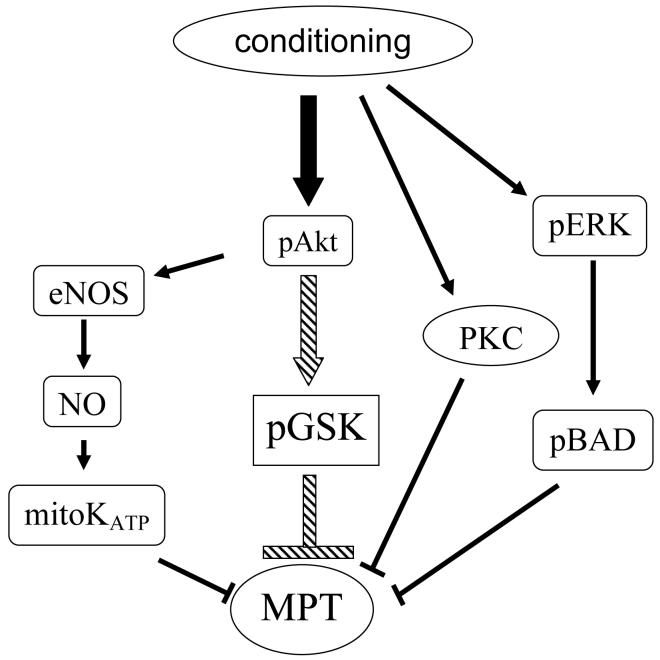
Preconditioning (PreC) and postconditioning (PostC) have been shown to initiate a number of signaling cascades that reduce cell death. However the mechanisms by which these signals reduce cell death have been elusive (1). PreC has been shown to phosphorylate and thereby inhibit GSK-3β, and perfusion with GSK inhibitors have been shown to reduce cell death induced by ischemia-reperfusion, when added before ischemia (2) or when added at the start of reperfusion (3,4,5). These studies are consistent with data in other tissues showing that inhibition of GSK-3β reduces apoptosis. Information regarding the mechanism by which inhibition of GSK protects was provided by Juhaszova et al (6) who reported that inhibition of GSK-3β delays the opening of the mitochondrial permeability transition pore (MPT) (see figure). The MPT is a large conductance pore in the inner mitochondrial membrane which is opened under conditions associated with ischemia-reperfusion such as high matrix reactive oxygen species and high matrix calcium. Pharmacological inhibitors of the MPT have been shown to reduce ischemia-reperfusion injury, suggesting that activation of MPT might have a role in ischemia-reperfusion mediated cell death. However the molecular components of the MPT have not been identified (7).
Nishino et al (8) raise two questions: 1) whether inhibition of GSK is required for protection in mice, and 2) whether inhibition of GSK is protective in mouse hearts. Pre- and Post-conditioning activate a number of redundant signaling pathways that lead to inhibition of MPT. The relative importance of different pathways may vary depending on the model and species. Previous studies (6) have suggested that inhibition of GSK is a major signaling pathway that results in inhibition of MPT; this is illustrated with the large arrow. However other pathways exist and it is not surprising that under different conditions that these pathways can dominate. Also transgenic mice may have alterations in these signaling pathways that can alter the response. Based on the number of redundant pathways, it is not surprising that conditions occur where a particular kinase is not required for protection. However, Nishino et al (8) also report that direct inhibition of GSK was not protective in mouse. This result is surprising since GSK inhibitors protect in other species. This result also conflicts with recent report by Gomez et al (5) who found that inhibitors of GSK reduced infarct size in mouse heart.
Juhaszova et al (6) showed that myocytes isolated from mice with cardiac specific overexpression of a constitutively active form of GSK-3β, in which the serine 9 is replaced with alanine, are not protected by PreC or diazoxide. Juhaszova et al also decreased GSK-3β using interfering RNA and showed that this was protective while decreasing GSK-3α was without effect. These data agree with data from other groups showing that inhibitors of GSK protect and that many types of cardioprotection result in increased phosphorylation of GSK-3β (2-6). However, the obligatory role of phosphorylation and/or inhibition of GSK in cardioprotection has been questioned by Nishino et al in this issue of Circulation Research (8). Nishino et al used GSK-3α/β knock-in (KI) mice in which the phosphorylation sites on GSK-3α (ser 21) and GSK-3β (ser 9) are changed to alanine, and wild-type mice that were inbred from the same colony, but were not littermates. In the GSK-double KI mice, infarct size, measured in a Langendorff model of global ischemia and reperfusion, was significantly lower in PreC (21.9%) and PostC (22.2%) hearts compared to non-conditioned hearts (39.5%), calling into question whether phosphorylation or inhibition of GSK is required for protection in mice. The authors further test the involvement of GSK inhibition in cardioprotection using pharmacologic GSK inhibitors and find that GSK inhibitors are not protective in this species, even though they observe protection in rats. Thus, these data suggest a species difference in the role of GSK in ischemia-reperfusion injury.
In contrast to the study by Nishino et al (8), others have found that GSK inhibition is critical for cardioprotection in mice (5, 6). In addition to the study by Sollott and coworkers, a recent study by Gomez et al (5) found that infarct size was markedly reduced by GSK inhibitors in mice, and that PostC was not protective in transgenic mice with overexpression of the same non-inhibitable GSK-3β as used by Sollott and coworkers. Thus there are conflicting data on the role of GSK-3β in ischemia-reperfusion injury in the mouse heart.
The role of GSK in cardioprotection has been addressed in two ways. One approach is through genetic manipulation and the other approach is to use pharmacologic agents. Each approach has advantages and disadvantages.
There are two genetic models that have been used to study the role of GSK in myocardial ischemia/reperfusion injury. Two groups (Juhaszova et al (6) and Gomez et al (5)) have employed transgenic mice with cardiac specific expression of a constitutively active form of GSK-3β, which cannot be phosphorylated. These studies find that many types of cardioprotection such as PreC and PostC do not confer protection in hearts and cardiomyocytes with constitutively active GSK-3β. Nishino et al (8) use a different model; a genetically modified mouse with a knock-in of a GSK-3α and GSK-3β that cannot be phosphorylated. With this mouse model, PreC and PostC are not blocked; both reduce infarct size. Nishino et al have suggested that in the mouse model used by Juhaszova et al and Gomez et al that the overexpression of constitutively active GSK-3β results in an increase in ANF and other factors that might alter cardioprotective signaling independent of GSK-3β. Indeed Gomez et al found a decrease in fractional shortening in the mice overexpressing active GSK-3β. However, one might also question whether protective compensatory pathways are activated in the double KI mice used by Nishino based on the reduced infarct size observed in the double KI (39.5%) compare to WT (61.1%) hearts from the same colony. The finding of protection in the KI mice in the absence of PreC or PostC might suggest that protective pathways are activated in these mice, although PreC and PostC can enhance this protection and the enhanced protection by PreC and PostC is not lost in the KI mice. It is possible that the double KI mice have activation of protective signaling pathways and that these protective pathways intervene at a point downstream from GSK (see figure). Thus loss of GSK inhibition would not inhibit protection if the signaling pathway downstream of GSK was endogenously activated in the KI mice. It is unfortunate that the complex breeding strategy did not allow direct comparison with WT littermates. It should also be noted that Gomez et al did not use littermates. Thus we are left with two different genetic models that give different results. Unfortunately both genetic models are likely to have compensatory changes that can influence the interpretation. When genetically modified mice were first introduced they were hailed as a way to test the effects of inhibiting pathways without the non-specific side effects that can occur with inhibitors. However, as we have learned over the years, genetic modification, particularly those that are present through the life of the animal, result in many compensatory changes that make it difficult to draw unambiguous conclusions. So unfortunately, the question of whether GSK inactivation is required for cardioprotection in the mouse or other species is not resolved and will require further study. In addition to the possibility that compensatory changes are being induced, there is also the concern that true wild-type littermates have not been used in both of the directly conflicting studies. It has been shown that genetic background is important in the time-course of ischemia/reperfusion injury in mouse heart, and this could potentially be a factor in either of the conflicting studies. Furthermore, because the genetic alteration is present continuously, it is possible that compensatory changes have occurred in pathways unrelated to GSK that allow the animals to survive, and may play a role in ischemia/reperfusion injury or cardioprotection in these genetically altered animals that would not be important in wild-type, and therefore, both genetic models may provide results that are not applicable to wild-type.
The second area of conflicting results highlighted in the study by Nishino et al (8) is the effect of GSK inhibitors on ischemia/reperfusion injury in wild-type animals. Earlier studies of the role of GSK inhibition in ischemia/reperfusion injury have used primarily rats and rabbit hearts, studied in vivo, as isolated perfused hearts, and isolated cardiomyocytes, and have reported protective effects of GSK inhibitors (2-6). In contrast to these studies, Nishino et al (8) found that GSK inhibitors did not protect in isolated perfused WT C57B6 mouse hearts (these were not the WT from the inbred colony). Interestingly, Nishino et al performed some studies showing that addition of GSK inhibitors result in protection in rat hearts. Based on these data, Nishino et al conclude that GSK inhibitors are not protective in mouse. However, others have reported that GSK inhibitors are protective in mouse heart. A recent study by Gomez et al (5) in Circulation reported that in C57B6 mice (the same strain of mice as used by Nishino et al) GSK inhibitors were protective when added at the start of reperfusion in an in vivo model of ischemia-reperfusion. Gomez et al (5) reported that PostC and GSK inhibition with SB216763 on reperfusion resulted in a similar reduction in infarct size (39% in PostC, 37% with SB vs. 58% untreated). Gomez et al used an in vivo model while Nishino et al examined infarct size using a global model of ischemia in a Landendorff heart.
So what can we conclude regarding the role of GSK in cardioprotection? It is not unusual for there to be conflicting results concerning the role of kinases in cardioprotection and reduction in apoptosis. Until we better understand the details of the mechanisms by which myocytes die, it will be difficult to conclusively prove a requirement for any kinase. The majority of studies seem to suggest a role for GSK in cardioprotection. The lack of protection in the study by Nishino et al (8) might reflect compensatory protection in the GSK-KI mice that might have protected at a point downstream of GSK, or might reflect a lack of optimization of the dosing, although the authors did show that these concentrations of GSK inhibitors blocked phosphorylation of glycogen synthase. As illustrated in the figure, it is also possible that GSK inhibition can initiate protection, but it is not unique and there are other signaling pathways that can mediate protection. If these other pathways are upregulated in the KI mice, it might be that under these conditions, GSK inhibition is not required. It is also possible that the exact mix of protective kinases that are required for protection is model and species dependent. In other words, there may be multiple mechanisms that can result in inhibition of the MPT. Under some conditions, inhibition of GSK is a major and essential part of this protection; however under other conditions other pathways may become more important and thus GSK is not required.
In summary, the study by Nishino et al (8) questions whether GSK inhibition is required for protection in mouse hearts. The lack of protection by GSK inhibitors observed by Nishino et al (8) contrasts with that observed in other species (in fact Nishino et al found GSK inhibitors protect in rat), and with the protection reported by Gomez et al (5) in mouse. Additional studies will be needed to resolve the role of GSK inhibition in mouse. Interestingly the use of genetically modified mice to address the role of GSK in cardioprotection has not resolved this issue due to compensatory mechanism that appear to be present in the genetically modified mice. So how do we resolve the role of GSK, or any kinase in protection? Clearly a mix of genetically modified mice and pharmacologic inhibitors is the current state-of-the-art. Additional studies, possibly using conditional KI of non-phosphorylatable GSK, or adenoviral transfection of hearts or myocytes along with pharmacologic inhibitors will be needed to resolve this issue.
Acknowledgements and Disclosure
Nothing to disclose. EM is supported by the NHLBI, NIH intramural program. CS is supported by NIH-RO1-HL-39752.
References
- 1.Murphy E, Steenbergen C. Preconditioning: the mitochondrial connection. Annu. Rev. Physiol. 2007;69:51–67. doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 2.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3b beta during preconditionoing through a phosphatidylinositol-3-kinase dependent pathway is cardioprotective. Circ. Res. 2002;90:377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 3.Gross ER, Hsu AK, Fross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta during reperfusion in intact rat hearts. Circ. Res. 2004;94:960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 4.Pagel PS, Krolikowski JG, Neff DA, Weihrauch D, Bienengraeber M, Kersten JR, Wartlier DC. Inhibition of glycogen synthase kinases enhances isoflurane-induced protetion against myocardial infarction during early reperfusion in vivo. Anesth Analg. 2006;102:1348–1354. doi: 10.1213/01.ane.0000202379.61338.37. [DOI] [PubMed] [Google Scholar]
- 5.Gomez l, Paillard M, Thibault H, Derumeauz G, Ovize M. Inhibition of GSK3-beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117:2761–2768. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- 6.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Zimin BD, Wang S, Ytrehus K, Antos CL, Olsen EN, Sollott SJ. Glycogen synthesis kinase 3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J. Clin. Invest. 2004;113:1534–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishino Y, Webb IG, Davidson SM, Ahmed A, Clark JE, Jacquet S, Shah A, Miura T, Yellon DM, Avkiran M, Marber M. Glycogen synthase kinase-3 inactivation is not required for ischemic preconditioning or postconditioning in the mouse. Circ. Res. 2008 doi: 10.1161/CIRCRESAHA.107.169953. [DOI] [PubMed] [Google Scholar]