Abstract
Background
The current study examined the neuro-cognitive network of visual word rhyming judgment in 14 children with dyslexia and 14 age-matched control children (8- to 14-year-olds) using functional magnetic resonance imaging (fMRI).
Methods
In order to manipulate the difficulty of mapping orthography to phonology, we used conflicting and non-conflicting trials. The words in conflicting trials either had similar orthography but different phonology (e.g., pint-mint) or similar phonology but different orthography (e.g., jazz-has). The words in non-conflicting trials had similar orthography and phonology (e.g., gate-hate) or different orthography and phonology (e.g., press-list).
Results
There were no differences in brain activation between the controls and children with dyslexia in the easier non-conflicting trials. However, the children with dyslexia showed less activation than the controls in left inferior frontal gyrus (BA 45/44/47/9), left inferior parietal lobule (BA 40), left inferior temporal gyrus/fusiform gyrus (BA 20/37) and left middle temporal gyrus (BA 21) for the more difficult conflicting trials. For the direct comparison of conflicting minus non-conflicting trials, controls showed greater activation than children with dyslexia in left inferior frontal gyrus (BA 9/45/46) and medial frontal gyrus (BA 8). Children with dyslexia did not show greater activation than controls for any comparison.
Conclusions
Reduced activation in these regions suggests that children with dyslexia have deficient orthographic representations in ventral temporal cortex as well as deficits in mapping between orthographic and phonological representations in inferior parietal cortex. The greater activation for the controls in inferior frontal gyrus could reflect more effective top-down modulation of posterior representations.
Keywords: Brain imaging, dyslexia, learning difficulties, magnetic resonance imaging, phonological processing, reading disorder
Dyslexia is perhaps the most common neurobehavioral disorder in children, with prevalence rates ranging from 5 to 17.5% (Shaywitz et al., 1998). Converging behavioral evidence suggests that a central problem in patients with dyslexia is a deficit in phonological processing, especially in identifying and manipulating the sound structure of a word (Brady & Shankweiler, 1991; Bruck, 1992; Stanovich & Siegel, 1994; Shankweiler et al., 1995). Neuroimaging studies show that patients with dyslexia exhibit less intense activation than controls in left temporo-parietal regions, including the posterior part of the superior temporal gyrus and inferior parietal cortex (Rumsey et al., 1997a, 1997b; Shaywitz et al., 1998; Brunswick, McCrory, Price, Frith, & Frith, 1999; Helenius, Uutela, & Hari, 1999; Pugh et al., 2000b; Simos et al., 2000b; Shaywitz et al., 2002, 2003). Under-activation in these regions may reflect deficits that patients with dyslexia have in phonological processing in superior temporal gyrus and in mapping from orthographic to phonological representations in inferior parietal cortex.
Neuroimaging studies have also found that patients with dyslexia show less activation than controls in left fusiform gyrus (Rumsey et al., 1997b; Shaywitz et al., 1998; Brunswick et al., 1999; Paulesu et al., 2001; Shaywitz et al., 2002, 2003). Research suggests that left fusiform gyrus is important for processing the orthographic structure of well-learned visual word forms (Price, Wise, & Frackowiak, 1996; Tarkiainen, Helenius, Hansen, Cornelissen, & Salmelin, 1999; Cohen et al., 2000; Xu et al., 2001; Cohen et al., 2002). Although there is a debate on whether the left fusiform gyrus is sensitive to lexicality or just familiarity, most agree that this region includes orthographic representations that are linguistically structured (Pugh et al., 2000b). Although reduced activation in left fusiform gyrus for patients with dyslexia may reflect a deficit in orthographic processing, some studies have shown normal activation in this region during letter rhyming judgment (Temple et al., 2001) or non-word reading (Georgiewa et al., 1999, 2002).
In addition to the activation deficits described above, neuroimaging studies of adults with dyslexia show either greater activation (Shaywitz et al., 1998; Brunswick et al., 1999) or no difference (Rumsey et al., 1997b; Paulesu et al., 2001; Shaywitz et al., 2003) from controls in left inferior frontal gyrus. This over-activation in the left inferior frontal gyrus has been interpreted as a compensatory mechanism involving sub-vocal phonological rehearsal by adults with dyslexia. In children with dyslexia, however, only one study found greater activation in the left inferior frontal gyrus (Georgiewa et al., 2002), whereas most studies show either less activation (Georgiewa, 1999; Shaywitz et al., 2002) or no difference (Georgiewa et al., 1999; Temple et al., 2001; Shaywitz et al., 2002). One study showed that there was a positive correlation between activation in left inferior frontal gyrus and age during a nonword rhyming judgment task in children with dyslexia (Shaywitz et al., 2002). Taken together, these studies suggest that adults with dyslexia show over-activation whereas children with dyslexia show under-activation in the left inferior frontal gyrus.
In the current study, we manipulated the difficulty of a visual rhyming task by including word pairs with conflicting (e.g., pint-mint, jazz-has) or non-conflicting (e.g., gate-hate, press-list) orthographic and phonological information in children. Research generally shows that spelling and rhyming judgments are more difficult for conflicting than for non-conflicting pairs (Polich, McCarthy, Wang, & Donchin, 1983; Johnston & McDermott, 1986; Kramer & Donchin, 1987; Rugg & Barrett, 1987; Levinthal & Hornung, 1992) and that patients with dyslexia have greater difficulty with conflicting pairs as compared to non-conflicting pairs (Rack, 1985; McPherson, Ackerman, & Dykman, 1997), but no neuroimaging studies have compared patients with dyslexia to controls on conflicting versus non-conflicting pairs. The goal of this study was to determine if trials that placed greater demands on orthographic and phonological processing would produce larger group differences. We expected the more difficult conflicting trials to generate greater behavioral and activation differences between children with dyslexia and controls as compared to the non-conflicting trials. Consistent with previous reports, we expected children with dyslexia to show less activation in left temporo-parietal cortex, left fusiform gyrus, and left inferior frontal gyrus.
Materials and methods
Participants
Fourteen children with dyslexia (M age = 11.6, range: 8.8-14.10; 12 males, 2 females) and fourteen chronological age-matched children (M age = 11.5, range: 8.9-14.11; 8 males, 6 females) participated in this study. Children with dyslexia met the following inclusionary criteria: (1) full scale IQ (Wechsler, 1999) was above 75, (2) mean on word and nonword reading accuracy and naming speed tests (Torgesen, Wagner, & Rashotte, 1999; Woodcock, McGrew, & Mather, 2001) was below 95. All dyslexics had a diagnosis in the past of learning disability by an appropriate professional. However, some dyslexics had received remediation and this may account for their relatively high scores on some of the standardized tests. The chronological age-matched control children met the following criteria: (1) difference of age with matched children with dyslexia was less than four months (M = 1.8 months, range: 0-4 months), (2) full scale IQ (Wechsler, 1999) was above 75, (3) mean on word and nonword reading accuracy and naming speed tests (Torgesen et al., 1999; Woodcock et al., 2001) was above 95. There were significant differences between children with dyslexia and controls in IQ and reading achievement (see Table 1). Because of the significant difference between groups in full scale IQ, this was used as a covariate of no interest for the fMRI data analysis. Parents of all children were given an informal interview to insure that the children met the following inclusionary criteria: (1) native English speaker, (2) right-handedness, (3) free of neurological disease or psychiatric disorders, and (4) no attention deficit hyperactivity disorder (ADHD). The Institutional Review Board at Northwestern University and Evanston Northwestern Healthcare Research institute approved the informed consent procedures.
Table 1.
Means (standard deviations, ranges) for the standardized tests for children with dyslexia and controls
| Dyslexia | Control | |
|---|---|---|
| Verbal IQ (WASI)* | 96 (14, 71-127) | 109 (11, 79-123) |
| Performance IQ (WASI)** | 96 (10, 79-112) | 109 (11, 79-124) |
| Full Scale IQ (WASI)** | 95 (12, 76-115) | 110 (12, 85-124) |
| Word Identification (WJ-III)*** | 86 (7, 72-102) | 112 (9, 96-126) |
| Word Attack (WJ-III)*** | 85 (9, 74-101) | 104 (7, 93-112) |
| Single Word Reading Efficiency (TOWRE)*** | 85 (6, 77-99) | 105 (8, 93-118) |
| Pseudoword Decoding Efficiency (TOWRE)*** | 78 (10, 59-96) | 101 (7, 89-110) |
| Reading Average*** | 83 (7, 73-94) | 106 (6, 98-112) |
p < .001
p < .01
p < .05 in independent-sample t-tests. Reading average: average of four standardized reading tests.
Functional activation task
Rhyming task
Two words were visually presented in sequential order and the participant had to determine whether the two words rhymed (Table 2). If the word pair rhymed, the participant pressed a button with the right index finger; if the word pair did not rhyme, the participant pressed another button with the right middle finger. Each word was presented for 800 msec followed by a 200 msec blank interval. A red fixation-cross appeared on the screen after the second word, indicating the need to make a response during the subsequent 2,600 msec interval.
Table 2.
Examples of stimuli in four lexical trial types for the visual rhyming task. Stimuli with similar orthography and dissimilar phonology or vice versa were the conflicting trials
| Similar phonology (P+) |
Dissimilar phonology (P-) |
|
|---|---|---|
| Similar orthography (O+) | Gate-hate | Pint-mint |
| Different orthography (O-) | Has-jazz | Press-list |
Half of the word pairs rhymed and half did not. Half of the word pairs had similar orthography and the other half did not. The combination of these factors resulted in four trials, namely: similar orthography similar phonology (O+P+), similar orthography different phonology (O+P-), different orthography similar phonology (O-P+) and different orthography different phonology (O-P-) (see Table 2). There were 24 word pairs for each trial type. The four trial types were matched for their written word frequency based on child and adult norms (Educators Word Frequency Guide, 1996). All words and symbols (see below) were presented in lower case, at the center of the screen, with a .5 letter offset of position between the first and second stimulus.
Control trials
Two perceptual control trial types were used in which two symbol strings were presented visually in sequential order and the participant had to determine whether the strings matched. In the ‘Simple’ trials, the symbol string consisted of a single symbol, while in the ‘Complex’ trials the symbol string consisted of three different symbols. Timing parameters were the same as for the lexical trials. Twenty-four items were presented in each perceptual trial type, with half of them matching. In addition to the perceptual control trials, 72 fixation trials were included as null events. In the null trials, a black fixation-cross was presented for the same duration as the stimuli in the lexical and perceptual trials and participants were instructed to press a button when the black fixation-cross turned red. We used null trials as the baseline for our fMRI analysis because the difference in behavioral performance between groups was the smallest for these trials.
Experimental procedure
After standardized tests were administered, participants were given a practice session in which they performed one run experimental task in a simulator. Different stimuli were used for the practice session. Scanning took place within a week from the practice session. In the scanning session, the task was administered in two 108 trial runs (8 minute), in which the order of lexical, perceptual and fixation trials and was optimized for event-related design using OptSeq (http://surfer.nmr.mgh.harvard.edu/optseq) (Burock, Buckner, Woldorff, Rosen, & Dale, 1998). The order of stimuli within task was fixed for all subjects.
MRI data acquisition
Images were acquired using a 1.5 Tesla GE scanner, using a standard head coil. The BOLD functional images were acquired using the EPI (echo planar imaging) method. The following parameters were used for scanning: TE = 35 msec, flip angle = 90°, matrix size = 64 × 64, field of view = 24 cm, slice thickness = 5 mm, number of slices = 24; TR = 2000 msec. Two runs, with 240 repetitions each, were administered for the functional images. In addition, structural T1 weighted 3D images were acquired (SPGR, TR = 21 ms, TE = 8 msec, flip angle = 20°, matrix size = 256 × 256, field of view = 22 cm, slice thickness = 1 mm, number of slices = 124).
Image analysis
Data analysis was performed using SPM2 (Statistical Parametric Mapping) (http://www.fil.ion.ucl.ac.uk/spm). The images were spatially realigned to the first volume to correct for head movements. No individual runs had more than 3 mm maximum displacement in either X, Y or Z translation (M = 1.41, SD = .92 for children with dyslexia; M = .87, SD = .45 for controls). Movement was not significantly different between children with dyslexia and controls (t = 1.96, p = .06). Sinc interpolation was used to minimize timing-errors between slices. The functional images were co-registered with the anatomical image, and normalized (12 linear affine parameters for brain size and position, 8 non-linear iterations and 2 × 2 × 2 nonlinear basis functions) to the standard T1 template volume (MNI). The data was then smoothed with a 10 mm isotropic Gaussian kernel (Xiong et al., 2000). Statistical analyses at the first level were calculated using an event-related design, separately for each task, with 4 lexical trial types, 2 perceptual trial types, and null trial types as trials of interest. A high pass filter with a cutoff period of 128 seconds was applied. Word pairs were treated as individual events for analysis and modeled using a canonical HRF. We used global normalization to scale the mean of each scan to a common value. Both correct and incorrect trials were used in the statistical analysis.
Parameter estimates from contrasts in single subject models were entered into random-effects analysis using one-sample t-tests across all participants in each group to determine whether activation within a group was significant and using two-sample t-tests across groups (children with dyslexia, controls) to determine whether there were reliable group differences. We also examined the correlation between behavioral performance and activation separately in controls and children with dyslexia by adding accuracy as a covariate of interest. All reported areas of activation were significant using p < .001 uncorrected at the voxel level and containing a cluster size greater than 20 voxels for the contrasts within each group and greater than 15 voxels for the contrasts between groups.
Results
Behavioral performance
Table 3 presents accuracy and reaction time for controls and children with dyslexia on the visual rhyming judgment in the scanner. We calculated trial type (conflicting, non-conflicting) by group (controls, children with dyslexia) ANOVAs separately for accuracy and reaction time on correct trials. There were significant main effects for group showing that children with dyslexia were less accurate, F(1,26) = 94.16, p < .001, and slower, F(1,26) = 9.437, p < .01, than controls. There were significant main effects for trial type showing that the conflicting trials were less accurate, F(1,26) = 81.09, p < .001, and slower, F(1,26) = 18.44, p < .001, than the non-conflicting trials. There was a significant interaction between group and trial type for accuracy, F(1,26) = 9.35, p < .01, but not for reaction time. Although the difference between controls and children with dyslexia was clearly larger for the conflicting trials, t-tests showed that children with dyslexia were less accurate than controls in non-conflicting trials, t(26) = 5.00, p < .001, as well as conflicting trials, t(26) = 9.50, p < .001. Although children with dyslexia were near chance on the conflicting trials, the fact that they had slower reaction times than for the nonconflicting trials suggests that they were not just responding randomly.
Table 3.
Means (and standard deviations) for accuracy and reaction time in children with dyslexia and controls in the conflicting and non-conflicting trials
| Conflicting | Non-conflicting | |
|---|---|---|
| Accuracy (%) | ||
| Controls | 79.5 (7.3) | 94.5 (5.2) |
| Dyslexia | 44.8 (11.5) | 75.2 (13.5) |
| Reaction time (ms) | ||
| Controls | 1240 (351) | 1119 (348) |
| Dyslexia | 1593 (272) | 1480 (282) |
Brain activation patterns
Table 4 shows reliable activation for conflicting minus null and non-conflicting minus null contrasts within each group, and Figures 1 and 2 shows the brain activation maps for these comparisons. Table 5 shows greater activation for controls than for children with dyslexia on conflicting trials, and Figure 3 shows the brain activation maps for this comparison. Controls showed greater activation than children with dyslexia in the left (BA 45/47/46/9) and right (BA 9) inferior frontal gyrus, left inferior temporal gyrus extending into the fusiform gyrus (BA 20/37), left inferior parietal lobule (BA 40), and left middle temporal gyrus (BA 21). Controls did not show significantly different activation than children with dyslexia for the non-conflicting trials. Table 5 also shows greater activation for controls than for children with dyslexia on the conflicting minus non-conflicting using the inclusive mask of brain activation for the controls on the conflicting minus null, and Figure 4 shows the brain activation maps for this comparison. Controls showed greater activation than children with dyslexia in left inferior and middle frontal gyrus (BA 9/45/46), medial frontal gyrus (BA8) and lingual gyrus. Children with dyslexia did not show greater activation than controls for any contrast. We also correlated behavioral performance with activation for controls and children with dyslexia for the more difficult conflicting trials. None of the correlations overlapped with the effect of greater activation for controls compared to children with dyslexia in the conflicting trials, suggesting that the effect of differential activation between groups may not be due to differences in accuracy on this specific lexical task.
Table 4.
Activations in controls and children with dyslexia in conflicting minus null (Conflict) and non-conflicting minus null (Non) contrasts
| Contrast | Region | H | BA | z-test | voxels | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Control (Conflict) | Inferior/middle frontal gyrus | L | 47/45/44/46/9/6/8 | 5.99 | 1503 | -42 | 24 | -3 |
| Inferior frontal gyrus | R | 47 | 5.26 | 334 | 36 | 27 | -3 | |
| Middle frontal gyrus | R | 9 | 3.87 | 27 | 39 | 6 | 27 | |
|
Medial frontal gyrus/ superior frontal gyrus/ anterior cingulate |
L/R | 8/32 | 5.68 | 687 | 9 | 18 | 48 | |
| Inferior parietal lobule | L | 40 | 3.65 | 38 | -36 | -33 | 45 | |
|
Fusiform/middle occipital/ inferior temporal gyrus |
L | 37/19/18 | 5.28 | 391 | -42 | -54 | -18 | |
|
Middle occipital/fusiform/ inferior temporal gyrus |
R | 18/19/37 | 5.13 | 421 | 36 | -90 | -3 | |
| Cuneus/posterior cingulate | R/L | 18/19/30 | 4.26 | 331 | 15 | -75 | 12 | |
| Caudate nucleus/thalamus | R/L | - | 5.48 | 290 | 9 | 12 | 6 | |
| Dyslexia (Conflict) | Inferior frontal gyrus | L | 45/44/9 | 4.16 | 252 | -33 | 33 | 6 |
|
Medial frontal gyrus/ superior frontal gyrus/ anterior cingulate |
L/R | 32 | 3.59 | 29 | 0 | 18 | 42 | |
| Fusiform/inferior occipital gyrus | L | 37/19 | 3.89 | 134 | -39 | -54 | -12 | |
| Control (Non) | Inferior frontal gyrus | L | 45/44/46/47 | 4.37 | 302 | -42 | 27 | 12 |
| Inferior frontal gyrus | L | 9/6 | 4.08 | 124 | -45 | 6 | 33 | |
| Superior frontal gyrus | L | 6/8 | 4.07 | 48 | -3 | 12 | 60 | |
|
Middle occipital/fusiform/ inferior temporal gyrus |
L | 19/37/18 | 4.95 | 349 | -36 | -81 | -3 | |
| Inferior/middle occipital gyrus | R | 19 | 4.84 | 281 | 36 | -81 | -9 | |
| Dyslexia (Non) | Precentral/inferior frontal gyrus | L | 6/9 | 4.16 | 76 | -57 | 0 | 36 |
| Inferior occipital/fusiform gyrus | L | 19/37 | 4.92 | 308 | -39 | -75 | -6 | |
| Inferior occipital/fusiform gyrus | R | 19/37 | 4.68 | 133 | 42 | -75 | -6 | |
| Lingual gyrus | R | 18 | 3.63 | 22 | 12 | -81 | 0 |
Note: Peaks of activation are listed in bold for areas spanning different regions; H = hemisphere, L = left, R = right; BA = Brodmann Area.
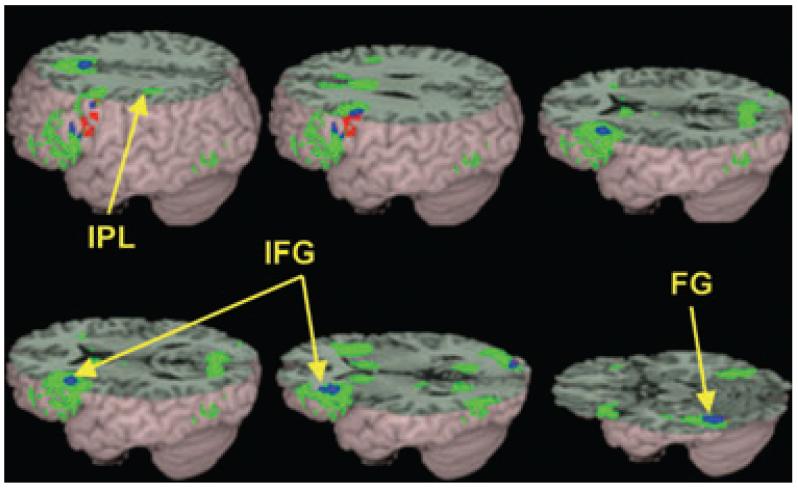
Figure 1.
Brain activations for the conflicting versus ‘null’ trials in controls group (green) and in children with dyslexia (red). The overlap between the groups is represented in blue. Both controls and children with dyslexia showed activation in left inferior frontal gyrus (IFG) and left fusiform gyrus (FG). Only controls showed activation in left inferior parietal lobule (IPL). See Table 3 for a full listing of activations
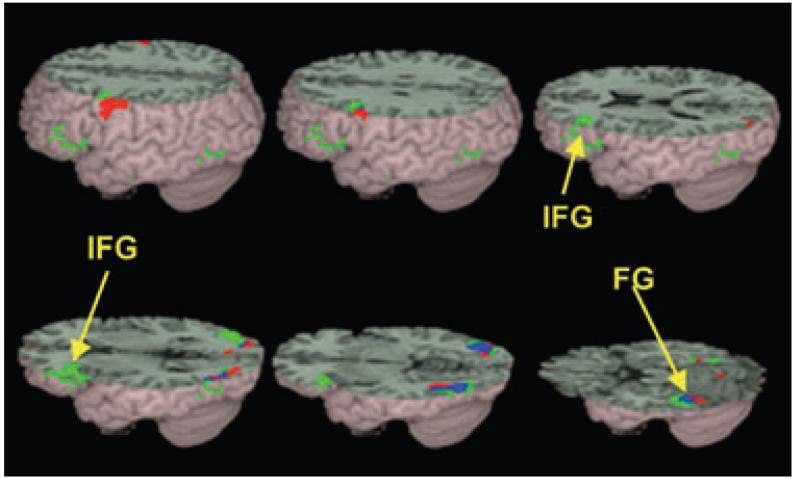
Figure 2.
Brain activations for non-conflicting versus ‘null’ trials in controls (green) and in children with dyslexia (red). The overlap between the groups is represented in blue. Both controls and children with dyslexia showed activation in left inferior frontal gyrus (IFG) and left fusiform gyrus (FG). See Table 3 for a full listing of activations
Table 5.
Direct comparisons between controls and children with dyslexia during conflicting minus null, nonconflicting minus null, and conflicting minus non-conflicting
| Contrast | Region | H | BA | z-test | voxels | x | Y | z |
|---|---|---|---|---|---|---|---|---|
| Conflicting - Null | Inferior/middle frontal gyrus | L | 45/47/46 | 4.73 | 203 | -57 | 21 | 6 |
| Precentral/inferior frontal gyrus | L | 6/9 | 4.14 | 79 | -42 | 3 | 36 | |
| Middle/inferior frontal gyrus | R | 10/9 | 3.55 | 19 | 36 | 36 | 18 | |
| Inferior parietal lobule | L | 40 | 3.65 | 18 | -30 | -39 | 33 | |
| Inferior temporal/fusiform gyrus | L | 20/37 | 4.18 | 20 | -42 | -21 | -21 | |
| Middle temporal gyrus | L | 21 | 3.94 | 27 | -60 | -54 | 3 | |
| Non-conflicting - Null | - | - | - | - | - | - | - | - |
| Conflicting - Non-conflicting | Middle/inferior frontal gyrus | L | 9,45,46 | 3.73 | 82 | 42 | 9 | 30 |
| Medial frontal gyrus | L/R | 8 | 3.50 | 25 | -3 | 21 | 48 | |
| Lingual gyrus | R | 17/18 | 3.85 | 47 | 12 | -39 | -12 |
Note: See Table 4 note.
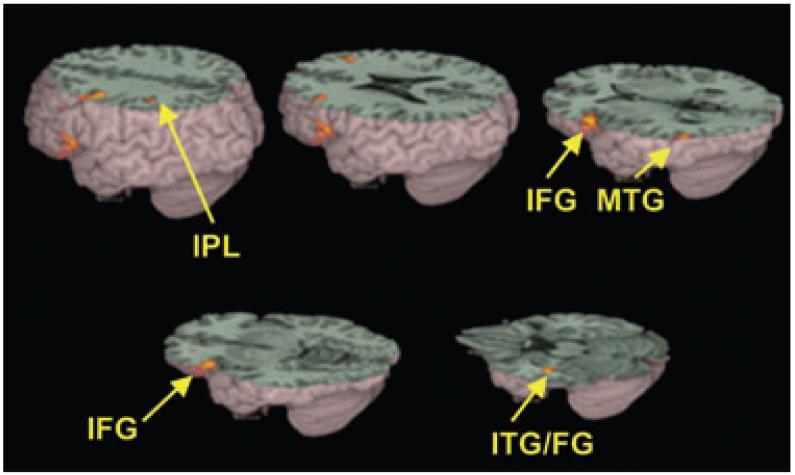
Figure 3.
Significantly greater activations for controls than for children with dyslexia in conflicting versus ‘null’ trials included the left inferior frontal gyrus (IFG), left inferior parietal lobule (IPL) and left inferior temporal gyrus/fusiform gyrus (ITG/FG). See Table 4 for a full listing of activations
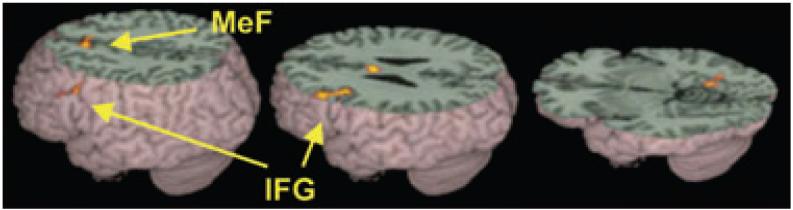
Figure 4.
Significantly greater activations for controls than for children with dyslexia in conflicting versus the non-conflicting trials (masked by controls conflicting - ‘null’ trials) included left inferior frontal gyrus (IFG) and medial frontal gyrus (MeF). See Table 5 for a full listing of activations
We recalculated group differences for the conflicting minus null, non-conflicting minus null and the conflicting minus non-conflicting contrasts for correct trials only. Table 6 shows that the similar group differences emerged for all contrasts and in all regions, except for inferior parietal lobule, when a more liberal significance threshold was applied (p < .01 uncorrected, 10 or greater voxels). Inferior parietal lobule showed a group difference at p < .05 uncorrected, 10 or greater voxels.
Table 6.
Direct comparisons between controls and children with dyslexia during conflicting minus null, non-conflicting minus null, and conflicting minus non-conflicting for correct trials only
| Contrast | Region | H | BA | z-test | voxels | x | Y | z |
|---|---|---|---|---|---|---|---|---|
| Conflicting - Null |
Inferior frontal/middle frontal/ precentral gyrus |
L | 47/45/46/6/9 | 3.68 | 388 | -48 | 21 | -3 |
| -42 | 3 | 36 | ||||||
| Inferior/middle frontal gyrus | R | 10/9 | 2.84 | 33 | 36 | 33 | 15 | |
| *Inferior parietal lobule | L | 40 | 2.36 | 102 | -36 | -36 | 39 | |
| Inferior temporal/fusiform gyrus | L | 20/37 | 3.22 | 48 | -42 | -24 | -21 | |
| Middle temporal gyrus | L | 21 | 4.01 | 70 | -60 | -57 | 3 | |
| Non-conflicting - Null | - | - | - | - | - | - | - | - |
| Conflicting - Non-conflicting | Inferior/middle frontal gyrus | L | 9/45/46 | 3.06 | 28 | -39 | 6 | 27 |
| Medial frontal gyrus | L/R | 8 | 3.03 | 38 | 3 | 33 | 48 | |
| Culmen/Lingual Gyrus | R | 20 | 3.36 | 67 | 12 | -42 | -21 |
Note: See Table 4 note.
inferior parietal lobule was only activated at p < .05 uncorrected.
Discussion
In the conflicting trials, both children with dyslexia and controls activated a network that has been previously implicated in lexical processing, including bilateral inferior frontal gyrus, inferior temporal/fusiform gyrus and medial frontal gyrus. Only controls showed activation in inferior parietal lobule and this activation was significantly greater than that of children with dyslexia for the conflicting trials compared to baseline. Controls also showed significantly greater activation in bilateral inferior frontal gyrus, left middle temporal gyrus and left fusiform gyrus for the conflicting trials compared to baseline and greater activation in left inferior frontal gyrus and medial frontal gyrus for the conflicting trials compared to non-conflicting trials. All significant differences in the magnitude of brain activation between controls and children with dyslexia appeared for the more difficult conflicting word pairs (e.g., pint-mint, has-jazz) and not for the non-conflicting pairs (e.g., gate-hate, press-list). This is consistent with previous research that has shown smaller group differences in activation for easier phonological tasks (Shaywitz et al., 1998). It is also consistent with behavioral results showing larger group differences in conflicting compared to non-conflicting pairs (Rack, 1985; McPherson et al., 1997). Although there was no difference between children with dyslexia and controls for the non-conflicting trials in brain activation, there was a difference in behavioral performance. It could be that there were differences in functional or effective connectivity between brain regions that caused differences in behavioral performance (Pugh et al., 2000a).
Inferior temporal gyrus/fusiform gyrus
Previous research has implicated the inferior temporal gyrus and fusiform gyrus in orthographic processing. Dehaene et al. (2004) suggested that moving in a posterior-to-anterior progression within the ventral occipito-temporal cortex results in progressively larger units, from visual features in extrastriate cortex to graphemes, syllables, and eventually entire words in temporal cortex. Posterior areas have been suggested to contain location specific letter detectors because they show case-invariant, location-specific priming. Middle areas have been suggested to encode partial words because they show significant priming effects for words only when they are repeated at the same location. Anterior regions have been suggested to encode whole words because they show both location-independent and case-invariant repetition effects. Anterior regions in the banks of the inferior temporal sulcus have also been shown to respond to both visual and auditory word forms. This has been referred to as ‘lateral inferotemporal multimodal area’ (LIMA) and has been suggested to be involved in supramodal word processing (Cohen, Jobert, Le Bihan, & Dehaene, 2004). Our study found that children with dyslexia produced less activation in an anterior region of the ventral temporal cortex than is probably involved in whole word or supramodal word processing. The lack of differences in posterior regions suggests that children with dyslexia in our study may have intact orthographic processing for letters and partial words, with an orthographic deficit only for whole word representations. However, some research have argued that left fusiform gyrus may be involved in semantic processing because activation in this region was correlated with performance accuracy on an auditory semantic decision task in young children (5-10 years) (Balsamo, Xu, & Gaillard, in press).
Inferior parietal lobule
Our study revealed that children with dyslexia produced less activation than controls in left inferior parietal lobule. Our findings are consistent with previous studies that have found under-activation in left inferior parietal cortex in children with dyslexia (Simos et al., 2000b; Temple et al., 2001). The inferior parietal lobule has been implicated in mapping between orthographic and phonological representations (Xu et al., 2001; Booth et al., 2002, 2003), and is sensitive to conflict between orthographic and phonological information (Bitan et al., in press). Control children produced greater activation in this region during rhyming judgments for conflicting pairs (e.g., pint-mint, has-jazz) than for non-conflicting pairs (e.g., gate-hate, press-list) (Bitan et al., in press). Group differences between children with dyslexia and controls may only emerge when the demands on mapping orthographic to phonological representations are enhanced (Shaywitz et al., 1998; Pugh et al., 2000b) as in the case of conflicting pairs.
Inferior frontal gyrus
Activation in the dorsal left inferior frontal gyrus (BA 44) has been found in tasks that involve effortful selection, retrieval or manipulation of phonological representations such as reading of pseudo-words (Fiez, Balota, Raichle, & Petersen, 1999; Fiebach, Friederici, Müller, & von Cramon, 2002). The inferior frontal gyrus may act as an executive system that controls the access, retrieval, selection and gating of information by the modulation or reactivation of representations in posterior brain regions (Wagner, Desmond, Demb, Glover, & Gabrieli, 1997; Poldrack et al., 1999). Studies of children with dyslexia have found either under-activation or normal activation in left inferior frontal gyrus (Georgiewa et al., 1999; Temple et al., 2001; Shaywitz et al., 2002). Under-activation in children seems to be most pronounced in tasks that involve difficult phonological processing (Shaywitz et al., 2002). Our finding of less activation in inferior frontal gyrus for children with dyslexia during the difficult conflicting pairs is consistent with this previous literature. The location of the difference between the controls and children with dyslexia in our study is close to a region that was sensitive to the conflict effect in a large group of control children (Bitan et al., in press), consistent with a deficit specific to difficult phonological processing. Effective connectivity analysis of the rhyming task has shown that the inferior frontal gyrus is involved in the top-down modulation of posterior regions involved in task-specific integration (Bitan et al., 2005). Children with dyslexia may not effectively recruit the inferior frontal gyrus for this top-down modulation of phonological representations in posterior temporal cortex. The reduced activation could therefore be a neural correlate of the wellestablished behavioral deficits in phonological processing in patients with dyslexia (Brady & Shankweiler, 1991; Bruck, 1992; Stanovich & Siegel, 1994; Shankweiler et al., 1995).
Middle temporal gyrus
Children with dyslexia in our study produced less activation than controls in the posterior region of the middle temporal gyrus. This finding is consistent with previous neuroimaging studies of patients with dyslexia (Rumsey et al., 1997b; Simos et al., 2000a; Paulesu et al., 2001; Shaywitz et al., 2002). Left middle temporal gyrus activation has been shown during a variety of semantic tasks (Pugh et al., 1996; Price, Moore, Humphreys, & Wise, 1997; Friederici, Opitz, & von Cramon, 2000). In addition, increases in accuracy have been associated with greater activation in middle temporal gyrus during association judgments (Blumenfeld, Booth, & Burman, 2006) and improvement in accuracy during semantic training has similarly resulted in greater activation in middle temporal gyrus (Sandak et al., 2004). The conflicting word pairs in our experiment were difficult to process, so perhaps the control children relied more on semantics for accessing the phonological representation in order to perform the rhyming judgment. Behavioral studies have shown increasing involvement of semantics for linguistic tasks when word reading becomes more difficult as when processing low frequency words (Becker, 1979; Stanovich & West, 1981; Borowski & Besner, 1993). Children with dyslexia may not have been able to effectively use their semantic representations as a compensatory mechanism because of their lower verbal abilities.
Conclusion
This study demonstrates that children with dyslexia have a deficit in a left hemisphere network involved in reading, including inferior frontal gyrus, inferior parietal lobule, and middle and ventral temporal cortex. The reduction of activation in the temporal regions could reflect deficits in orthographic representations and less reliance on semantic representations when processing the difficult conflicting pairs. The reduction in the parietal and frontal regions could reflect deficits in mapping between orthographic and phonological representations and in top-down modulation of these representations. Significant group differences were noted only for the more difficult conflicting trials, suggesting that task difficulty may be an important consideration for experimental designs intending to identify abnormal activation patterns in dysfunctional groups.
Acknowledgements
This research was supported by grants from the National Institute of Child Health and Human Development (HD042049) and by the National Institute of Deafness and Other Communication Disorders (DC06149) to JRB.
Abbreviations
- IPL
inferior parietal lobule
- IFG
inferior frontal gyrus
- FG
fusiform gyrus
- ITG
inferior temporal gyrus
- MeF
medial frontal gyrus
- BA
Brodmann Area
- EPI
echo planar imaging
- SPGR
spoiled gradient recalled acquisitions in the steady state
- SPM2
statistical parametric mapping
- HRF
hemodynamic response function
- TE
Time of echo
- TR
Time of repetition.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- Balsamo LM, Xu B, Gaillard WD. Language lateralization and the role of the fusiform gyrus in semantic processing in young children. NeuroImage. doi: 10.1016/j.neuroimage.2006.01.027. (in press) [DOI] [PubMed] [Google Scholar]
- Becker CA. Semantic context and word frequency effects in visual word recognition. Journal of Experimental Psychology: Human Perception and Performance. 1979;5:556–566. doi: 10.1037//0096-1523.5.2.252. [DOI] [PubMed] [Google Scholar]
- Bitan T, Booth JR, Choy J, Burman DD, Gitelman DR, Mesulam MM. Shifts of effective connectivity within a language network during rhyming and spelling. Journal of Neuroscience. 2005;25:5397–5403. doi: 10.1523/JNEUROSCI.0864-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Chou TL, Dong L, Cone NE, Cao F, Bigio JD, Booth JR. The interaction of orthographic and phonological information in children: An fMRI study. Human Brain Mapping. doi: 10.1002/hbm.20313. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld HK, Booth JR, Burman DD. Differential prefrontal-temporal neural correlates of semantic processing in children. Brain and Language. 2006 doi: 10.1016/j.bandl.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Functional anatomy of intra- and cross-modal lexical tasks. NeuroImage. 2002;16:7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Zhang L, Choy J, Gitelman DR, Parrish TR, Mesulam MM. Modality-specific and -independent developmental differences in the neural substrate for lexical processing. Journal of Neurolinguistics. 2003;16:383–405. [Google Scholar]
- Borowski R, Besner D. Visual word recognition: A multistage activation model. Journal of Experimental Psychology: Learning Memory and Cognition. 1993;19:812–940. doi: 10.1037//0278-7393.19.4.813. [DOI] [PubMed] [Google Scholar]
- Brady SA, Shankweiler DP. Phonological processes in literacy: A tribute to Isabelle Y. Liberman. Erlbaum; Hillsdale, NJ: 1991. [Google Scholar]
- Bruck M. Persistence of dyslexic's phonological awareness deficits. Developmental Psychology. 1992;28:874–886. [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics. Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. NeuroReport. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff M, Michel F. The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L, Jobert A, Le Bihan D, Dehaene S. Distinct unimodal and multimodal regions for word processing in the left temporal cortex. NeuroImage. 2004;23:1256–1270. doi: 10.1016/j.neuroimage.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline JB, Le Bihan D, Cohen L. Letter binding and invariant recognition of masked words: Behavioral and neuroimaging evidence. Psychological Science. 2004;15:307–313. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Fiebach C, Friederici AD, Müller K, von Cramon DY. fMRI evidence for dual routes to the mental lexicon in visual word recognition. Journal of Cognitive Neuroscience. 2002;14:11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24:205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Opitz B, von Cramon DY. Segregating semantic and syntactic aspects of processing in the human brain: An fMRI investigation of different word types. Cerebral Cortex. 2000;10:698–705. doi: 10.1093/cercor/10.7.698. [DOI] [PubMed] [Google Scholar]
- Georgiewa P, Rzanny R, Gaser C, Gerhard UJ, Vieweg U, Freesmeyer D, Mentzel HJ, Kaiser WA, Blanz B. Phonological processing in dyslexic children: A study combining functional imaging and event related potentials. Neuroscience Letters. 2002;318:5–8. doi: 10.1016/s0304-3940(01)02236-4. [DOI] [PubMed] [Google Scholar]
- Georgiewa P, Rzanny R, Hopf JM, Knab R, Glauche V, Kaiser WA, Blanz B. fMRI during word processing in dyslexic and normal reading children. Neuroreport: An International Journal for the Rapid Communication of Research in Neuroscience. 1999;10:3459–3465. doi: 10.1097/00001756-199911080-00036. [DOI] [PubMed] [Google Scholar]
- Helenius P, Uutela K, Hari R. Auditory stream segregation in dyslexic adults. Brain. 1999;122(Pt 5):907–913. doi: 10.1093/brain/122.5.907. [DOI] [PubMed] [Google Scholar]
- Johnston RS, McDermott EA. Suppression effects in rhyme judgement tasks. Quarterly Journal of Experimental Psychology A. 1986;38A:111–124. [Google Scholar]
- Kramer AF, Donchin E. Brain potentials as indices of orthographic and phonological interaction during word matching. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13:76–86. doi: 10.1037//0278-7393.13.1.76. [DOI] [PubMed] [Google Scholar]
- Levinthal CF, Hornung M. Orthographic and phonological coding during visual word matching as related to reading and spelling abilities in college students. Reading and Writing. 1992;4:1–20. [Google Scholar]
- McPherson BW, Ackerman PT, Dykman RA. Auditory and visual rhyme judgements reveal differences and similarities between normal and disabled adolescent readers. Dyslexia. 1997;3:63–77. [Google Scholar]
- Paulesu E, Demonet JF, Fazio F, McCrory E, Chanoine V, Brunswick N, Cappa SF, Cossu G, Habib M, Frith CD, Frith U. Dyslexia: Cultural diversity and biological unity. Science. 2001;291:2165–2167. doi: 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull M, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Polich J, McCarthy G, Wang WS, Donchin E. When words collide: Orthographic and phonological interference during word processing. Biological Psychology. 1983;16:155–80. doi: 10.1016/0301-0511(83)90022-4. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Wise RJS. Segregating semantic from phonological processes during reading. Journal of Cognitive Neuroscience. 1997;9:727–733. doi: 10.1162/jocn.1997.9.6.727. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJS, Frackowiak RJS. Demonstrating the implicit processing of visually presented words and pseudowords. Cerebral Cortex. 1996;6:62–78. doi: 10.1093/cercor/6.1.62. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AJ, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Mental Retardation and Developmental Disabilities Research Reviews. 2000b;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Shankweiler DP, Katz L, Fletcher JM, Gore JC. Cerebral organization of component processes in reading. Brain. 1996;119:1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Lee JR, Katz L, Frost SJ, Shaywitz SE, Shaywitz BA. The angular gyrus in developmental dyslexia: Task specific differences in functional connectivity within posterior cortex. Psychological Science. 2000a;11:51–56. doi: 10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- Rack JP. Orthographic and phonetic coding in developmental dyslexia. British Journal of Psychology. 1985;76:325–340. doi: 10.1111/j.2044-8295.1985.tb01956.x. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Barrett SE. Event-related potentials and the interaction between orthographic and phonological information in a rhyme-judgment task. Brain and Language. 1987;32:336–361. doi: 10.1016/0093-934x(87)90132-5. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Donohue BC, Brady DR, Nace K, Giedd JN, Andreason P. A magnetic resonance imaging study of planum temporale asymmetry in men with developmental dyslexia. Archives of Neurology. 1997a;54:1481–1489. doi: 10.1001/archneur.1997.00550240035010. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Nace K, Donohue B, Wise D, Maisog JM, Andreason P. A positron emission tomographic study of impaired word recognition and phonological processing in dyslexic men. Archives of Neurology. 1997b;54:562–573. doi: 10.1001/archneur.1997.00550170042013. [DOI] [PubMed] [Google Scholar]
- Sandak R, Mencl WE, Frost SJ, Rueckl JG, Katz L, Moore DL, Mason SA, Fulbright RK, Constable RT, Pugh KR. The neurobiology of adaptive learning in reading: A contrast of different training conditions. Cognitive, Affective and Behavioral Neuroscience. 2004;4:67–88. doi: 10.3758/cabn.4.1.67. [DOI] [PubMed] [Google Scholar]
- Shankweiler D, Crain S, Katz L, Fowler AE, Liberman AM, Brady SA, Thornton R, Lundquist E, Dreyer L, Fletcher JM, Stuebing KK, Shaywitz SE, Shaywitz BA. Cognitive profiles of reading-disabled children: Comparison of language skills in phonology, morphology, and syntax. Psychological Science. 1995;6:149–156. [Google Scholar]
- Shaywitz B, Shaywitz S, Pugh KR, Mencl WE, Fulbright RK, Skudlarksi P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Pugh KR, Holahan JM, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Neural systems for compensation and persistence: Young adult outcome of childhood reading disability. Biological Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, Shankweiler DP, Liberman AM, Skudlarski P, Fletcher JM, Katz L, Marchione KE, Lacadie C, Gatenby C, Gore JC. Functional disruption in the organization of the brain for reading in dyslexia. Proceedings of the National Academy of Sciences. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Bergman E, Papanicolaou AC. Cerebral mechanisms involved in word reading in dyslexic children: A magnetic source imaging approach. Cerebral Cortex. 2000a;10:809–816. doi: 10.1093/cercor/10.8.809. [DOI] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Wheless JW, Maggio WW, Fletcher JM, Castillo EM, Papanicolaou AC. Brain mechanisms for reading: The role of the superior temporal gyrus in word and pseudoword naming. Neuroreport. 2000b;11:2443–2447. doi: 10.1097/00001756-200008030-00021. [DOI] [PubMed] [Google Scholar]
- Stanovich KE, Siegel LS. Phenotypic performance profile of children with reading disabilities: A regression based approach to the phonological-core variable-difference model. Journal of Educational Psychology. 1994;86:24–53. [Google Scholar]
- Stanovich KE, West RF. The effect of sentence context on ongoing word recognition: Tests of a two-process theory. Journal of Experimental Psychology: Human Perception and Performance. 1981;7:568–672. [Google Scholar]
- Tarkiainen A, Helenius P, Hansen PC, Cornelissen PL, Salmelin R. Dynamics of letter string perception in the human occipitotemporal cortex. Brain. 1999;122:2119–2131. doi: 10.1093/brain/122.11.2119. [DOI] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Salidis J, Deutsch GK, Tallal P, Merzenich MM, Gabrieli JDE. Disrupted neural responses to phonological and orthographic processing in dyslexic children: An fMRI study. Neuroreport: An International Journal for the Rapid Communication of Research in Neuroscience. 2001;12:299–307. doi: 10.1097/00001756-200102120-00024. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of word reading efficiency. PRO-ED, Inc.; Austin, TX: 1999. [Google Scholar]
- Wagner AD, Desmond JE, Demb JB, Glover GH, Gabrieli JDE. Semantic repetition priming for verbal and pictorial knowledge: A functional MRI study of left inferior prefrontal cortex. Journal of Cognitive Neuroscience. 1997;9:714–726. doi: 10.1162/jocn.1997.9.6.714. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; Austin, TX: 1999. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. The Riverside Publishing Company; Itasca, IL: 2001. [Google Scholar]
- Xiong R, Rao S, Jerabek P, Zamarripa F, Woldorff M, Lancaster J, Fox PT. Intersubject variability in cortical activations during a complex language task. NeuroImage. 2000;12:326–339. doi: 10.1006/nimg.2000.0621. [DOI] [PubMed] [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega-Bermudez F, Pietrini P, Reeves-Tyer P, DiCamillo P, Theodore W. Conjoint and extended neural networks for the computation of speech codes: The neural basis of selective impairment in reading words and pseudowords. Cerebral Cortex. 2001;11:267–277. doi: 10.1093/cercor/11.3.267. [DOI] [PubMed] [Google Scholar]