Abstract
Background
The usefulness of a low butyrylcholinesterase activity on admission for predicting severity in acute organophosphorus insecticide poisoning has long been debated. Previous studies have been confounded by the inclusion of multiple insecticides with differing inhibitory kinetics.
Aim
We aimed to assess the usefulness of admission butyrylcholinesterase activity, together with plasma organophosphorus concentration, for predicting death with identified organophosphorus insecticides.
Design
A prospective cohort of self-poisoned patients
Methods
We prospectively studied 91 and 208 patients with proven dimethoate or chlorpyrifos self-poisoning treated using a standard protocol. Plasma butyrylcholinesterase activity and organophosphorus concentration were measured on admission and clinical outcomes recorded.
Results
The usefulness of a plasma butyrylcholinesterase activity <600mU/ml on admission varied markedly - while highly sensitive in chlorpyrifos poisoning (sensitivity 11/11 deaths; 100%, 95%CI 71.5-100), its specificity was only 17.7% (95%CI 12.6-23.7). In contrast, while poorly sensitive for deaths in dimethoate poisoning (12/25 patients; 48%, 95%CI 27.9-68.7) it was reasonably specific (86.4%, 95%CI 75.7-93.6). A high organophosphorus concentration on admission was associated with worse outcome; however, a clear threshold concentration was only present for dimethoate poisoning.
Conclusions
Plasma butyrylcholinesterase activity on admission can provide useful information; however, it must be interpreted carefully. It can only be used to predict need for critical care and death when the insecticide ingested is known and its sensitivity and specificity for that insecticide has been studied. Plasma concentration of some organophosphorus insecticides predicts outcome. The development of rapid bedside tests for organophosphorus detection may aid early assessment of severity.
Introduction
Organophosphorus (OP) insecticide self-poisoning is a major global health problem, 1,2 with hundreds of thousands of deaths each year.3,4 Identifying patients with a poor prognosis who will benefit from intensive care support is currently difficult.5
OPs inhibit acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) enzymes. 5 AChE inhibition causes clinical features due to overstimulation of cholinergic synapses in the parasympathetic system, neuromuscular junction, and central nervous system.6 BuChE inhibition, by contrast, appears not to result in clinical features.5 However, BuChE activity is more easily measured than AChE activity and BuChE assays are widely available and therefore commonly recommended in the early assessment of OP pesticide poisoned patients. In 1971, Namba and colleagues proposed a severity scoring system that included the measurement of BuChE:7 mild poisoning had BuChE 20-50% of normal activity, moderate poisoning 10-20%, and severe poisoning <10%.
The usefulness of this approach has been much debated. Despite studies showing BuChE to be a poor predictor of outcome and response to therapy,8-10 some clinicians continue to use it in the early assessment of OP pesticide severity.11-19 The clinical studies that have been done to assess its usefulness, however, have been confounded by the presence of multiple OP pesticides.14,20-26
It is possible that BuChE activity may be useful for particular OPs, if they can be confidently identified on admission. The aim of this study was therefore to determine the usefulness of admission plasma BuChE activity or OP concentration for predicting need for critical care or death, using patients proven to have ingested dimethoate or chlorpyrifos - two OP pesticides that are common problems across rural Asia.
Methods
Patients
Patients were seen on admission to three Sri Lankan hospitals as part of an RCT of acute self-poisoning that started 31 Mar 2002 in Anuradhapura, 4 Jun 2002 in Polonnaruwa, and 23 Nov 2002 in Kurunegala. Patients for this study were seen until 19 Feb 2003 in Kurunegala and 31 Dec 2003 in Anuradhapura and Polonnaruwa. Ethics approval was obtained from the Oxfordshire Clinical Research Ethics Committee and Faculty of Medicine Ethics Committee, Colombo.
The management of these patients was uniform on the two hospitals and has been previously described.27,28 Briefly, patients were resuscitated on arrival with fluids and atropine, and administered pralidoxime chloride 1g q6h for 1-3 days. Patients remained under the care of the responsible consultant physician and were treated using protocols agreed between ward doctors and study team.27 All decisions were made on the basis of the patient’s clinical condition and not the particular OP ingested.
Once resuscitated, patients or their relatives were approached concerning recruitment to a RCT of activated charcoal (ISRCTN02920054) that was nested into the cohort; informed signed consent was obtained from patients or relatives. The RCT was stopped in October 2004 after the planned final interim analysis showed no effect of activated charcoal on death.29
Consecutive patients recruited to the study were included in this study if they had a history of chlorpyrifos or dimethoate ingestion (as indicated by patient or relatives, transferring doctor, or pesticide bottle) and either OP was subsequently detected in a plasma sample taken on admission. Patients who ingested more than one OP or other poisons in addition to the OP (except for alcohol) were excluded from the study.
Toxicological analysis
Blood samples were taken on admission from patients recruited to the RCT until December 2003. Samples were placed at 4C immediately after phlebotomy, plasma extracted after centrifugation within 10 minutes, and the samples placed at -23C.
Plasma from 376 patients (240 chlorpyrifos, 136 dimethoate) were then assayed for BuChE activity and OP concentration. All analyses were done in Munich. BuChE activity was assessed as described.30,31 BuChE activity in unexposed people is known to vary widely.32 In plasma samples collected in an identical fashion from patients in the RCT with Thevetia peruviana poisoning (n=72), but no known exposure to OP or carbamate pesticides, we found a median value of 4500 mU/mL (3200 to 5200 IQR, Eyer, unpublished observations). Given the likelihood of unperceived exposure we conservatively took 600 mU/mL as 10% of normal activity. OPs in plasma were quantified by reversed phase HPLC and UV detection. The lower limits of quantitation were 0.1 and 1.0 nmol/ml plasma for chlorpyrifos and dimethoate, respectively.28
Statistics
The primary data analysis was performed in GraphPad Prism (version 4). Clinical characteristics were summarised using counts (percentages) for categorical variables and the median (interquartile range [IQR]) for non-normally distributed continuous variables. Logistic regression to examine for confounding effects of time from ingestion for blood sampling was performed using Stata v10.
Results
Between 31 Mar 2002 and 31 Dec 2003, admission blood samples for 376 patients with a history of dimethoate or chlorpyrifos self-poisoning were analysed for BuChE activity and OP concentration. The relevant OP could not be detected in 77 patients; these patients are not further discussed.
208 patients had ingested chlorpyrifos (median OP concentration 1.24 uM [inter-quartile range (IQR) 0.38 to 3.14]; median BuChE activity 34 mU/mL [IQR 0 to 300]) and 91 had ingested dimethoate (median OP concentration 342 uM [IQR 159 to 643]; median BuChE activity 1277 mU/mL [IQR 678 to 2349]). Both median BuChE activity and median OP concentration were significantly different between OPs (P<0.0001 Mann Whitney test).
Twenty five of 91 (27.5%, 95%CI 18.6 to 37.8) dimethoate poisoned patients died compared to 11/208 (5.3%, 95%CI 2.7 to 9.3) with chlorpyrifos poisoning. Patients who died from both pesticides were older and more likely to be male; however, there was no difference in time to admission between fatal and non-fatal cases (table 1). Blood OP concentrations were greater in patients who died compared to those who survived for both OPs (figure 1, table 1, both P<0.0001).
Table 1. Demographic and admission characteristics for patients with non-fatal and fatal OP self-poisoning.
| OP INSECTICIDE | Chlorpyrifos | Dimethoate | |||
|---|---|---|---|---|---|
| Non-fatal (N = 197) | Fatal (N = 11) | Non-fatal (N = 66) | Fatal (N = 25) | ||
| Demographics | |||||
| Male [n (%)] | 147 (74.6) | 11 (100) | 47 (71.2) | 24 (96.0) | |
| Age [yrs, median (interquartile range [IQR])] | 28 (22 to 38) | 42 (32 to 55) | 30 (23 to 40) | 40 (30 to 52) | |
| Time to presentation [hrs, median, (IQR)] * | 3.71 (2.39 to 4.99) | 2.92 (2.78 to 4.08) | 3.07 (2.16 to 4.49) | 2.84 (2.20 to 3.71) | |
| Admission characteristics | |||||
| Glasgow Coma Score (GCS) [median (IQR)] | 15 (14 to 15) | 11 (3 to 13) | 14 (10 to 15) | 3 (3 to 6) | |
| BuChE level [mU/ml; median, (IQR)] | 34 (0 to 331) | 6 (0 to 94) | 1496 (857 to 2909) | 735 (269 to 1240) | |
| OP plasma concentration [μM; median, (IQR)] | 1.1 (0.37 to 2.93) | 4.73 (3.58 to 5.90) | 245 (114 to 380) | 846 (657 to 1183) | |
Figure 1.
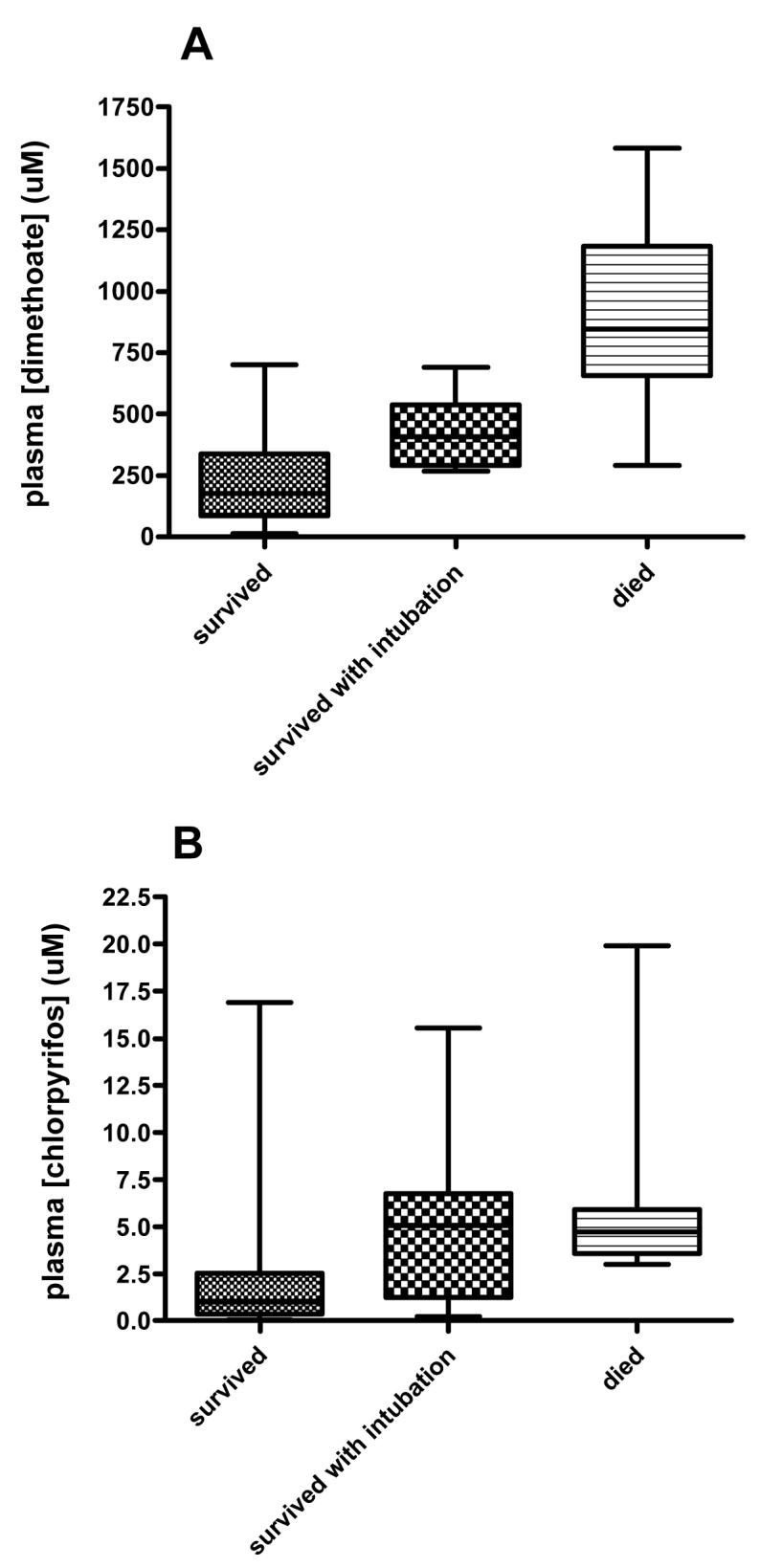
Median and range of plasma blood concentration on admission for patients poisoned by A) dimethoate and B) chlorpyrifos.
BuChE activity on admission
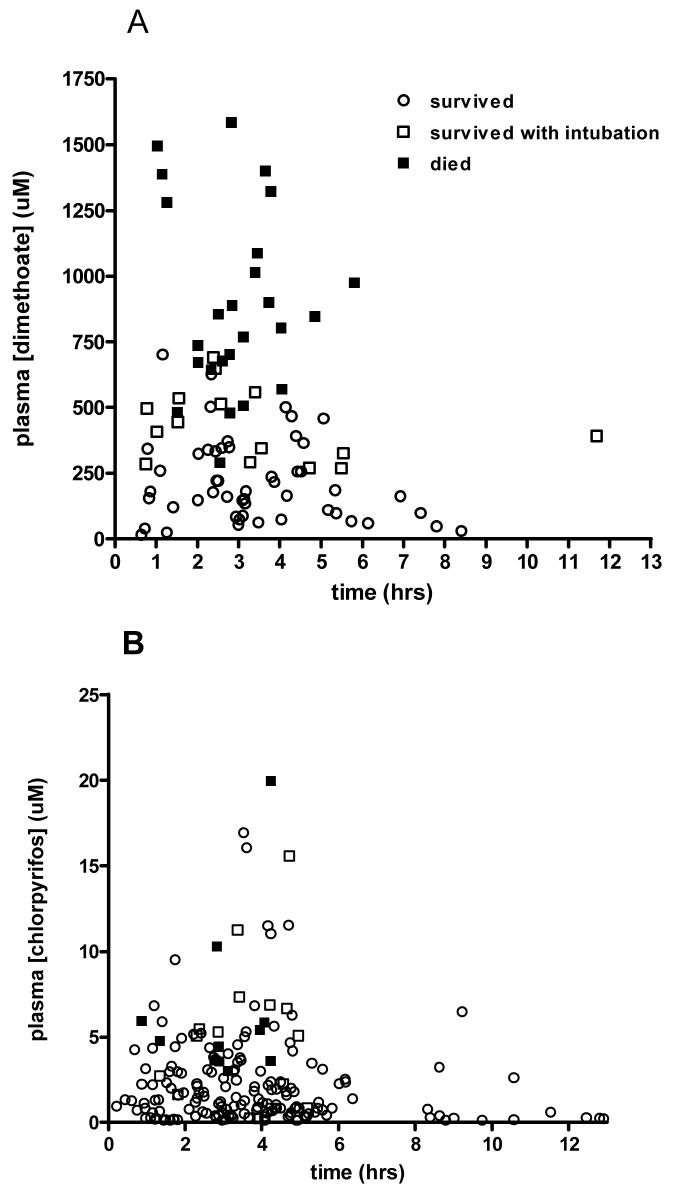
The usefulness of plasma BuChE activity on admission differed between OPs (figure 2). A value less than 600 mU/ml (10% of normal activity in our laboratory) was highly sensitive for death in chlorpyrifos patients (sensitivity 11/11; 100%, 95%CI 71.5 to 100) but not specific, since 163/198 survivors also had values <600 mU/ml (specificity 17.7%, 12.6 to 23.7). In contrast, a value of <600 had low sensitivity to predict deaths from dimethoate poisoning (12 deaths out of 25 patients; 48%, 27.9 to 68.7) but was reasonably specific (86.4%, 75.7 to 93.6) since only 9 of 66 survivors had a value <600 mU/ml on admission.
Figure 2.
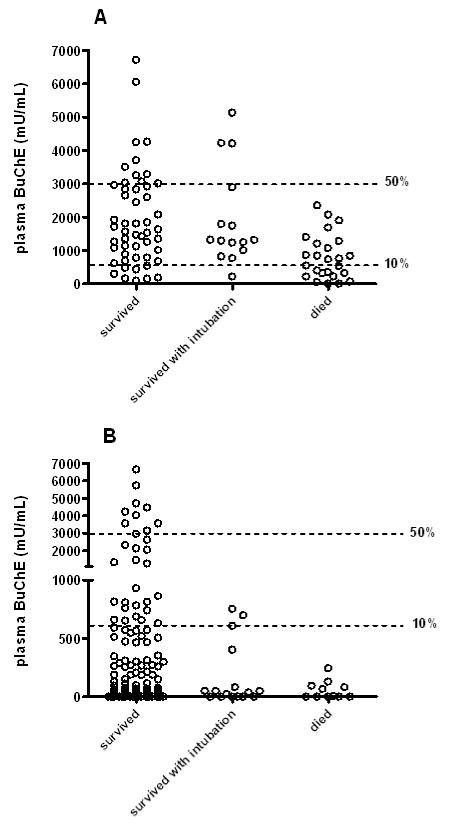
Plasma BuChE activity on admission for individual patients poisoned by A) dimethoate and B) chlorpyrifos. The lines mark BuChE at 50% and 10% of estimated normal activity (6000 mU/ml).
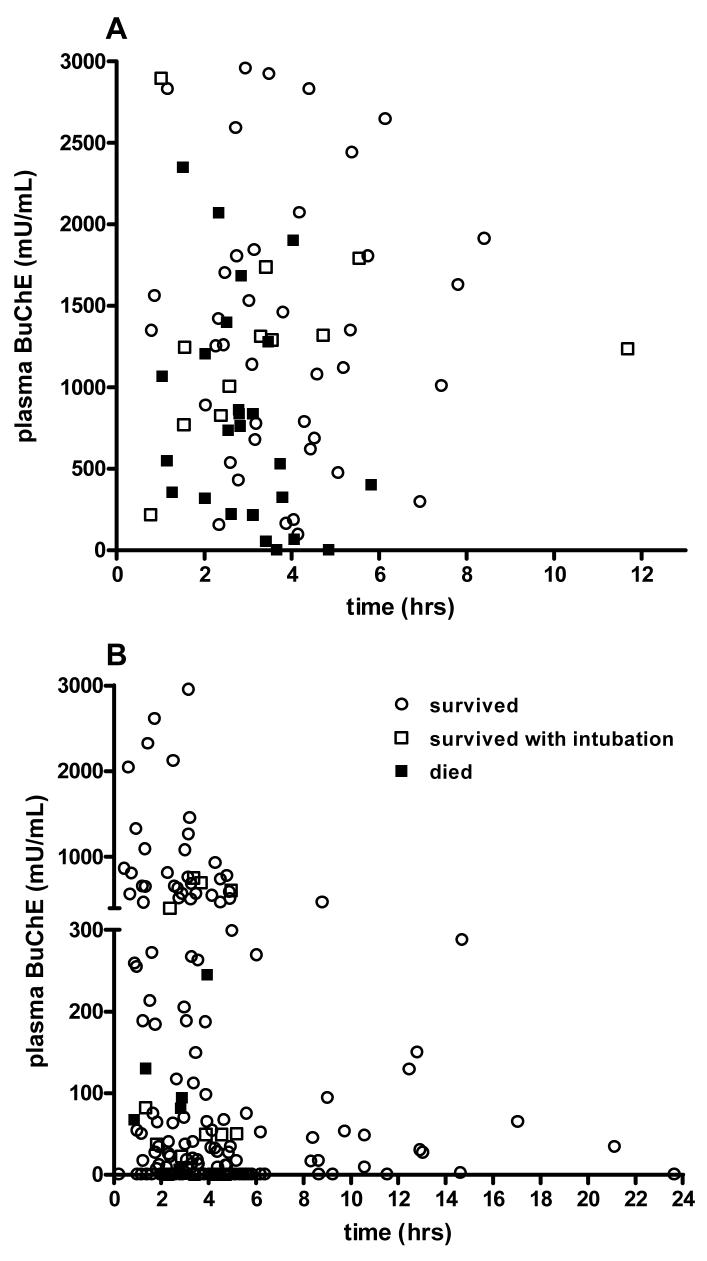
We constructed plots of BuChE activity (figure 3) against time since ingestion for both dimethoate and chlorpyrifos to assess whether time since ingestion was a factor affecting its usefulness on admission. Visual inspection and logistic regression analysis indicated that incorporating time post ingestion did not improve prediction of outcome using BuChE activity (dimethoate: pseudo r2 = 0.21, 0.17; chlorpyrifos: pseudo r2 = 0.11, 0.05; with and without time as a factor, respectively).
Figure 3.
Plot of BuChE activity on admission versus time since ingestion for patients poisoned by A) dimethoate and B) chlorpyrifos.
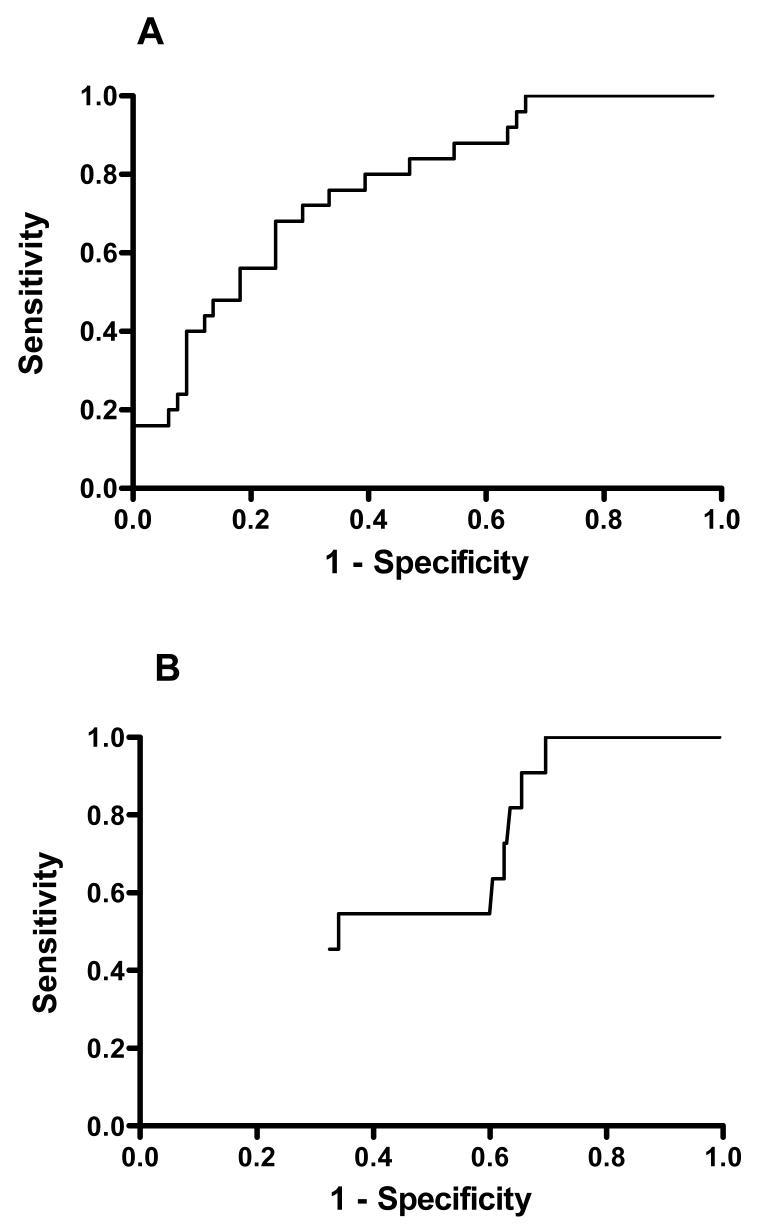
We performed ROC analysis of the specificity and sensitivity of BuChE activity on analysis in predicting a lethal outcome (figure 4). BuChE activity performed poorly with an AUC of 0.77 (95%CI 0.66 to 0.87) for dimethoate and 0.60 (04.5 to 0.75) for chlorpyrifos. There were very different optimal cut-off points (according to Youden’s index) for the two OPs: chlorpyrifos <250 mU/ml (or 4% of normal) vs dimethoate <875 mU/ml (or 15% of normal).
Figure 4.
ROC analysis of BuChE activity (A,B) or OP concentration (C,D) on admission versus time since ingestion for patients poisoned by dimethoate (A,C) and chlorpyrifos (B,D).
OP concentration on admission
We also assessed the usefulness of OP concentration on admission for predicting death. We used Youden’s index to identify cut-points with the highest combined value of sensitivity and specificity. For dimethoate a concentration cut-off of >475 μM gave a sensitivity of 96% (95%CI: 80 to 100%) and a specificity of 85% (74 to 92%) and a positive likelihood ratio of 6.3. For chlorpyrifos concentration, the optimal cut-off was >3.0 μM which gave a sensitivity of 100% (95%CI: 72 to 100%) and a specificity of 77% (70 to 82%) and a positive likelihood ratio of 4.3.
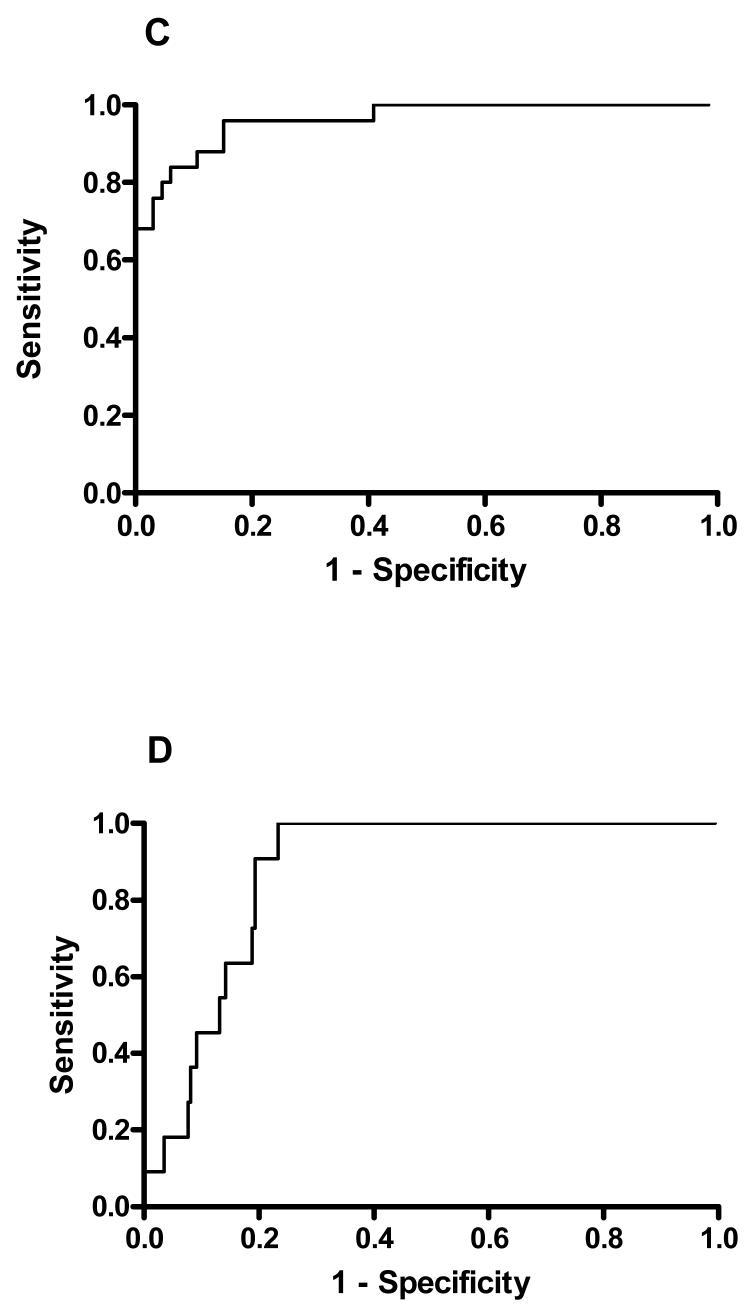
We constructed plots of OP concentration (figure 5) against time since ingestion for both dimethoate and chlorpyrifos to assess whether time since ingestion was a factor affecting the usefulness of either value on admission. Visual inspection and logistic regression analysis indicated that incorporating time post ingestion did not improve prediction of outcome (dimethoate: r2 = 0.637, 0.637; chlorpyrifos: r2 = 0.16 and 0.13; with and without time as a factor, respectively).
Figure 5.
Plot of OP concentration on admission versus time since ingestion for patients poisoned by A) dimethoate and B) chlorpyrifos.
ROC analysis of the specificity and sensitivity of OP concentration on analysis in predicting a lethal outcome showed a different picture for the two OPs (figure 4). The dimethoate concentration was both highly sensitive and specific with an AUC of 0.96 (95%CI: 0.92 to 1.00). The AUC for chlorpyrifos concentration ROC was also good at 0.88 (95%CI 0.82 to 0.93), but as can be seen from figure 5 it lacked high specificity at any value where there was acceptable sensitivity.
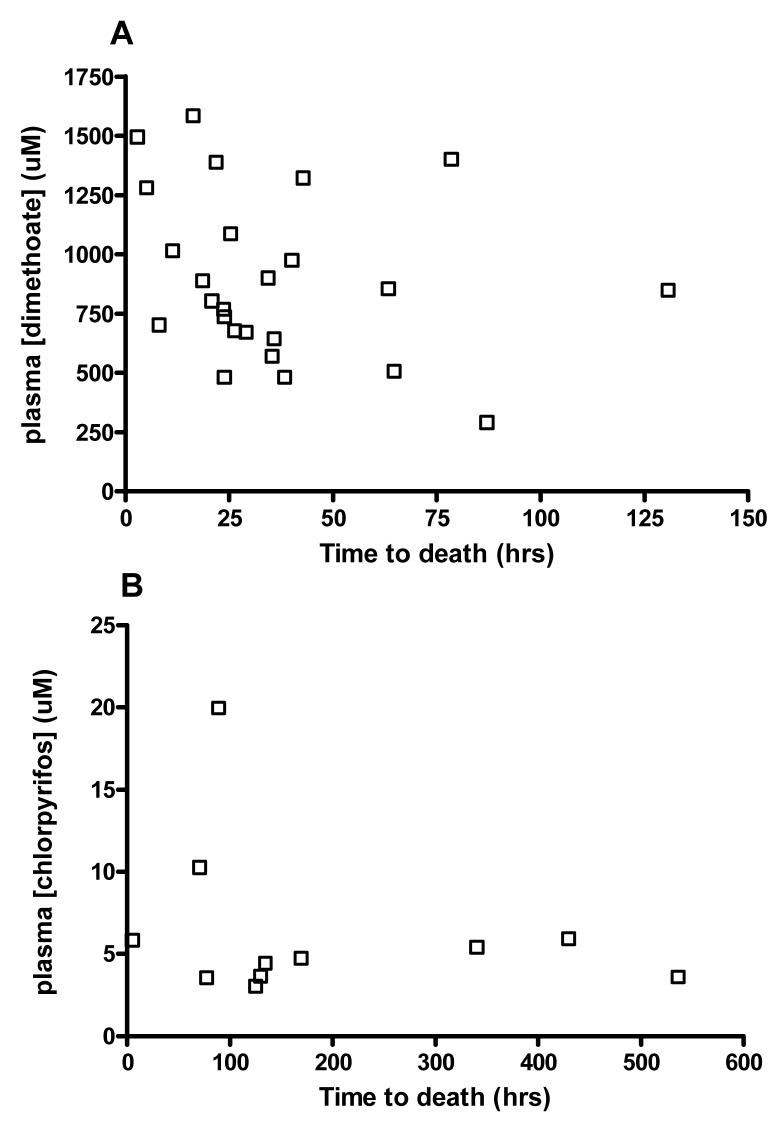
We also postulated there might be a relationship between higher OP concentration on admission and a shorter time to death in fatal cases; however, this was not apparent on review of the data (figure 6).
Figure 6.
Plot of OP concentration on admission versus time interval from ingestion to death for patients fatally poisoned by A) dimethoate and B) chlorpyrifos.
Discussion
Since Namba’s review of OP pesticide poisoning in 1971,7 a BuChE measurement on admission has been used by some clinicians to stratify severity - values <10% of normal indicating a severe poisoning. However, we did not find this cut-off to be helpful since different OPs inhibit BuChE to differing degrees compared to their inhibition of the clinically important AChE. BuChE activity therefore does not always reflect severity.
For example, the active metabolite of chlorpyrifos (chlorpyrifos-oxon) is >500-times more potent an inhibitor of BuChE than AChE;33 in consequence, all patients with sufficient AChE inhibition to produce clinical symptoms will have markedly depressed BuChE activity.28 In contrast, the dimethoate active metabolite (omethoate) inhibits cholinesterases more slowly,28,34 and BuChE activity can be near normal in symptomatic patients.28 Overall, a BuChE activity on admission is only useful when the OP pesticide has been identified and when its sensitivity and specificity is known for that particular OP.
BuChE activity must be interpreted carefully. Many patients with mild to moderate chlorpyrifos poisoning had severely inhibited BuChE on admission; by contrast, BuChE was not severely inhibited in 52% of patients with dimethoate poisoning who died. Nevertheless, plasma BuChE activity on admission was useful for some patients: in chlorpyrifos poisoning, an activity >600mU/ml accurately predicted good outcome, while an activity <600mU/ml predicted death in dimethoate poisoning. However, it was not useful for most patients - even when the OP was known and the best cut-off values chosen for each OP, the BuChE performed at a level unacceptable for a clinically useful prognostic test (figure 5).35
We found the OP concentration on admission to be potentially useful, particularly for dimethoate poisoned patients. All patients presenting with a dimethoate concentration of >750 uM died; two-thirds of fatal cases had a dimethoate concentration greater than this value on admission. In contrast, in chlorpyrifos poisoning, an OP concentration of >3 uM was sensitive for a poor outcome but this value had a poor specificity of ∼77%. Since the case fatality of chlorpyrifos poisoning is 6-7%, more than 80% of people with a concentration >3 uM on admission survive.
The reasons for this difference in the utility of concentration data are likely to be complex. AChE inhibition occurs more quickly in chlorpyrifos poisoning than in dimethoate poisoning, with patients becoming ill more quickly and requiring urgent therapy within just a few hours.28 Patients die from moderate poisoning if they do not reach hospital in time or get rapid resuscitation on arrival. Many chlorpyrifos deaths appear to be due to late respiratory complications, such as pneumonia or acute respiratory distress syndrome, not closely correlated with initial poisoning severity, that result from pre-hospital onset of poisoning and subsequent complications.
In contrast, severe dimethoate poisoning has a sub-acute presentation and most deaths occur from a cardiovascular cause after 12 hours that is not apparently modifiable by any treatment.28 In consequence, moderately poisoned dimethoate patients reach hospital alive and receive treatment in time, whereas severely poisoned patients die despite treatment.
Unexpectedly, despite the very strong correlation between dimethoate concentration and death, within these fatal cases we did not observe a strong relationship between higher OP concentration on admission and a shorter time to death. The variable time from ingestion to admission may be obscuring any relationship. Most of these samples were taken from 1-6 hrs - ie potentially during the absorption and disposition phases. It is not therefore possible to simply adjust for the time to sampling as concentrations may be both rising and falling during this time. A full exploration of this would require a much better understanding of the kinetics of dimethoate in overdose to adjust for the time of sampling with PKPD modelling.
In this study, a plasma dimethoate concentration of >750 μm was uniformly fatal, whatever the time between ingestion and blood sampling. If future research confirms this finding in more patients and in Western ICUs, this concentration fulfils an ‘All or None’ level of evidence for prognosis (level 1c, Oxford Centre for Evidence Based Medicine), i.e no patients with plasma concentration >750 μm survive. As a result, the effectiveness of interventions could be assessed outside of a randomised controlled trial.
In conclusion, plasma BuChE activity on admission can provide useful information but it must be interpreted critically when the particular OP is known. The development of rapid bedside tests for organophosphorus insecticide detection will greatly aid early assessment of severity.
Acknowledgements
We thank the directors, consultant physicians, and medical and nursing staff of the study hospitals for their support; Renate Heilmair, Bodo Pfeiffer and Elisabeth Topoll for technical assistance, and the Ox-Col study doctors for their immensely valuable work. ME is a Wellcome Trust Career Development Fellow funded by grant 063560. SACTRC is funded by the Wellcome Trust/National Health and Medical Research Council International Collaborative Research Grant 071669.
References
- (1).Jeyaratnam J. Acute pesticide poisoning: a major global health problem. Wld Hlth Statist Quart. 1990;43:139–44. [PubMed] [Google Scholar]
- (2).van der Hoek W, Konradsen F, Athukorala K, Wanigadewa T. Pesticide poisoning: a major health problem in Sri Lanka. Soc Sci Med. 1998;46:495–504. doi: 10.1016/s0277-9536(97)00193-7. [DOI] [PubMed] [Google Scholar]
- (3).Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. Q J Med. 2000;93:715–31. doi: 10.1093/qjmed/93.11.715. [DOI] [PubMed] [Google Scholar]
- (4).Eddleston M, Phillips MR. Self poisoning with pesticides. BMJ. 2004;328:42–4. doi: 10.1136/bmj.328.7430.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Lotti M. Clinical toxicology of anticholinesterase agents in humans. In: Krieger RI, Doull J, editors. Handbook of pesticide toxicology. Volume 2. Agents. 2 edn. San Diego: Academic Press; 2001. pp. 1043–85. [Google Scholar]
- (6).Ballantyne B, Marrs TC. Overview of the biological and clinical aspects of organophosphates and carbamates. In: Ballantyne B, Marrs TC, editors. Clinical and experimental toxicology of organophosphates and carbamates. 0 edn. Oxford: Butterworth heinemann; 1992. pp. 3–14. [Google Scholar]
- (7).Namba T, Nolte C, Jackrel J, Grob D. Poisoning due to organophosphate insecticides. Am J Med. 1971;50:475–92. doi: 10.1016/0002-9343(71)90337-8. [DOI] [PubMed] [Google Scholar]
- (8).Bardin PG, van Eeden SF, Moolman JA, Foden AP, Joubert JR. Organophosphate and carbamate poisoning. Arch Intern Med. 1994;154:1433–41. [PubMed] [Google Scholar]
- (9).Lotti M. Cholinesterase inhibition: complexities in interpretation. Clin Chem. 1995;41:1814–8. [PubMed] [Google Scholar]
- (10).Worek F, Diepold C, Eyer P. Dimethylphosphoryl-inhibited human cholinesterases: Inhibition, reactivation, and aging kinetics. Arch Toxicol. 1999;73:7–14. doi: 10.1007/s002040050580. [DOI] [PubMed] [Google Scholar]
- (11).Phillip AT. Cholinesterases: assay methods and significance. Clinical Toxicology Review. 1995 Apr;17(7):1–3. [Google Scholar]
- (12).Kwong TC. Organophosphate pesticides: biochemistry and clinical toxicology. Ther Drug Monitor. 2002;24:144–9. doi: 10.1097/00007691-200202000-00022. [DOI] [PubMed] [Google Scholar]
- (13).Abdullat IM, Battah AH, Hadidi KA. The use of serial measurement of plasma cholinesterase in the management of acute poisoning with organophosphates and carbamates. Forensic Sci Int. 2006;162:126–30. doi: 10.1016/j.forsciint.2006.06.074. [DOI] [PubMed] [Google Scholar]
- (14).Khan S, Hemalatha R, Jeyaseelan L, Oommen A, Zachariah A. Neuroparalysis and oxime efficacy in organophosphate poisoning: a study of butyrylcholinesterase. Hum Exp Toxicol. 2001;20:169–74. doi: 10.1191/096032701678766796. [DOI] [PubMed] [Google Scholar]
- (15).Lee P, Tai DYH. Clinical features of patients with organophosphate poisoning requiring intensive care. Intensive Care Med. 2001;27:694–9. doi: 10.1007/s001340100895. [DOI] [PubMed] [Google Scholar]
- (16).Chugh SN, Aggarwal N, Dabla S, Chhabra B. Comparative evaluation of atropine alone and atropine with pralidoxime (PAM) in the management of organophosphorus poisoning. J Indian Acad Clin Med. 2005;6:33–7. [Google Scholar]
- (17).Shivakumar S, Raghavan K, Ishaq RM, Geetha S. Organophosphorus poisoning : a study on the effectiveness of therapy with oximes. J Assoc Physicians India. 2006;54:250–1. [PubMed] [Google Scholar]
- (18).Dart RC, Hurlbut KM, Kuffner EK, Yip L. The five minute toxicology consult. Philadelphia: Lippincott Williams & Williams; 2000. [Google Scholar]
- (19).Tsao TCY, Juang YC, Lan RS, Shieh WB, Lee CH. Respiratory failure of acute organophosphate and carbamate poisoning. Chest. 1990;98:631–6. doi: 10.1378/chest.98.3.631. [DOI] [PubMed] [Google Scholar]
- (20).Brahmi N, Mokline A, Kouraichi N, Ghorbel H, Blel Y, Thabet H, et al. Prognostic value of human erythrocyte acetylcholinesterase in acute organophosphate poisoning. Am J Emerg Med. 2006;24:822–7. doi: 10.1016/j.ajem.2006.05.009. [DOI] [PubMed] [Google Scholar]
- (21).Nouira S, Abroug F, Elatrous S, Boujdaria R, Bouchoucha S. Prognostic value of serum cholinesterase in organophosphate poisoning. Chest. 1994;106:1811–4. doi: 10.1378/chest.106.6.1811. [DOI] [PubMed] [Google Scholar]
- (22).Aygun D, Doganay Z, Altintop L, Guven H, Onar M, Deniz T, et al. Serum acetylcholinesterase and prognosis of acute organophosphate poisoning. J Toxicol Clin Toxicol. 2002;40:903–10. doi: 10.1081/clt-120016962. [DOI] [PubMed] [Google Scholar]
- (23).Kar N. Lethality of suicidal organophosphorus poisoning in an Indian population: exploring preventability. Ann Gen Psychiatr. 2007;5:17. doi: 10.1186/1744-859X-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Cherian MA, Roshini C, Visalakshi J, Jeyaseelan L, Cherian AM. Biochemical and clinical profile after organophosphorus poisoning - a placebo-controlled trial using pralidoxime. J Assoc Physicians India. 2005;53:427–31. [PubMed] [Google Scholar]
- (25).Lin CL, Yang CT, Pan KY, Huang CC. Most common intoxication in nephrology ward. Organophosphate poisoning. Ren Fail. 2004;26:349–54. doi: 10.1081/jdi-120039816. [DOI] [PubMed] [Google Scholar]
- (26).Goswamy R, Chaudhuri A, Mahashur AA. Study of respiratory failure in organophosphate and carbamate poisoning. Heart & Lung. 1994;23:466–72. [PubMed] [Google Scholar]
- (27).Eddleston M, Dawson A, Karalliedde L, Dissanayake W, Hittarage A, Azher S, et al. Early management after self-poisoning with an organophosphorus or carbamate pesticide - a treatment protocol for junior doctors. Crit Care. 2004;8:R391–R397. doi: 10.1186/cc2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Eddleston M, Eyer P, Worek F, Mohamed F, Senarathna L, von Meyer L, et al. Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. Lancet. 2005;366:1452–9. doi: 10.1016/S0140-6736(05)67598-8. [DOI] [PubMed] [Google Scholar]
- (29).Eddleston M, Juszczak E, Buckley NA, Senarathna L, Mohamed F, Dissanayake W, et al. A randomised controlled trial of multiple dose activated charcoal in acute self-poisoning. Lancet. 2007 doi: 10.1016/S0140-6736(08)60270-6. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Eyer P. The role of oximes in the management of organophosphorus pesticide poisoning. Toxicol Rev. 2003;22:165–90. doi: 10.2165/00139709-200322030-00004. [DOI] [PubMed] [Google Scholar]
- (31).Worek F, Mast U, Kiderlen D, Diepold C, Eyer P. Improved determination of acetylcholinesterase activity in human whole blood. Clin Chim Acta. 1999;288:73–90. doi: 10.1016/s0009-8981(99)00144-8. [DOI] [PubMed] [Google Scholar]
- (32).den Blaauwen DH, Poppe WA, Tritschler W. Cholinesterase (EC 3.1.1.8) mit Butyrylthiocholin Iodid als Substrat: Referenzwerte in Abhängigkeit von Alter und Geschlecht unter Berücksichtigung hormonaler Einflüsse und Schwangerschaft. J Clin Chem Clin Biochem. 1983;21:381–6. [PubMed] [Google Scholar]
- (33).Amitai G, Moorad D, Adani R, Doctor BP. Inhibition of acetylcholinesterase and butyrylcholinesterase by chlorpyrifos-oxon. Biochem Pharmacol. 1998;56:293–9. doi: 10.1016/s0006-2952(98)00035-5. [DOI] [PubMed] [Google Scholar]
- (34).Buratti FM, Testai E. Evidences for CYP3A4 autoactivation in the desulfuration of dimethoate by the human liver. Toxicology. 2007;241:33–46. doi: 10.1016/j.tox.2007.08.081. [DOI] [PubMed] [Google Scholar]
- (35).Fan J, Upadhye S, Worster A. Understanding receiver operating characteristic (ROC) curves. Can J Emerg Med. 2006;8:19–20. doi: 10.1017/s1481803500013336. [DOI] [PubMed] [Google Scholar]