller MS. Effects of hypothermia or anesthetics on hippocampal glutamate and glycine concentrations after repeated transient global cerebral ischemia. Anesthesiology. 1994;80:177–186. doi: 10.1097/00000542-199401000-00025. [DOI] [PubMed] [Google Scholar]
- Jabaudon D, Scanziani M, Gahwiler BH, Gerber U. Acute decrease in net glutamate uptake during energy deprivation. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5610–5615. doi: 10.1073/pnas.97.10.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J. Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HP, Chamberlin AR, Bridges RJ. Nontransportable inhibitors attenuate reversal of glutamate uptake in synaptosomes following a metabolic insult. Mol. Pharmacol. 1999;55:1044–1048. doi: 10.1124/mol.55.6.1044. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol. Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Nelson RM, Lambert DG, Richard Green A, Hainsworth AH. Pharmacology of ischemia-induced glutamate efflux from rat cerebral cortex in vitro. Brain Res. 2003;964:1–8. doi: 10.1016/s0006-8993(02)03691-0. [DOI] [PubMed] [Google Scholar]
- Patel PM, Drummond JC, Cole DJ, Goskowicz RL. Isoflurane reduces ischemia-induced glutamate release in rats subjected to forebrain ischemia. Anesthesiology. 1995;82:996–1003. doi: 10.1097/00000542-199504000-00024. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Sakai H, Sheng H, Yates RB, Ishida K, Pearlstein RD, Warner DS. Isoflurane Provides Long-term Protection against Focal Cerebral Ischemia in the Rat. Anesthesiology. 2007;106:92–99. doi: 10.1097/00000542-200701000-00017. [DOI] [PubMed] [Google Scholar]
- Seki Y, Feustel PJ, Keller RW, Jr, Tranmer BI, Kimelberg HK. Inhibition of ischemia-induced glutamate release in rat striatum by dihydrokinate and an anion channel blocker. Stroke. 1999;30:433–440. doi: 10.1161/01.str.30.2.433. [DOI] [PubMed] [Google Scholar]
- Wang C, Lee J, Jung H, Zuo Z. Pretreatment with volatile anesthetics, but not with the nonimmobilizer 1,2-dichlorohexafluorocyclobutane, reduced cell injury in rat cerebellar slices after an in vitro simulated ischemia. Brain Res. 2007;1152:201–208. doi: 10.1016/j.brainres.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JX, Gelb AW. Free radicals, antioxidants, and neurologic injury: possible relationship to cerebral protection by anesthetics. J. Neurosurg. Anesthesiol. 2002;14:66–79. doi: 10.1097/00008506-200201000-00014. [DOI] [PubMed] [Google Scholar]
- Yuan H-B, Huang Y, Zheng S, Zuo Z. Hypothermic preconditioning increases survival of Purkinje neurons in rat cerebellar slices after an in vitro simulated ischemia. Anesthesiology. 2004;100:331–337. doi: 10.1097/00000542-200402000-00023. [DOI] [PubMed] [Google Scholar]
- Zheng S, Zuo Z. Isoflurane preconditioning reduces Purkinje cell death in an in vitro model of rat cerebellar ischemia. Neuroscience. 2003;118:99–106. doi: 10.1016/s0306-4522(02)00767-4. [DOI] [PubMed] [Google Scholar]